Slow-release enteric-coated sodium ferulate preparation and preparation method thereof
A technology of sodium ferulate and slow-release preparations, which is applied in the field of pharmaceutical preparations, can solve the problems of poor stability, great influence on stability, and low bioavailability of ordinary tablets, so as to improve bioavailability, reduce the number of times of taking medicine, Guarantee the effect of drug safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
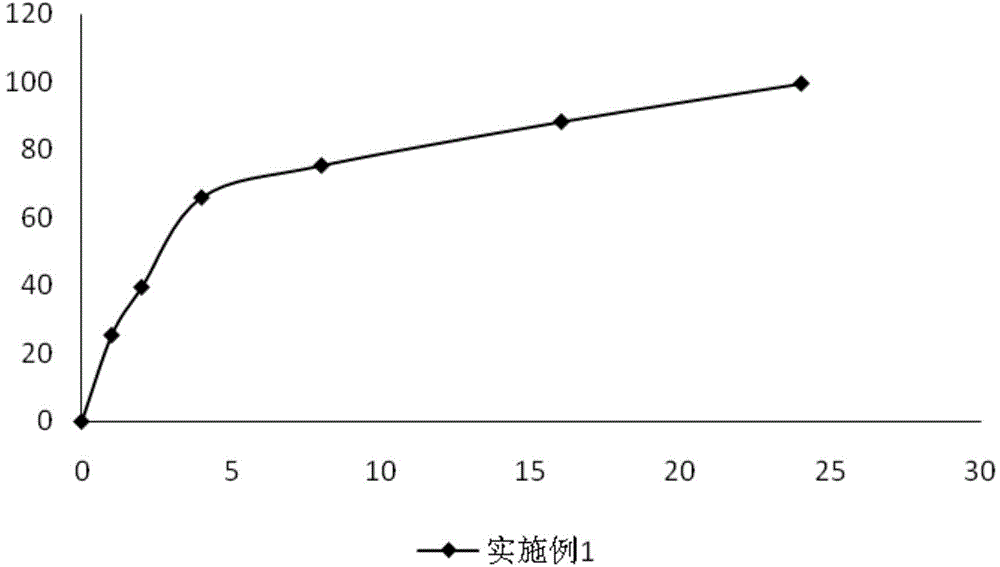
Embodiment 1
[0050]
[0051] Preparation method: pass sodium ferulate, polyvinylpyrrolidone, microcrystalline cellulose, and sucrose through a 100-mesh sieve respectively, weigh the sodium ferulate, polyvinylpyrrolidone, microcrystalline cellulose, and sucrose according to the prescription amount, and mix them in a mixer Evenly, place it in a centrifugal granulator, spray 75g of ethanol solution with a volume percentage of 80% to make small pellets, and prepare hypromellose slow-release coating solution according to the prescription of the slow-release coating solution to coat the core of the plain pill. Coating, sustained release coating liquid solid content 18% ~ 21%, weight gain 2.8% ~ 3.2%, dry air 60 ~ 80m 3 / h, the air inlet temperature is 55-60°C, the coating time is 3-3.5h, and then 800g of ethanol solution with a volume percentage of 75% is used to prepare the coating powder containing the enteric material cellulose acetate phthalate Dissolve the coating solution, and then coat...
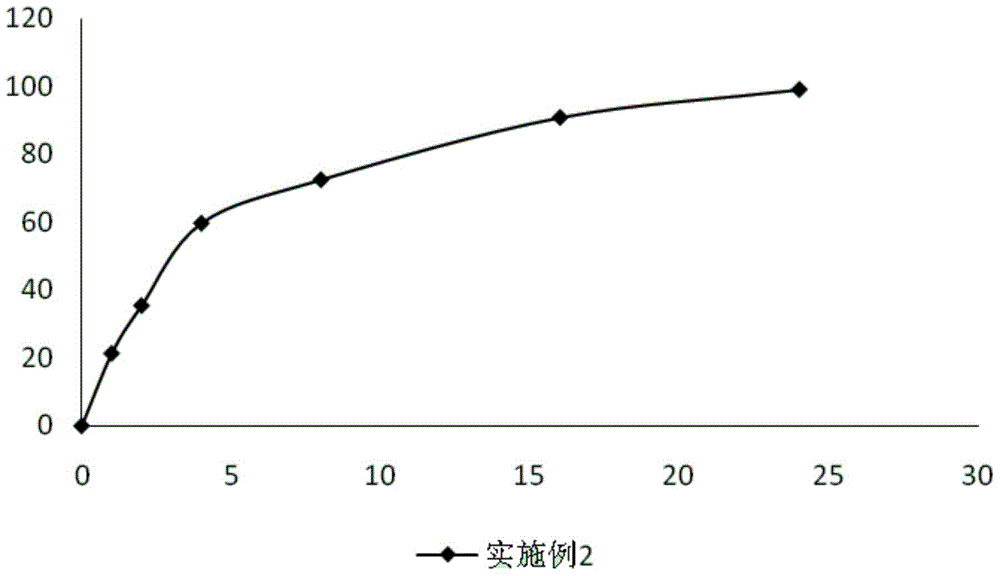
Embodiment 2
[0053]
[0054] Preparation method: pass sodium ferulate, polyvinylpyrrolidone, microcrystalline cellulose, and sucrose through a 100-mesh sieve respectively, weigh the sodium ferulate, polyvinylpyrrolidone, microcrystalline cellulose, and sucrose according to the prescription amount, and mix them in a mixer Evenly, place it in a centrifugal granulator, spray 75g of ethanol solution with a volume percentage of 80% to make small pellets, and prepare hypromellose slow-release coating solution according to the prescription of the slow-release coating solution to coat the core of the plain pill. Coating, sustained release coating liquid solid content 18% ~ 21%, weight gain 2.8% ~ 3.2%, dry air 60 ~ 80m 3 / h, the air inlet temperature is 55-60°C, the coating time is 3-3.5h, and then 800g of ethanol solution with a volume percentage of 75% is used to prepare the coating powder containing the enteric material cellulose acetate phthalate Dissolve the coating solution, and then coat...
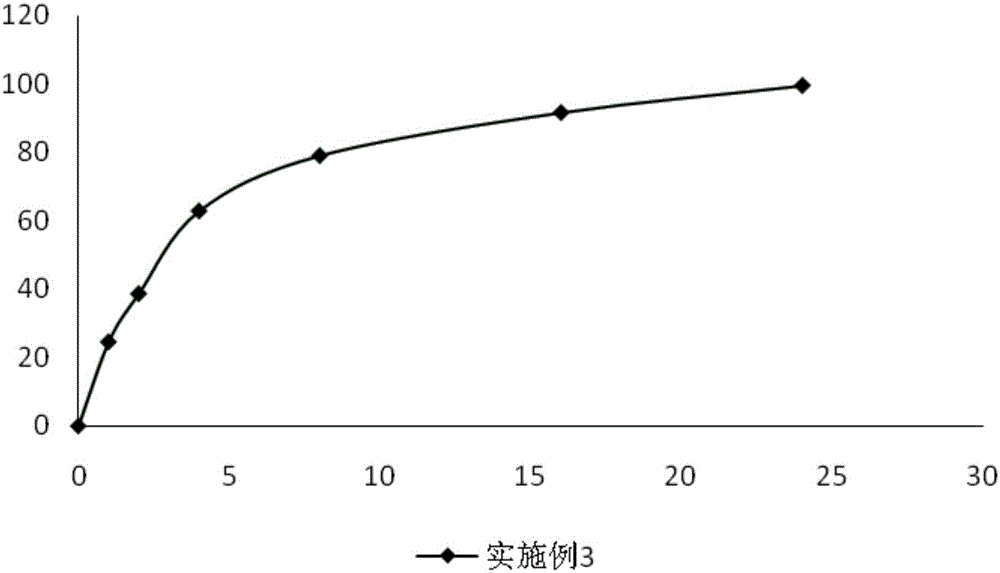
Embodiment 3
[0056]
[0057] Preparation method: pass sodium ferulate, polyvinylpyrrolidone, microcrystalline cellulose, and sucrose through a 100-mesh sieve respectively, weigh the sodium ferulate, polyvinylpyrrolidone, microcrystalline cellulose, and sucrose according to the prescription amount, and mix them in a mixer Uniformly, be placed in centrifugal granulator, spray into 75g volume percent and be the ethanol solution system pellet of 80%, be prepared into coating solution by slow-release material, prepare hypromellose slow-release coating solution according to prescription The core of the vegetarian pill is coated, the solid content of the slow-release coating liquid is 18% to 21%, the weight gain is 2.8% to 3.2%, and the dry air is 60 to 80m 3 / h, the air inlet temperature is 55-60°C, the coating time is 3-3.5h, and then 800g of ethanol solution with a volume percentage of 75% is used to prepare the coating powder containing the enteric material cellulose acetate phthalate Diss...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com