Sodium aescinate micro-emulsification injection and preparation method thereof
A escin sodium and microemulsification technology, which is applied in the direction of anti-inflammatory agents, pharmaceutical formulations, emulsion delivery, etc., can solve the problems of low drug efficacy, low bioavailability, and effective concentration, and achieve the goal of reducing phlebitis Production, high bioavailability, and the effect of reducing toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 7
[0040] The investigation of the oil-water partition coefficient of embodiment 1 sodium aescinate microemulsion injection
[0041] The n-octanol-water partition coefficient is the logarithm value of the ratio of the concentration of organic compounds in the n-octanol phase and the water phase at equilibrium. Saturate n-octanol with double distilled water, pH4.0 acetate buffer, pH6.8 phosphate buffer, pH9.0 phosphate buffer, and use the above-mentioned n-octanol-saturated water and buffer Prepare β-escin sodium, and measure the oil-water partition coefficient 3.Oh after mixing respectively. The results are shown in Table 1.
[0042] Table 1. Oil-water partition coefficient of β-escin sodium
[0043]
[0044] The results show that β-escin is weakly acidic, β-escin sodium is weakly alkaline, and β-escin sodium has strong hydrophilicity under neutral and alkaline conditions, and is free under acidic conditions to β - Aescin, showing a certain lipophilicity.
Embodiment 2 7
[0045] The investigation of embodiment 2 sodium aescinate microemulsion injection oil phase
[0046]The main instruments are: AL204-IC precision electronic balance (Mettler-Tomido Instrument Co.), BP211D precision electronic balance (Sartorius AG), 85-2 constant temperature magnetic stirrer (Shanghai Sile Instrument Factory), JSM -5900LV scanning electron microscope (JEOL, Japan), Zetasizer Nano ZS90 laser particle size analyzer / Zeta potential meter (Malvern, UK). The main reagents are: sodium aescinate (Wuxi Kaifu Pharmaceutical Co., Ltd.), soybean lecithin (SPC, Shanghai Taiwei Pharmaceutical Co., Ltd.), soybean oil (Tieling Beiya Pharmaceutical Oil Co., Ltd.), medium chain triglycerides Ester (Tieling Beiya Medicinal Oil Co., Ltd.), glycerin (Tieling Beiya Medicinal Oil Co., Ltd.), HS15 (Germany BASF), Pluronic F-68 (Germany BASF), etc., and other reagents are of analytical grade.
[0047] Prescription Screening of Blank Microemulsion
[0048] The key to preparing microem...
Embodiment 3
[0057] The screening of embodiment 3 emulsifiers
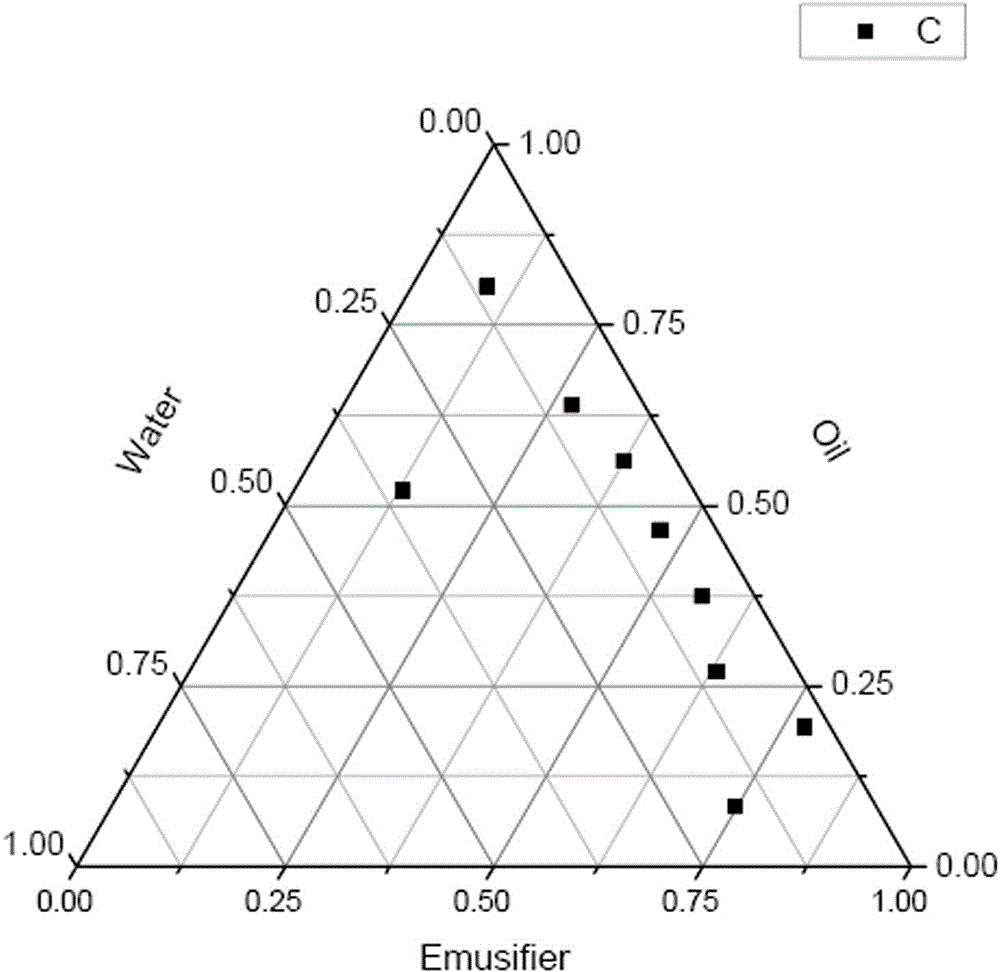
[0058] The screened medium-chain triglyceride is used as the oil phase. Respectively with Pluronic F-68, soybean lecithin, Pluronic F-68 + soybean lecithin (1:1), Pluronic F-68 + HS15 (1:1), soybean lecithin + HS15 (1:1) As the emulsifier to be selected, glycerin is used as the co-emulsifier, Km=2:1, and the mass ratio of the mixed surfactant and oil phase is 9:1, 8:2, 7:3, 6:4, 5 :5, 4:6, 3:7, 2:8, 1:9 mixed, and distilled water was added dropwise at 25°C until a transparent and clear microemulsion was formed. When the appearance is no longer clear, record the critical addition. According to the mass percentage of oil, water and mixed surfactant at the critical point, the curve is drawn in the pseudo ternary phase diagram to determine the O / W microemulsion area. The experimental data are shown in Table 4-8.
[0059] Table 4 Screening of emulsifiers (Pluronic F-68)
[0060]
[0061] Table 5 Screening of emulsifiers (so...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com