Recombinant L-arabinose isomerase as well as gene and application thereof
A technology of arabinose and isomerase, applied in the direction of isomerase, application, genetic engineering, etc., to achieve the effect of short cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
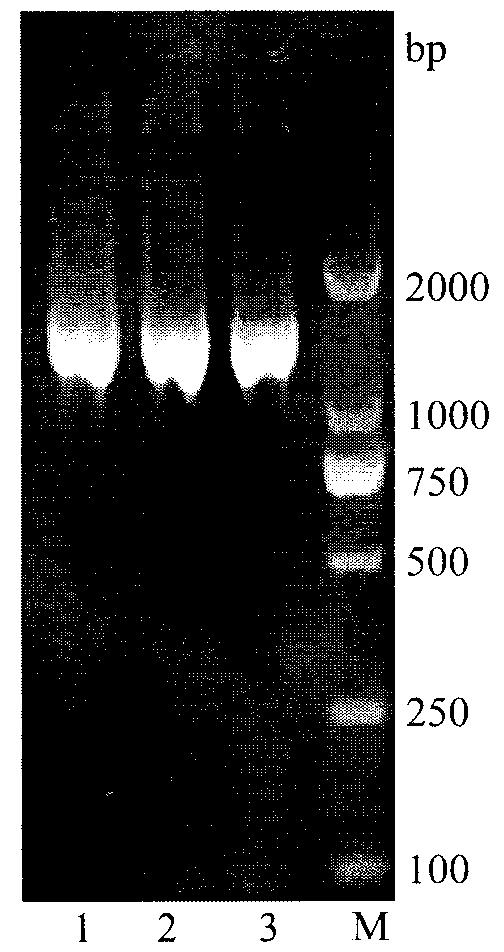
[0032] The construction of embodiment 1 recombinant plasmid pMA5-araA
[0033]1) Obtained the reported amino acid sequence of L-arabinose isomerase from the NCBI website, and obtained the L-arabinose isomerase that is beneficial to the expression in Bacillus subtilis by CluxtalX software comparison analysis, splicing and codon optimization Gene, the sequence of which is shown in the sequence listing SEQ ID NO.1. The optimized gene was synthesized by chemical synthesis, and the synthesized gene was connected to the cloning vector pUC57 (purchased from Shanghai Jingjing) to obtain the cloning vector pUC57-araA. synthesis).
[0034] 2) Design primers for PCR amplification of L-arabinose isomerase, the sequence of the upstream primer TG_F is 5'-GGAATTC CATATG ATGCTGTCATTACGTCC-3', the sequence of the downstream primer TG_R is 5'-GA GGATCC TTACCGCCCCCGCCAGAATACT-3'; with Nde I and BamH I restriction sites respectively.
[0035] 3) Using the cloning vector pUC57-araA containing...
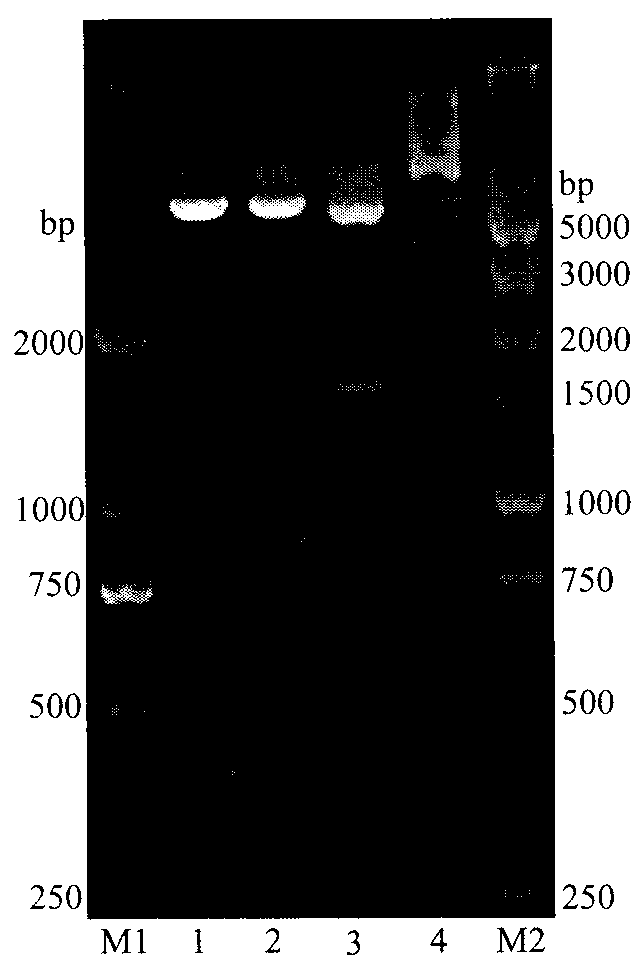
Embodiment 2
[0038] Example 2 Construction of recombinant Bacillus subtilis and induced expression of L-arabinose isomerase
[0039] 1) Prepare WB600 competent cells, the specific steps include:
[0040] a. Inoculate WB600 (Wu XC, Lee W, Tran L, Wong SL.Engineering a Bacillus subtilis expression-secretion system with a strain deficiency in six extracellular proteases.J Bacteriol.1991; 173(16):4952-4958) in 3mL In liquid medium (LB containing 0.5 mol / L sorbitol), culture overnight at 200 rpm at 37°C.
[0041] b. Take 2 mL of the overnight culture, inoculate it into 40 mL of liquid medium (LB containing 0.5 mol / L sorbitol), and culture at 200 rpm at 37°C until OD600=0.85-0.95.
[0042] c. Collect the cells by centrifugation at 5000 g for 5 min at 4°C, suspend the cells with 50 mL of pre-cooled shock buffer (0.5 mol / L sorbitol, 0.5 mol / L mannitol, 10% glucose), and rinse 4 times.
[0043] d. Centrifuge as above, suspend the cells with 1 mL of pre-cooled shock buffer, and aliquot 60 μL / tube....
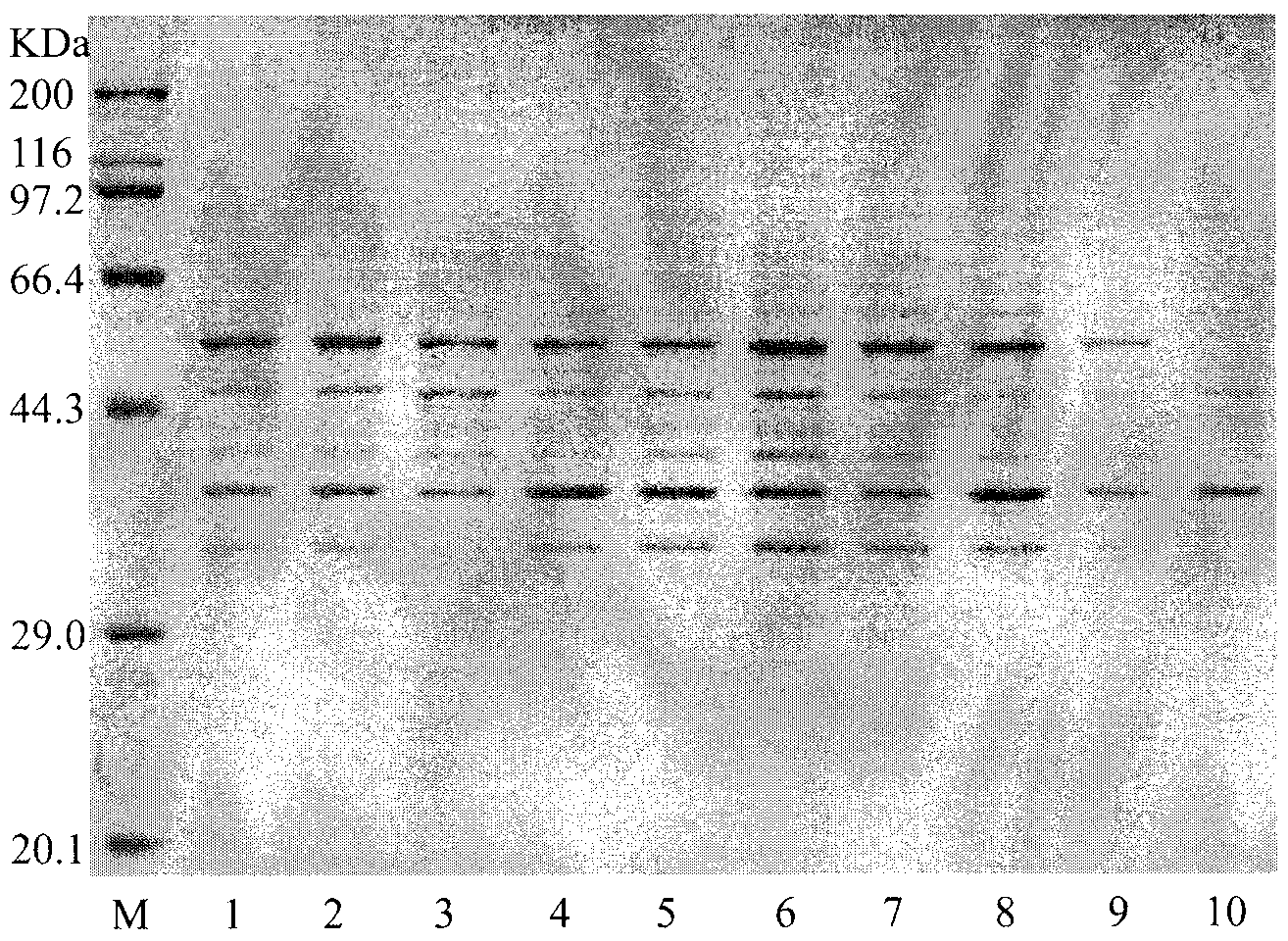
Embodiment 3
[0052] Example 3 Condition optimization for the preparation of tagatose by L-arabinose isomerase
[0053] 1) Determination of L-arabinose isomerase activity: take the bacteria after fermentation and culture, suspend in 70mM Tris-HCl (pH7.6) buffer at a concentration of 100mg / ml, add 100mg / ml substrate galactose, Shake at 60°C for 1 hour, centrifuge at 12,000 rpm for 5 minutes to collect the supernatant, and measure the change in absorbance at 560 nm using the improved cysteine-carbazole method after appropriate dilution. The specific steps are as follows:
[0054] a. Take 0, 0.2, 0.4, 0.6, 0.8mL tagatose standard solution (50mg / L) in a stoppered test tube, make up to 1.0mL with distilled water.
[0055] b. Take a 25mL stoppered test tube, add 1.0mL of appropriately diluted reaction solution and control solution, and then add 6.0mL of 70% (V / V) sulfuric acid solution, 0.2mL of 1.5% (W / V) half Cystine hydrochloride solution, shake well, immediately add 0.2mL 0.12% (W / V) carbazo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com