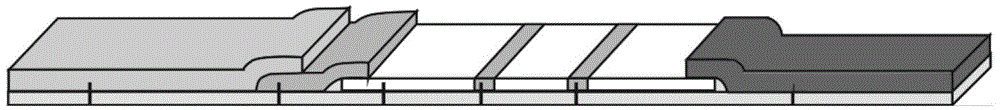
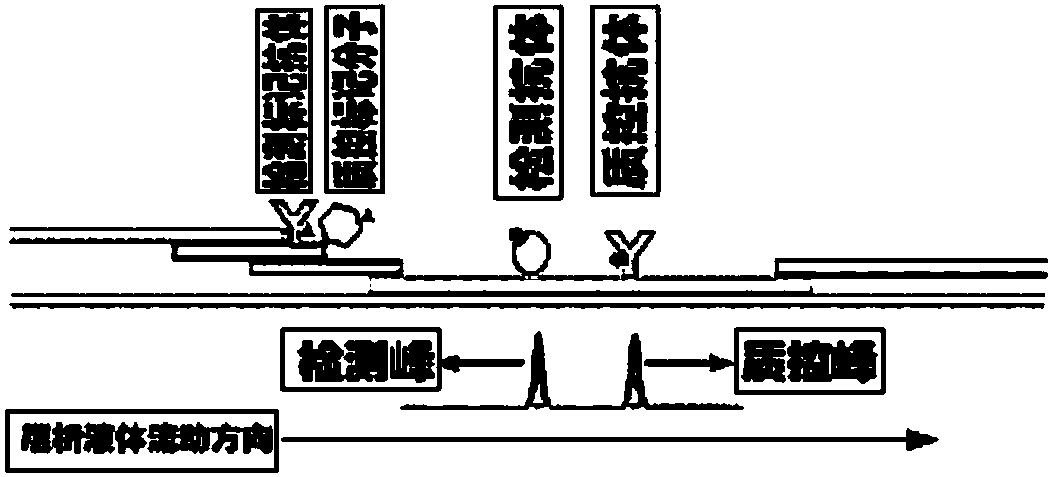
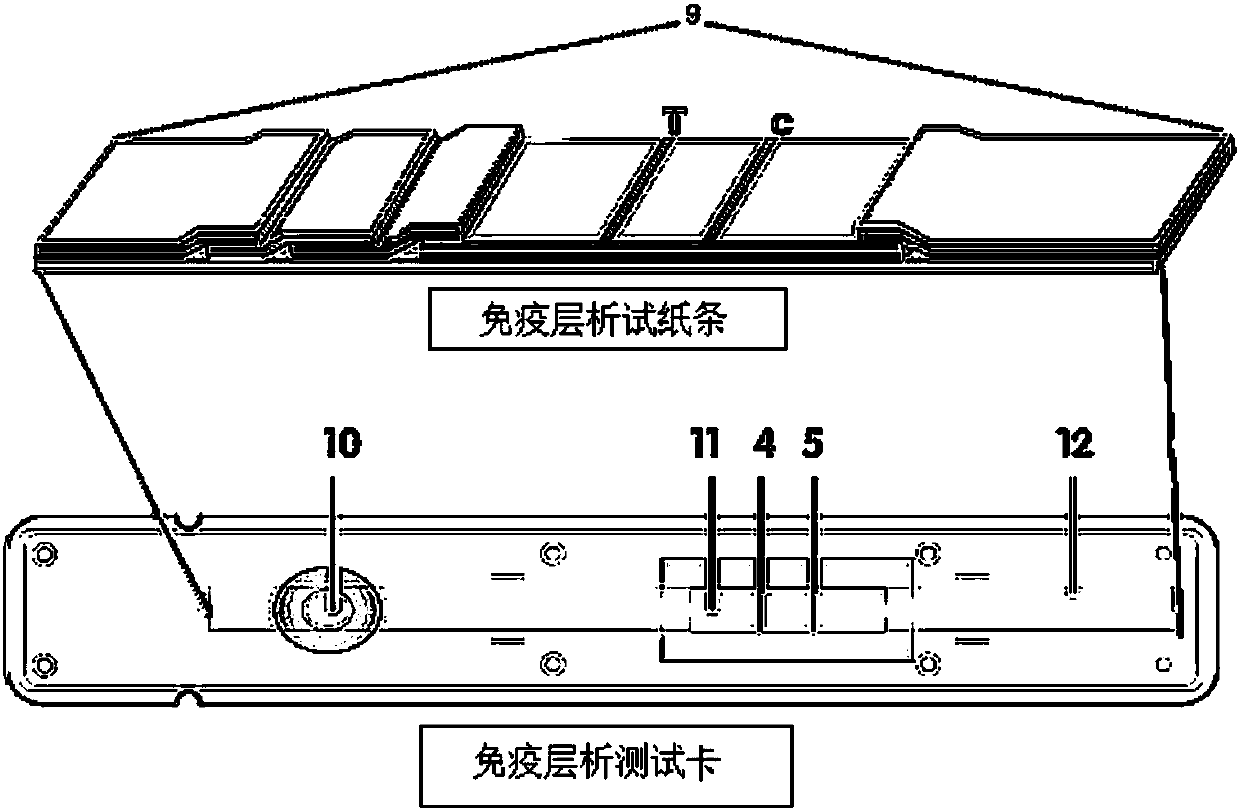
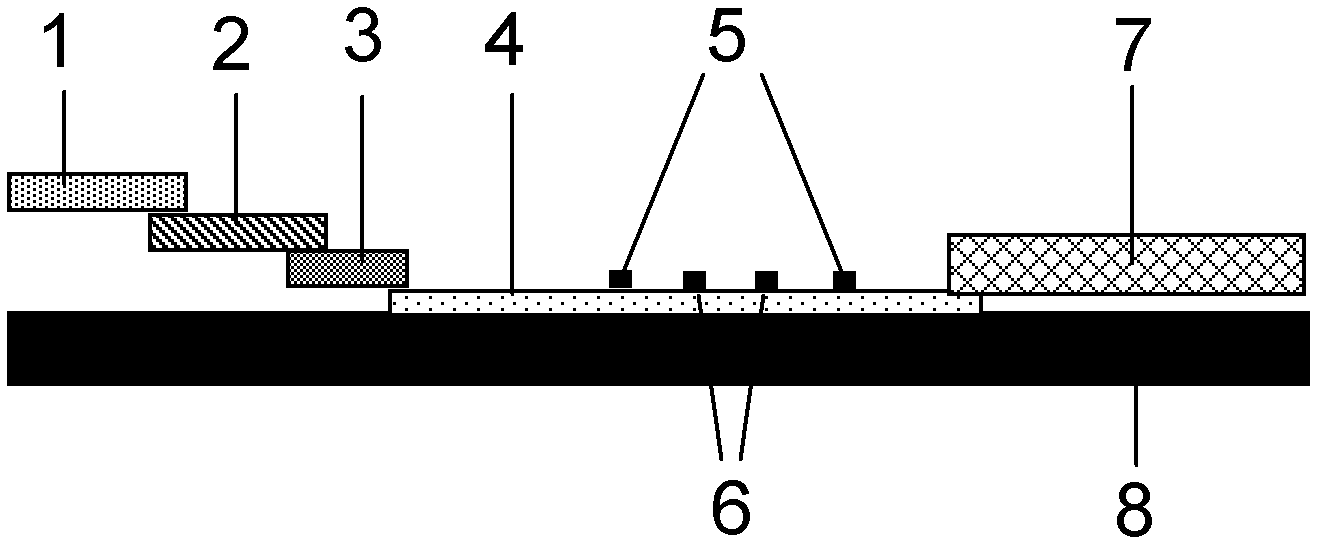
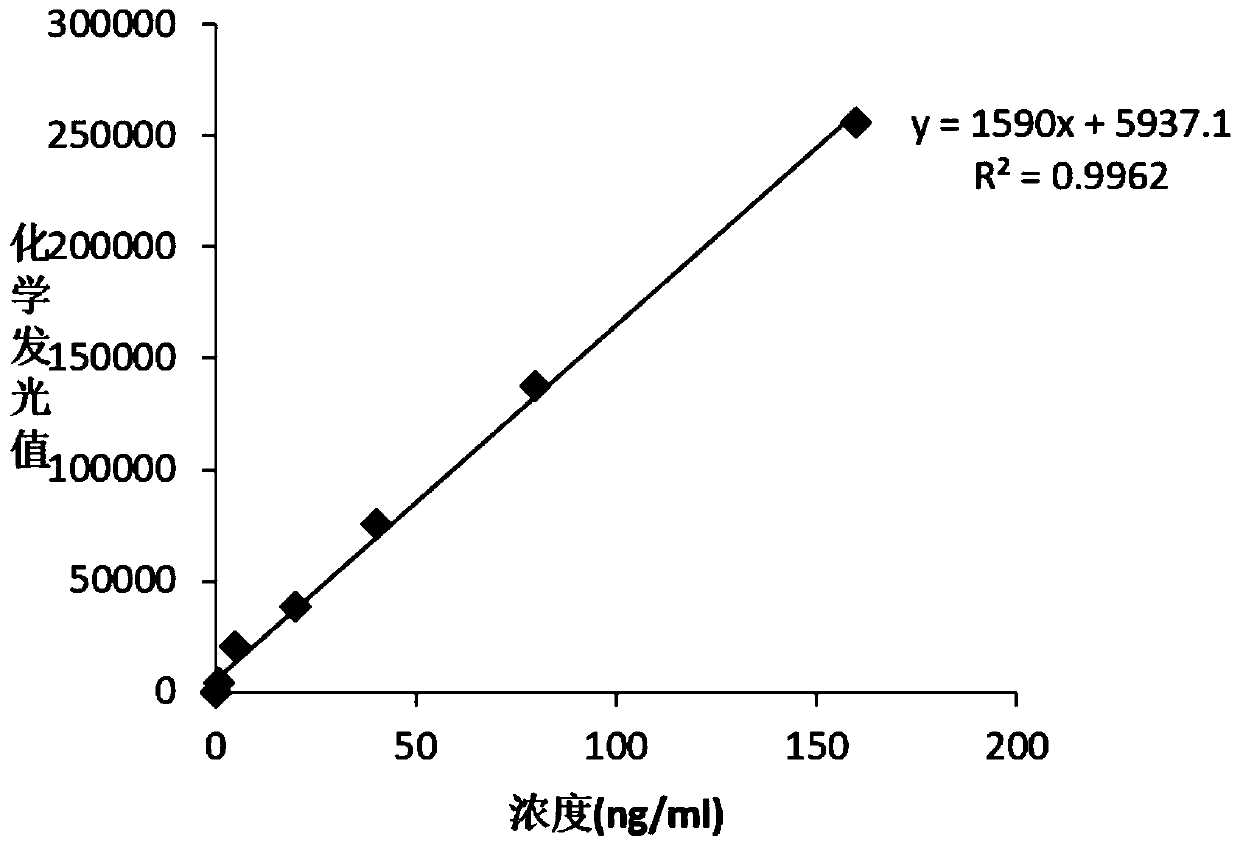
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
33results about How to "Good marking stability" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
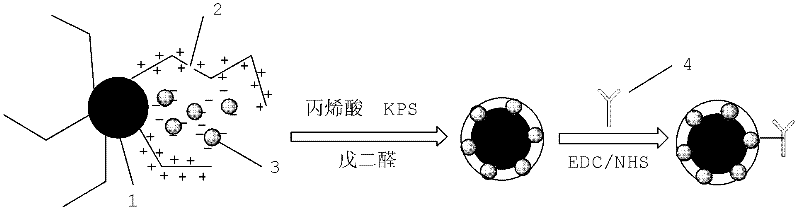
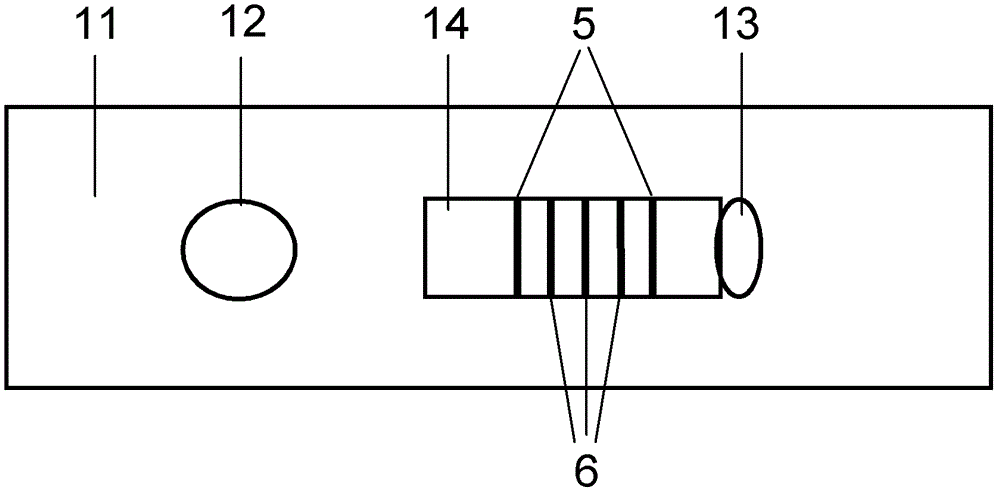
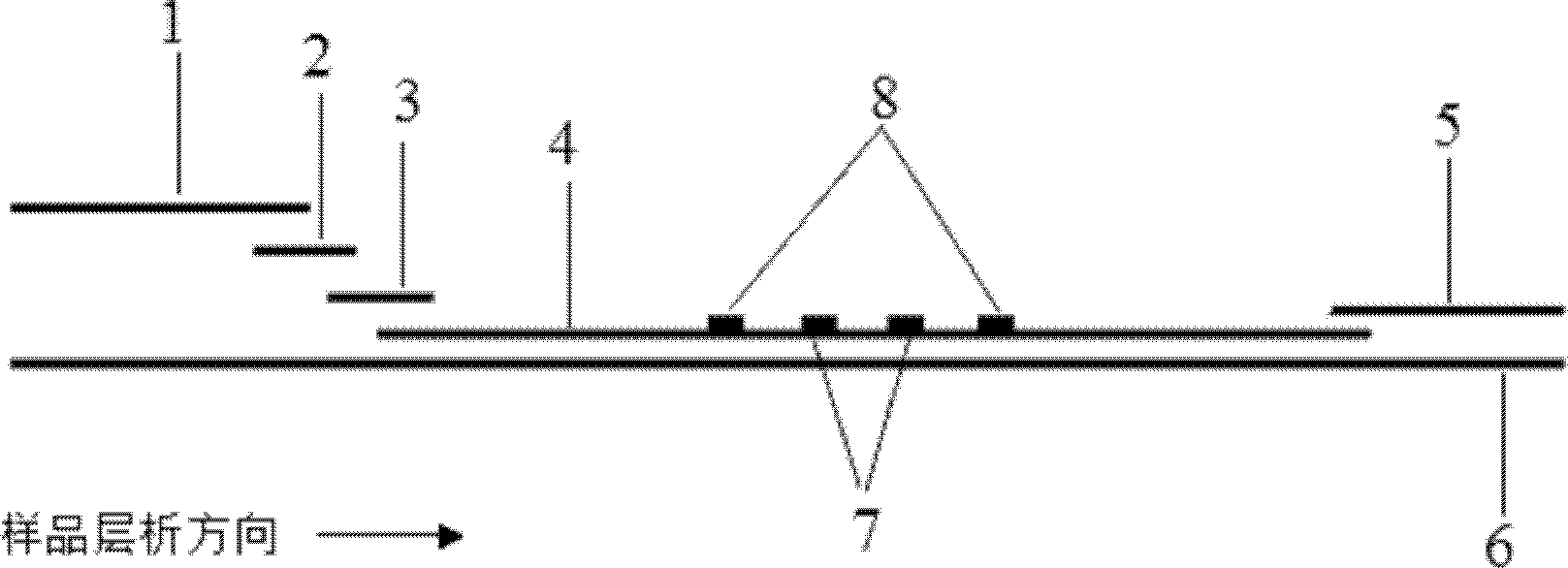
Method for detecting by using quantum dot fluorescence immunochromatographic test strips
ActiveCN103048460AHigh fluorescence intensityOperational securityMaterial analysisFluorescenceQuantum dot
The invention provides a method for detecting by using quantum dot fluorescence immunochromatographic test strips. The method comprises the following specific steps: (1) preparing a quantum dot labeled protein liquid; (2) preparing a quantum dot labeled protein detecting liquid; (3) preparing a reaction film coated with a detection index; (4) bonding a sample pad, the reaction film prepared in the step (3) and a water absorbing pad on a PVC backing in sequence so as to obtain a test paper board; cutting the test paper board into test strips; and loading the test strips into a test paper clip; and (5) qualitatively or quantitatively detecting. The method for detecting by using the quantum dot fluorescence immunochromatographic test strips, provided by the invention, is a rapid detecting method using the quantum dot fluorescence immunochromatographic test strips, which is featured by high sensitivity, synchronicity in multi-index detection, simplicity, convenience, intuitive nature, low price and wide application.
Owner:WUHAN JIAYUAN BIOMEDICAL ENG
Method for highly sensitive quantitative detection of quantum dot fluorescence immunochromatographic assay
ActiveCN102520165ASensitive quantitative detection fastRealize detectionMaterial analysisCritical illnessLinear range
The invention discloses a method for highly sensitive quantitative detection of quantum dot fluorescence immunochromatographic assay. The method includes: building a fluorescence immunochromatographic assay test strip on the basis of optimizing the structure of the test strip and components by the aid of excellent fluorescent characteristics of quantum dots and by means of combining quantum dot fluorescence labeling technology and immunochromatographic assay; detecting fluorescence signal strength of a quantitative belt and a quality control belt by the aid of a fluorescence quantometer and correcting the fluorescence strength of the quantitative belt by the aid of the quality control belt after immunochromatographic assay of the test strip; and further quantitatively detecting analyte according to a standard curve obtained by the fluorescence quantometer. The method is simple, rapid, accurate, low in cost and quite high in sensitivity. Compared with a conventional colloidal gold immunochromatographic assay method, the method has the advantages of fine labeling stability, low non-specificity, high sensitivity, wide linear range and accuracy in quantization. The method is applicable to samples such as blood samples, urine samples, spittle, excrement and the like, and can be applied to detection of critical illness, poison, food safety and the like.
Owner:BEIJING KANGMEI TIANHONG BIOTECH
Fluorescence immunochromatographic assay and kit for quantitative detection of troponin I/creatine kinase isoenzyme/myohemoglobin
ActiveCN102520192AHigh sensitivityHigh detection sensitivityBiological testingNon specificImmunochromatographic Assays
The invention discloses a quantum dot multicolor marking method for quantitative detection of various cardiovascular disease markers and a kit of troponin I / creatine kinase isoenzyme / myohemoglobin. The method realizes fluorescent quantitative detection by utilizing excellent fluorescent properties of quantum dots and combining a multicolour marking technology and an immunochromatographic assay on the basis of optimizing each component of a test strip. Compared with the common collaurum immunochromatographic assay, the method has the advantages of good mark stability, low nonspecificity, high sensitivity, wide linear range, small cross interference, and accuracy in quantification. The kit disclosed by the invention is used for carrying out quantification detection on the troponin I, the creatine kinase isoenzyme and the myohemoglobin simultaneously, is suitable for detection of whole blood, blood serum and plasma samples, can provide a reference for cardiovascular and cerebrovascular disease diagnosis, and is widely applied to primary hospitals and clinics.
Owner:SHENZHEN KANGMEI BIOTECH
Fluorescent immunochromatography method for whole quantitative detection of C-reactive protein and reagent kit thereof
ActiveCN102539785ASolve the backgroundSolve the signal indistinguishableBiological testingFluorescence/phosphorescenceBasic levelQuantum dot
The invention discloses a fluorescent immunochromatography method for whole quantitative detection of C-reactive protein and a reagent kit thereof. The fluorescent immunochromatography method for the whole quantitative detection of the C-reactive protein (CRP) utilizes excellent fluorescent characteristics of quantum dots, and combines double-color marking technology and immunochromatography technology to achieve fluorescent quantitative detection on the basis of optimizing each constituent elements of test paper. Compared with a conventional colloidal gold immunochromatography method, the fluorescent immunochromatography method for the whole quantitative detection of the CRP has the advantages of being good in stability, low in non-specificity, high in flexibility, wide in linear range and accurate in quantifying. The reagent kit of the fluorescent immunochromatography method can perform the whole quantifying and can simultaneously predict and evaluate infectious diseases, antibiotic effects and cardiovascular and cerebrovascular diseases. The fluorescent immunochromatography method for the whole quantitative detection of the CRP and the reagent kit of the fluorescent immunochromatography method are suitable for various-level hospitals, and particularly contribute to wide popularization in basic-level hospitals and clinics.
Owner:SHENZHEN KANGMEI BIOTECH
Magnetic fluorescent microsphere immunochromatography quantitative detection method
The invention discloses a magnetic fluorescent microsphere immunochromatography quantitative detection method. In the method, respective excellent characteristics of magnetic nano particles and quantum dots are fully utilized, and an immunochromatography technology is combined to realize fluorescent quantitative detection on the basis of optimizing the structure and ingredients of a test strip. The method has a function of amplifying signals; and compared with the conventional colloidal gold immunochromatography method, the method has the advantages of high mark stability, low non-specificity, high sensitivity, wide linear range and accurate quantification. The invention provides a simple, accurate, specific and cheap detection tool for blood samples, urine samples, spittle, excrement and the like, so the method can be widely applied to the fields of medical technology, food safety, veterinary drug residues, environmental monitoring, drug detection and the like.
Owner:BEIJING KANGMEI TIANHONG BIOTECH
Fluorescence immunochromatographic assay method for quantitatively detecting heart fatty acid binding protein and kit for quantitatively detecting same
ActiveCN102520194ASolve the backgroundSolve the signal indistinguishableBiological testingBlood plasmaBiology
The invention discloses a fluorescence immunochromatographic assay method for quantitatively detecting hFABP (heart fatty acid binding protein) and a kit for quantitatively detecting the same. The fluorescence immunochromatographic assay method for quantitatively detecting the hFABP realizes quantitative fluorescence detection on the basis of optimizing components of a test strip by the aid of excellent fluorescent characteristics of quantum dots and by means of combining bicolor labeling technique and immunochromatographic assay. Compared with a conventional colloidal gold immunochromatographic assay method, the fluorescence immunochromatographic assay method has the advantages of fine labeling stability, low non-specificity, high sensitivity, wide linear range and accuracy in quantization. The kit is used for quantitatively detecting the hFABP, can be used for simultaneously detecting whole blood, blood serum and plasma samples, serves as a simple, accurate, specific and inexpensive detecting tool for early screening and prognosis evaluation of acute myocardial infarction, is applicable to hospitals at all levels, and is particularly beneficial to wide popularization in primary hospitals and clinics.
Owner:SHENZHEN KANGMEI BIOTECH
Fluorescence immunochromatographic assay and kit for quantitatively detecting N-terminal pro brain natriuretic peptide
ActiveCN102565423ASolve the backgroundSolve the signal indistinguishableBiological testingFluorescence/phosphorescenceDiseaseN-terminal pro-Brain Natriuretic Peptide
Owner:SHENZHEN KANGMEI BIOTECH
Immunofiltration assay fluorescent quantitative detection method based on high-sensitivity quantum dot
ActiveCN102539771AHigh luminous intensityWide excitation spectrumFluorescence/phosphorescenceQuantum dotQuality control
The invention discloses an immunofiltration assay fluorescent quantitative detection method based on a high-sensitivity quantum dot; the immunofiltration assay fluorescent quantitative detection method comprises the following steps of: constructing a fluorescence immunofiltration array device by using the excellent fluorescent characteristic of the quantum dot in combination of a quantum dot fluorescence labeling technology and an immunofiltration array technology on the basis of optimizing constituent parts for the immunofiltration assay; and after immunofiltration array, detecting the strenght of fluorescent signals of the quantum dot and a quality control dot by using a fluorescence quantometer, correcting the fluorescence strenght of the quantum pot by using the quality control dot, and further realizing the quantitative detection of a tested object according to a standard curve obtained by using the fluorescence quantometer. The method is simple, rapid, accurate, low in cost and high in sensitiveness. Comapred with the conventional collodial gold immunofiltration array method, the immunofiltration assay fluorescent quantitative detection method has the advantages of good labeling stability, low non-specificity, high sensitivity, wide linear range and accurate quantification. The method is suitable for samples such as serums, urine, spittle, excrement and the like and can be applicable to the detection of serious illness, poisons, food safety and the like.
Owner:BEIJING KANGMEI TIANHONG BIOTECH
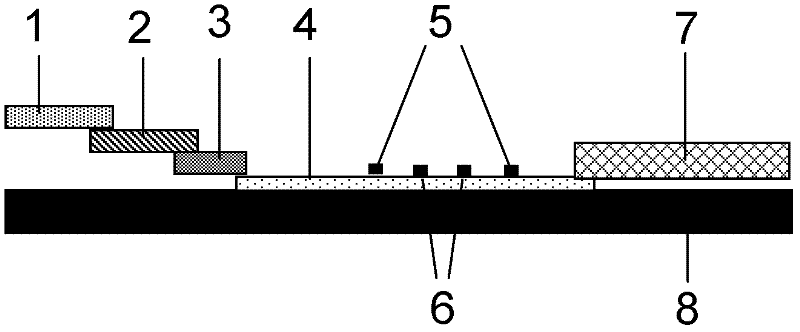
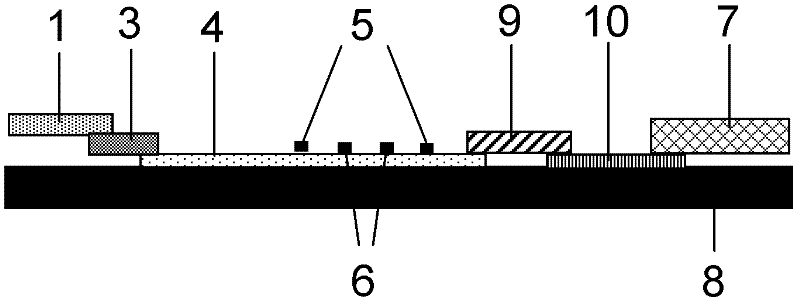
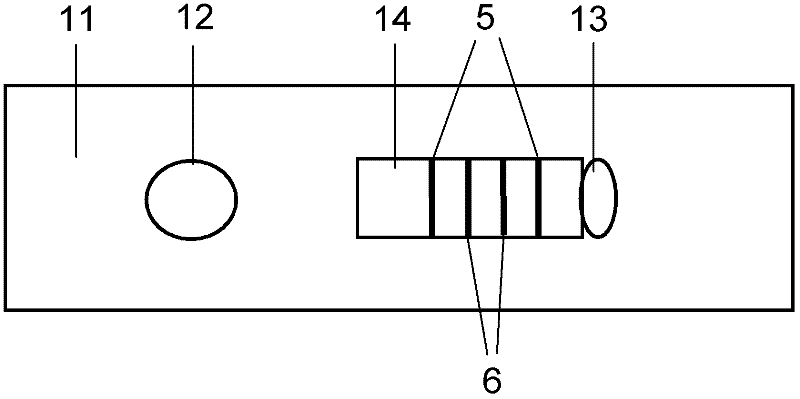
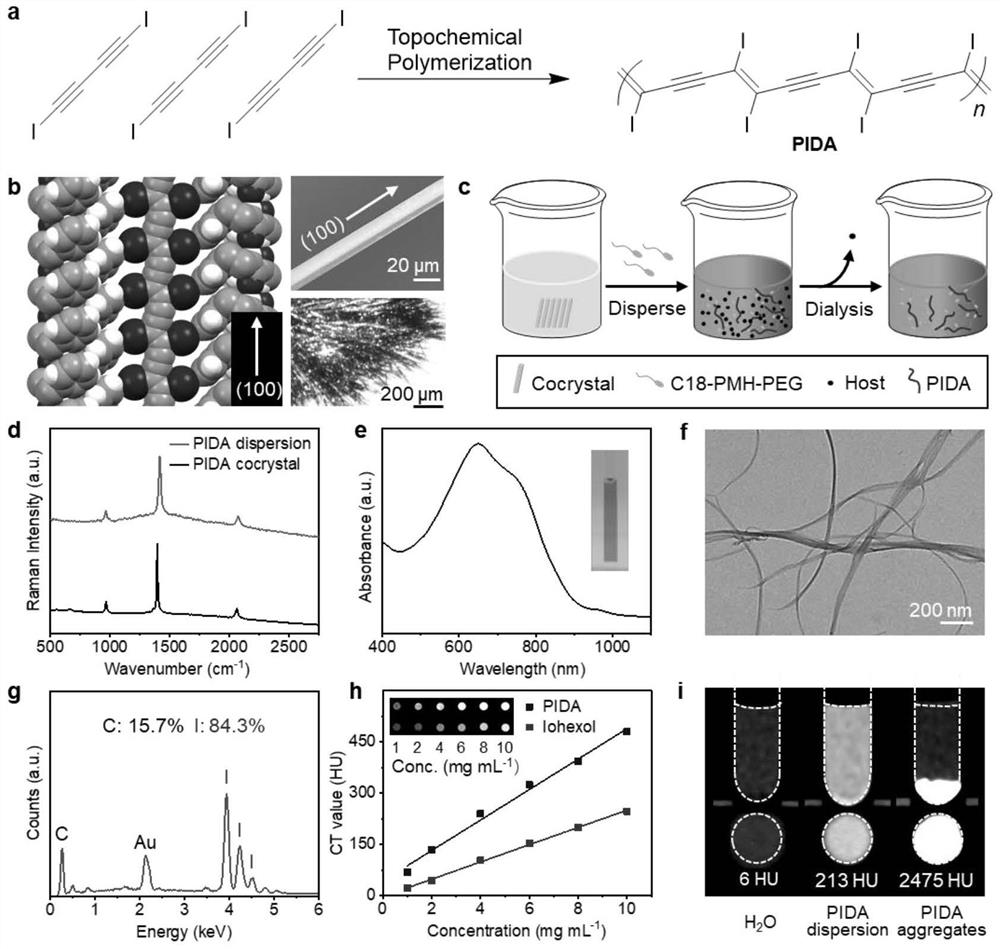
Conjugated carbon-iodine polymer and preparation thereof, and application of conjugated carbon-iodine polymer in preparation of positioning marker
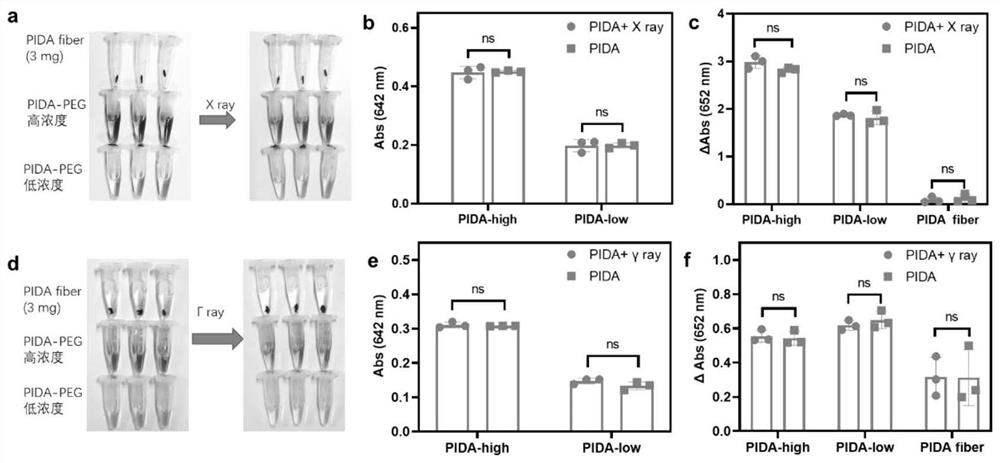
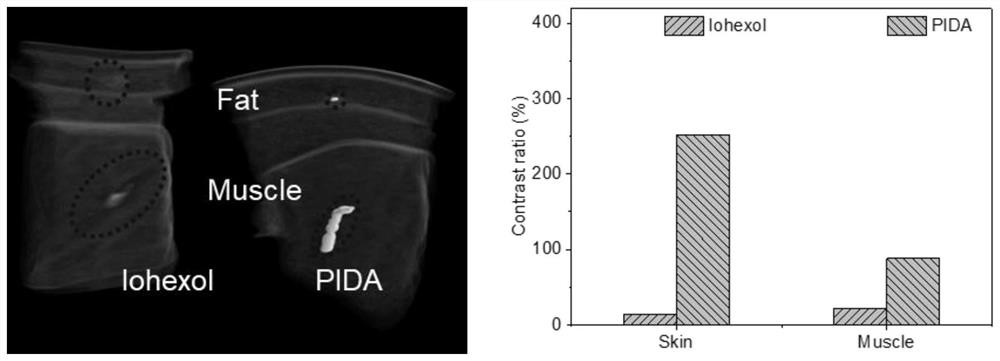
ActiveCN114149569AStrong absorption capacityPrecise resectionX-ray constrast preparationsPhotovoltaic energy generationMetal ArtifactImaging quality
The invention relates to a conjugated carbon-iodine polymer, preparation thereof and application of the conjugated carbon-iodine polymer in preparation of a positioning marker, and belongs to the technical field of imaging markers. According to the brand-new imaging marker based on the conjugated carbon-iodine polymer disclosed by the invention, due to the conjugated structure, the polymer has very strong absorption in a visible light region, and the iodine content up to 84.1% corresponds to the superstrong imaging capability of the polymer. In the tumor operation process, based on double guidance of polymer image marking and naked eye observation, the marking can better assist in determining the cutting edge of the tumor, so that accurate cutting of the tumor is achieved, and damage to surrounding normal tissue is reduced as much as possible. In the tumor cyber knife treatment process, the polymer can replace a clinical gold mark to provide ray marking guidance, the ray imaging quality and more accurate radiation dose distribution are improved due to no metal artifacts, the relative stability of the marking position is improved due to good biocompatibility, and the radiotherapy side effect can be further reduced.
Owner:HUAZHONG UNIV OF SCI & TECH
Fluorescence immunochromatographic assay and kit for quantitatively detecting cardiac troponin T
ActiveCN102565422ASolve the backgroundSolve the signal indistinguishableBiological testingFluorescenceBlood plasma
The invention discloses a fluorescence immunochromatographic assay and kit for quantitatively detecting cardiac troponin T (cTnT). The fluorescence immunochromatographic assay for quantitatively detecting cTnT realizes fluorescence quantitative detection on the basis of optimizing various constituent parts of test paper by using excellent fluorescence characteristic of quantum dots and combining a bicolor marking technology and an immunochromatographic technology. Compared with the conventional colloidal gold immunochromatographic assay, the fluorescence immunochromatographic assay disclosed by the invention has the advantages of good marking stability, low non-specificity, high sensitivity, wide linear range and quantifying accuracy. The fluorescence immunochromatographic kit disclosed by the invention is used for carrying out quantitative detection on the cTnT and detecting whole blood, blood serum and blood plasma samples and is suitable for different levels of hospitals and particularly good for wide popularization in primary hospitals and clinics.
Owner:SHENZHEN KANGMEI BIOTECH
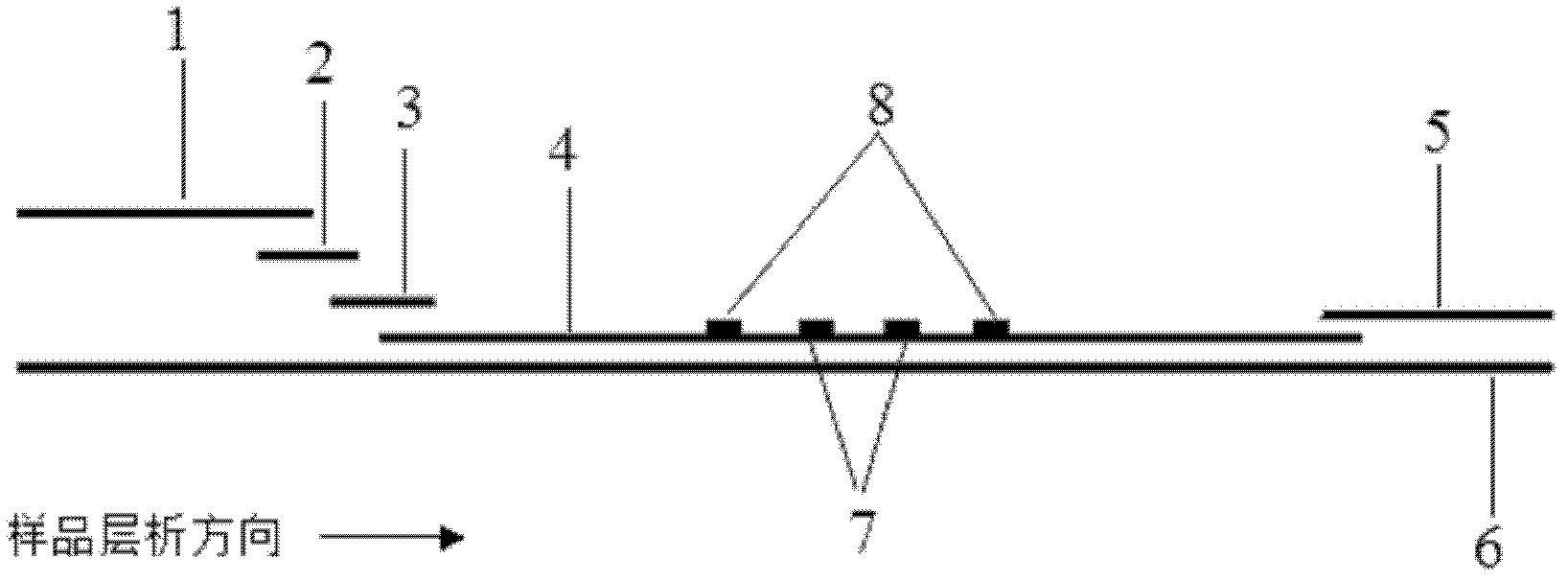
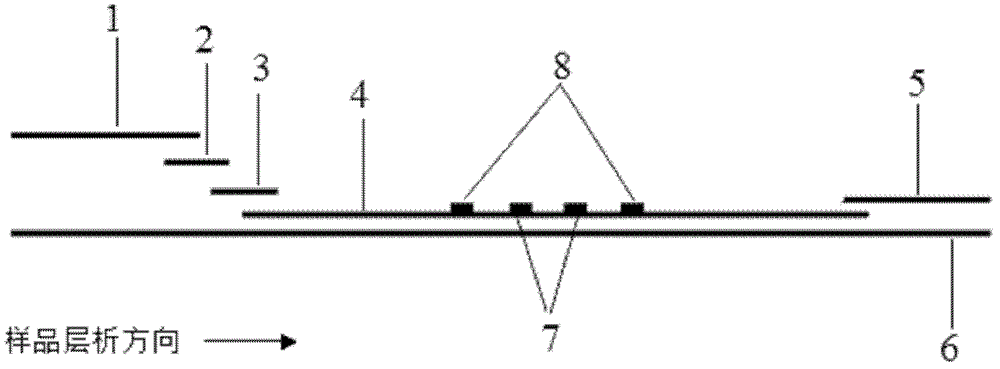
Preparation and application of immunochromatography method and test paper for quantitative detection of prealbumin
InactiveCN106680488AHigh sensitivityImprove capture efficiencyMaterial analysisMicrosphereFluorescence
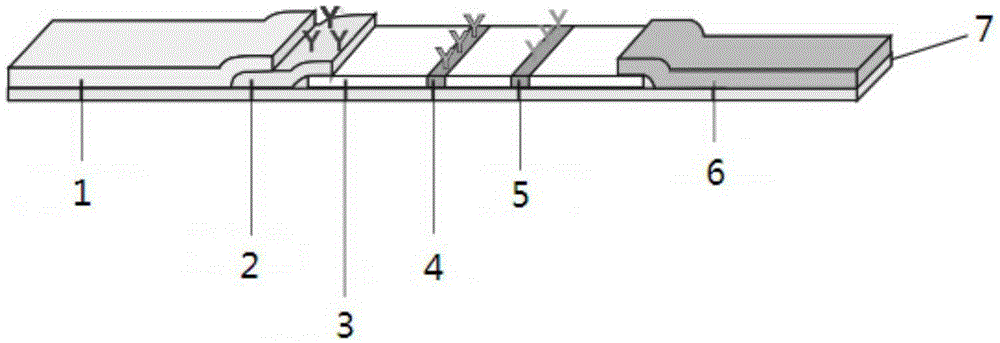
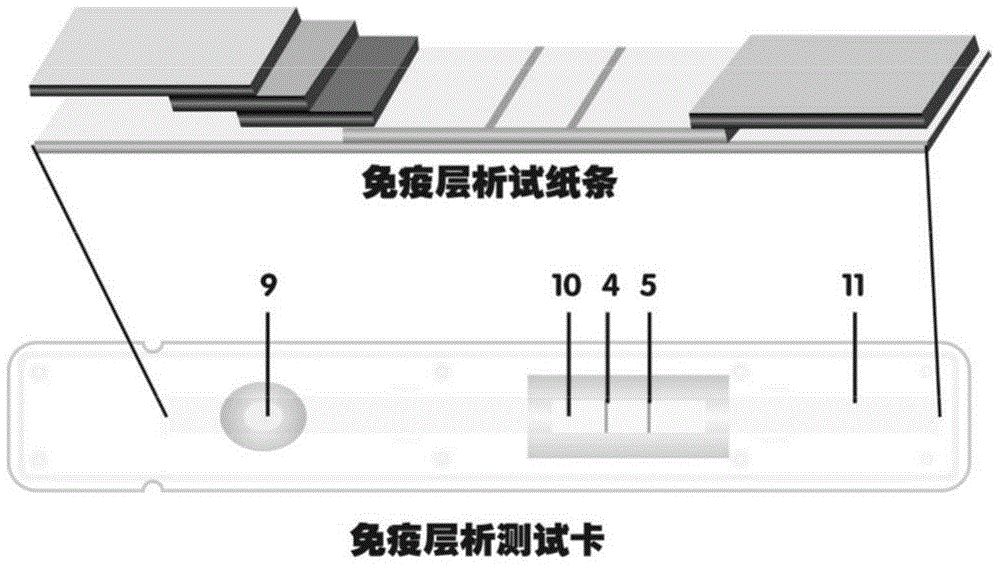
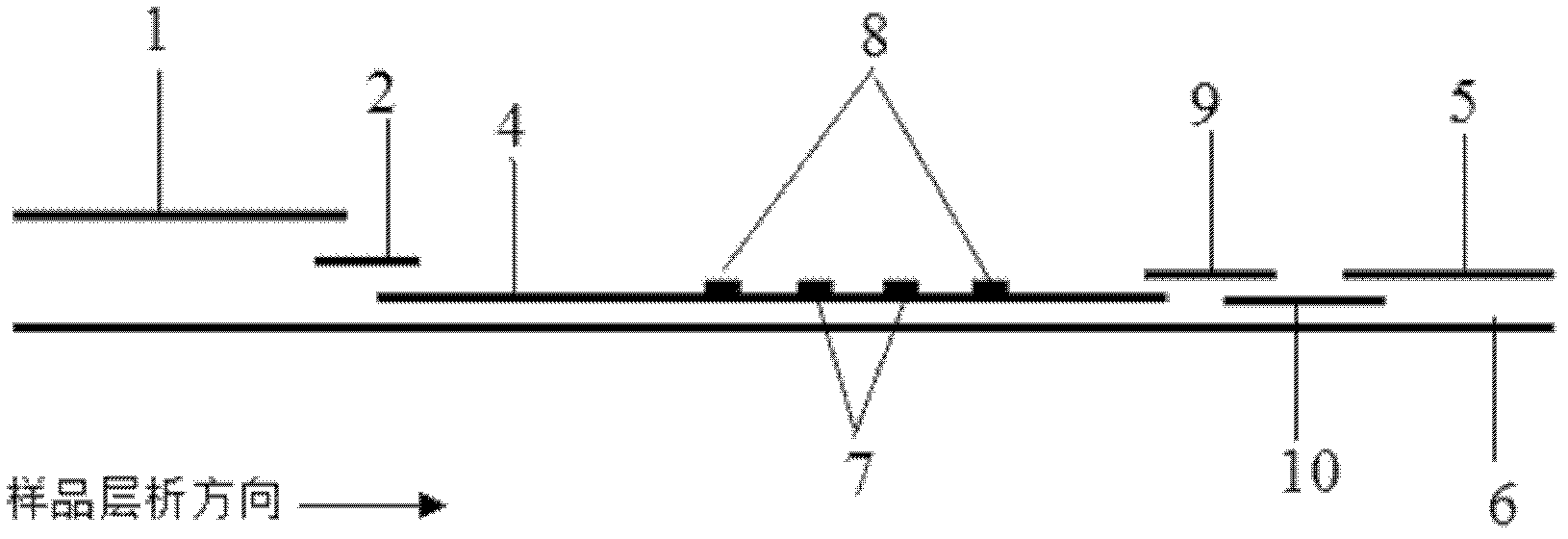
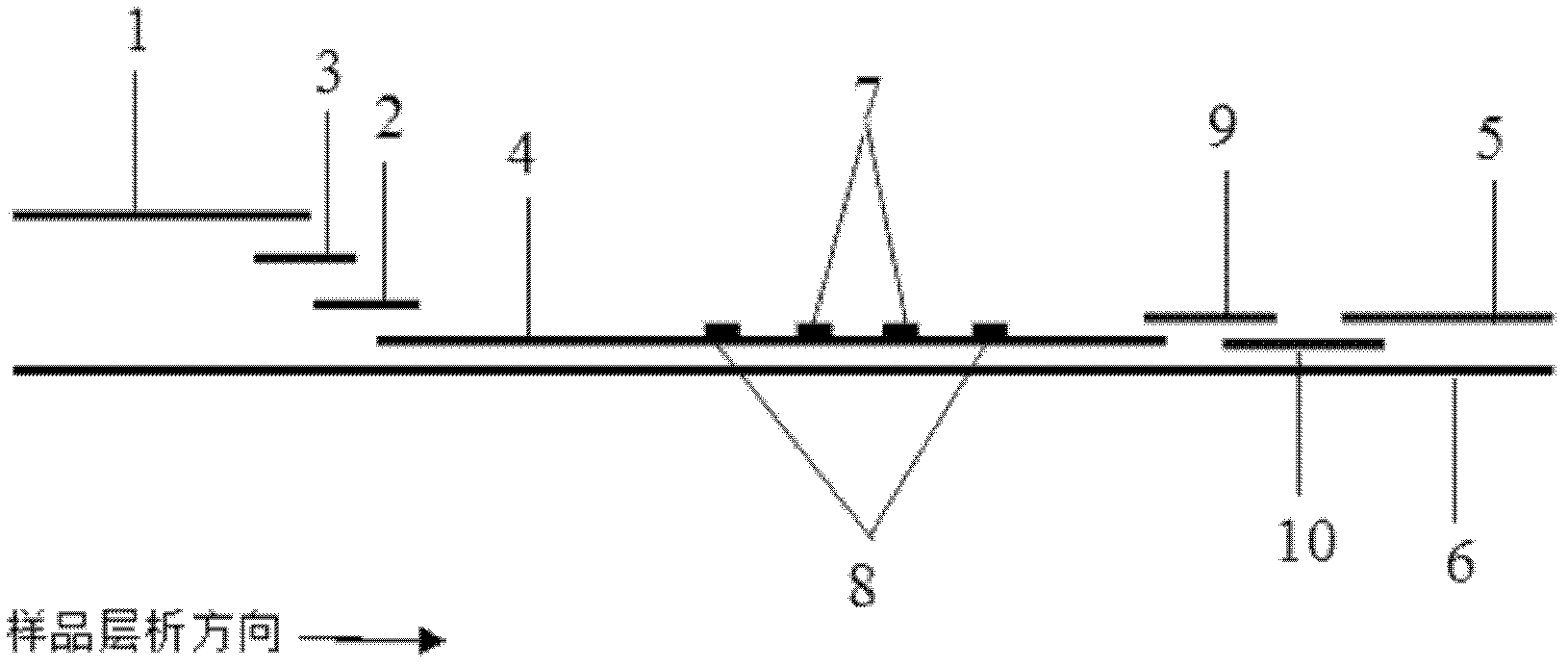
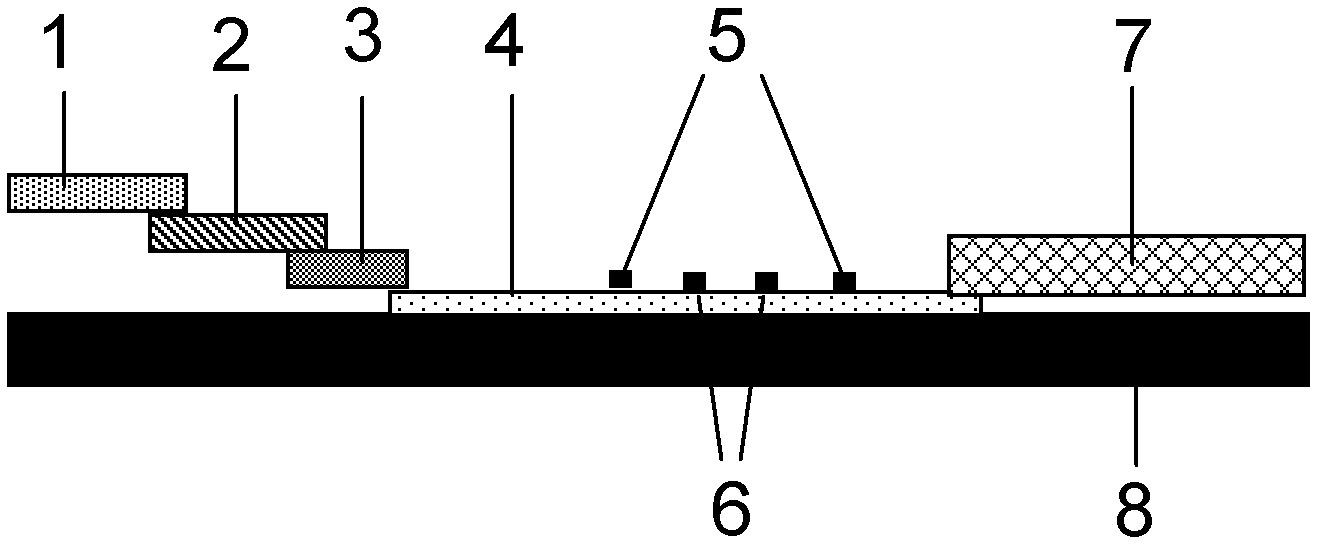
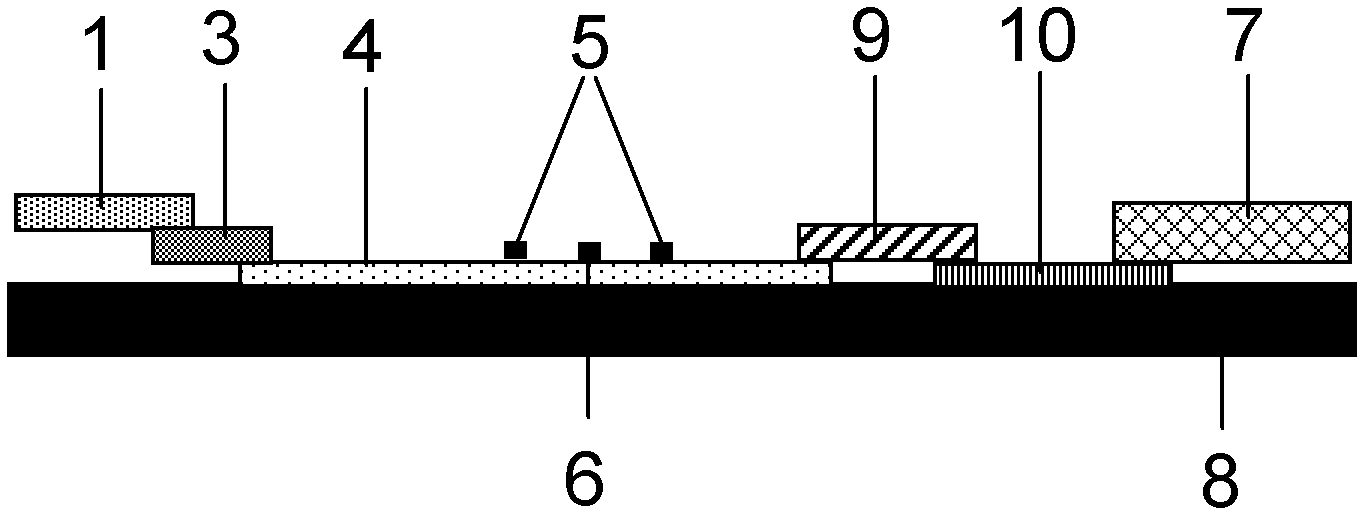
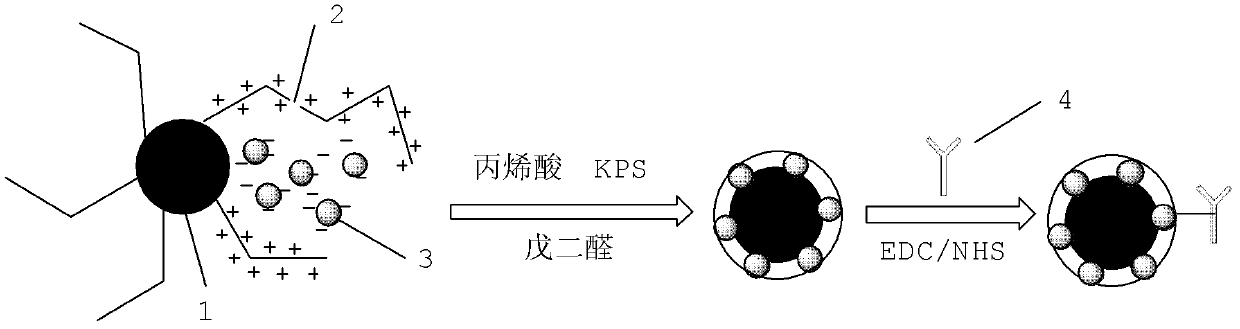
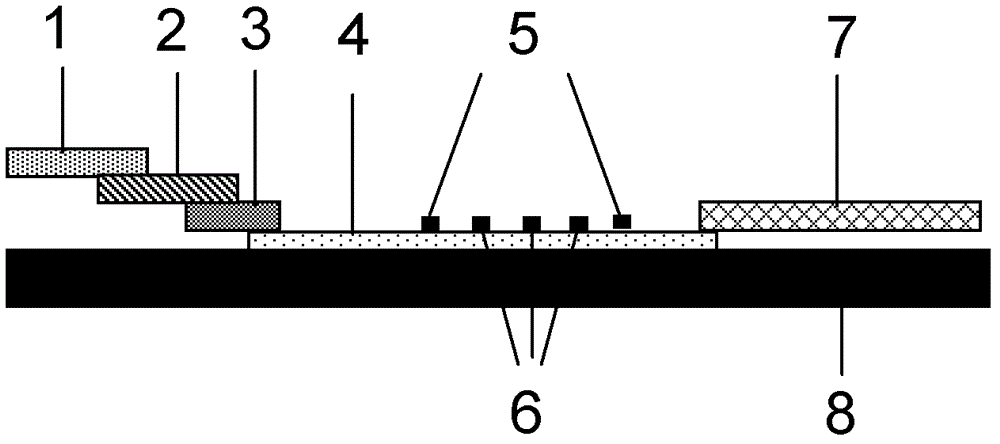
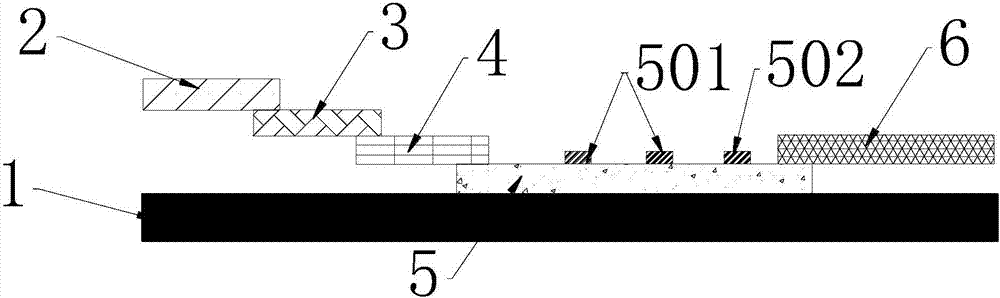
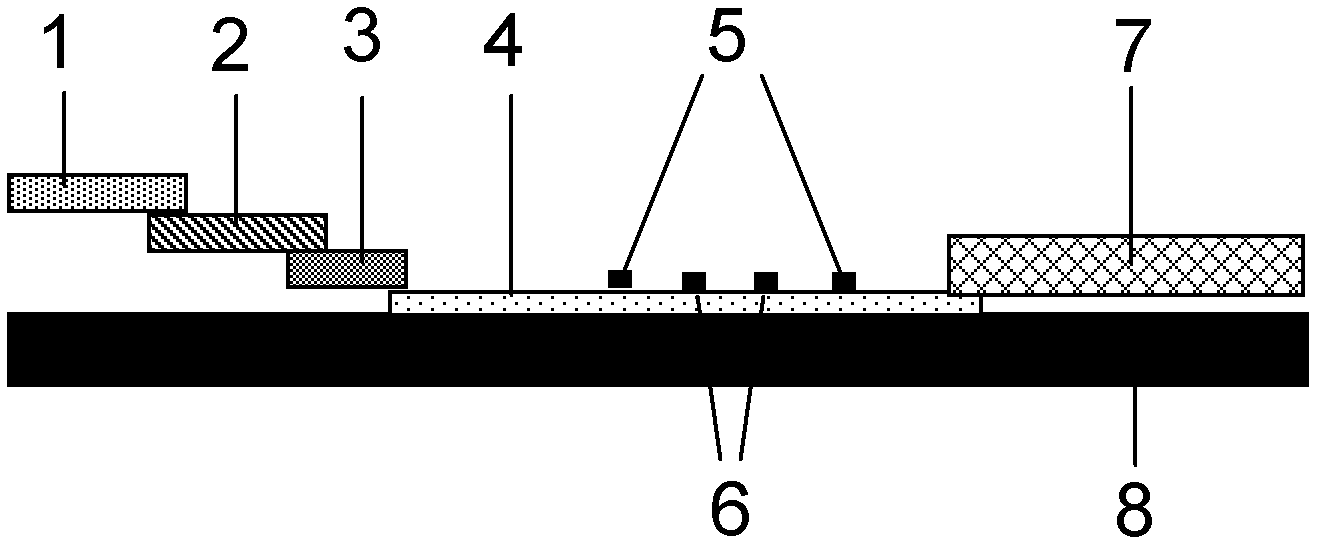
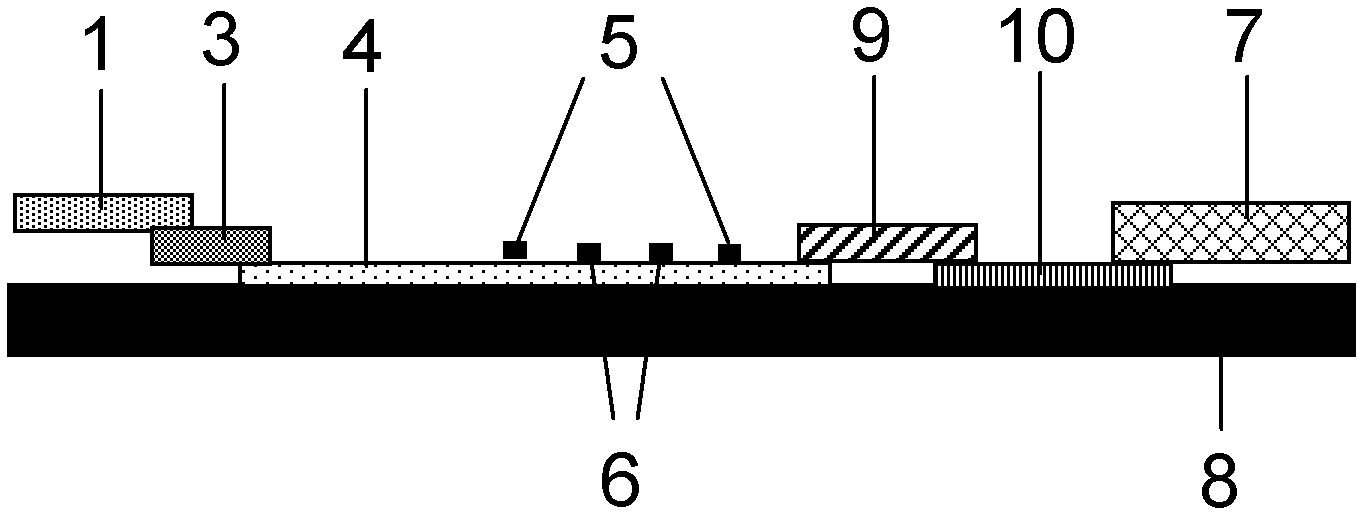
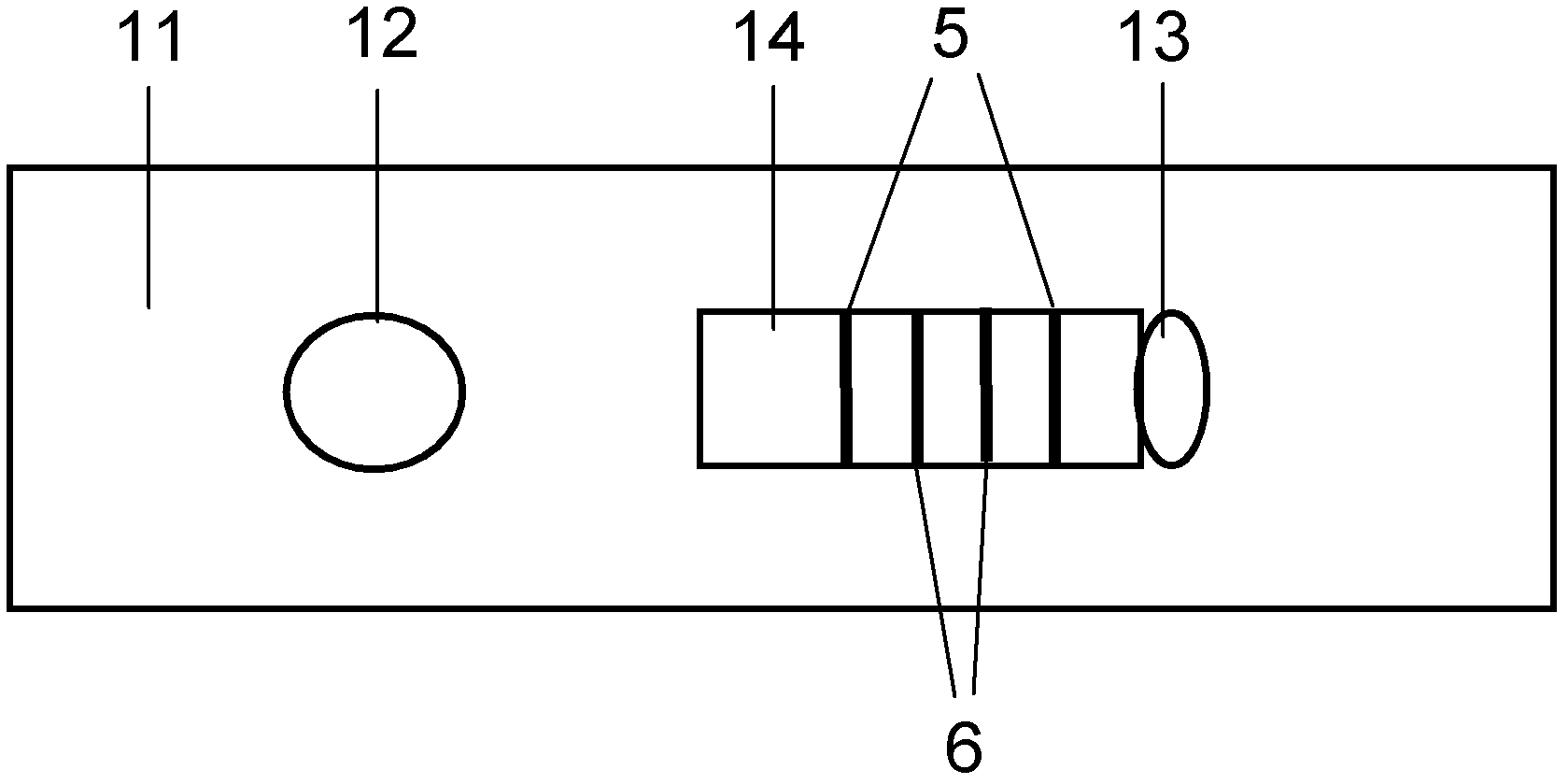
The invention provides preparation and application of an immunochromatography method and test paper for quantitative detection of prealbumin. The test paper consists of a bottom plate, a sample pad, a conjugate pad, a nitrocellulose membrane and a piece of water absorbing paper, wherein the sample pad, the conjugate pad, the nitrocellulose membrane and the water absorbing paper are sequentially adhered to the bottom plate in a lap joint manner and are respectively and partially overlapped; a detection line and a quality control line are arranged on the nitrocellulose membrane; the quality control line is wrapped with a donkey-anti-rat antibody. A method comprises the following steps: (1) adding a solution which is of known concentration and comprises a prealbumin standard substance onto the sample pad of the test paper, and drawing a standard curve; (2) adding a solution of a substance to be tested, performing reaction, and reading content according to standard curves. The test paper and the detection method provided by the invention are high in sensitivity, that is, up to pg / mL, wide in detection linearity, convenient to operate, and short in time, that is, results can be obtained within only 10-20 minutes.
Owner:北京中生金域诊断技术股份有限公司
Fluorescence immunochromatography kit for quantitatively detecting human epididymis secretory protein-4 and preparation method for fluorescence immunochromatography kit
InactiveCN104655858AHigh luminous intensityWide excitation spectrumDisease diagnosisBiological testingPlasma samplesFluorescence
The invention discloses a fluorescence immunochromatography kit for quantitatively detecting human epididymis secretory protein-4 by taking fluorescent dye as a marker. The fluorescence immunochromatography kit disclosed by the invention realizes fluorescence quantitative detection for the human epididymis secretory protein-4, has the advantages of being good in stability, wide in linear range, good in specificity, accurate to quantify, simple and quick, can be used for simultaneously detecting whole blood, blood serum and plasma samples, and is suitable for hospitals of various levels.
Owner:DEMAIJI BIOTECH BEIJING
Fluorescence immunochromatographic assay and kit for quantitative detection of human cardiac troponin I (cTnI)
ActiveCN102520193ASolve the backgroundSolve the signal indistinguishableBiological testingFluorescenceBlood plasma
The invention discloses a fluorescence immunochromatographic assay and kit for quantitative detection of cardiac troponin I (cTnI). The fluorescence immunochromatographic assay for quantitative detection of the cTnI realizes fluorescent quantitative detection by utilizing excellent fluorescent properties of quantum dots and combining a bicolour marking technology and an immunochromatographic assay on the basis of optimizing each component of a test strip. Compared with the common collaurum immunochromatographic assay, the fluorescence immunochromatographic assay has the advantages of good mark stability, low nonspecificity, high sensitivity, wide linear range and accuracy in quantification. The kit disclosed by the invention is used for carrying out quantification detection on the cTnI, can be used for detecting whole blood, blood serum and plasma samples simultaneously, and is applied to different levels of hospitals and is particularly favored to be widely popularized to primary hospitals and clinics.
Owner:SHENZHEN KANGMEI BIOTECH
Kit for rapidly and quantitatively detecting troponin and creatine kinase isozyme
ActiveCN106198997ASimple and fast operationRapid responseDisease diagnosisBiological testingBiotin-streptavidin complexCreatine kinase
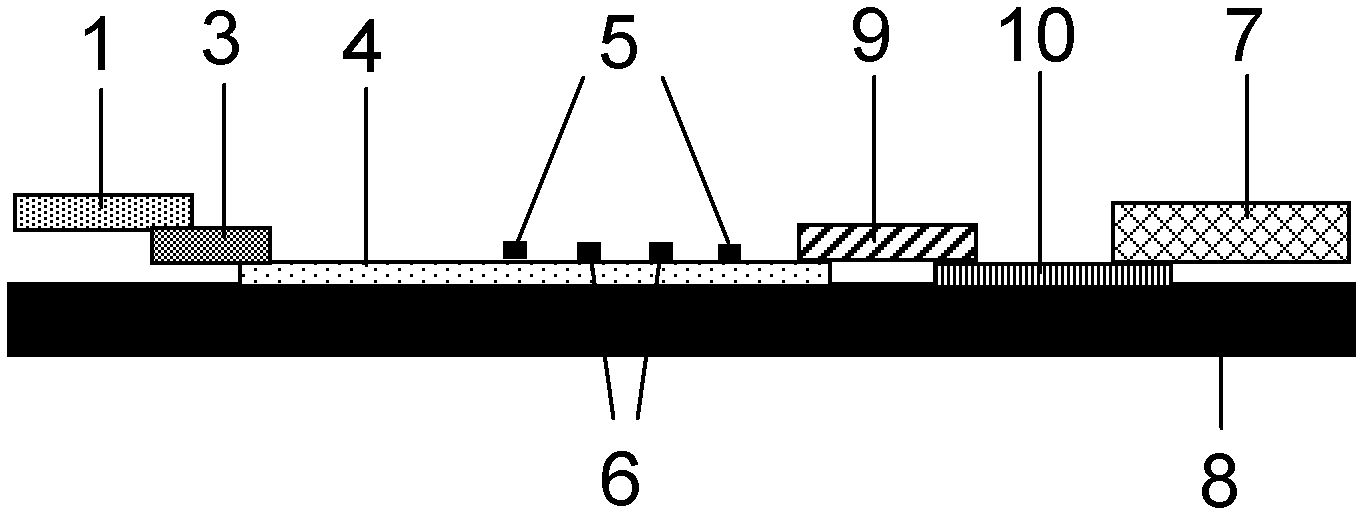
The invention belongs to the field of kits, and particularly relates to a kit for rapidly and quantitatively detecting troponin and creatine kinase isozyme, and a preparation method of the kit. The kit comprises a base plate, wherein a sample pad, an antibody binding pad I, an antibody binding pad II, an enveloped analysis film and a water absorption pad which are sequentially lapped are arranged on the base plate; an immunomagnetic bead which is coupled with biontin-marked anti-troponin I monoclonal antibody is enveloped on the antibody binding pad I, an immunomagnetic bead which is coupled with an anti-creatine kinase isozyme monoclonal antibody is enveloped on the antibody binding pad II, and the surface of the magnetic bead I is enveloped with streptavidin. The kit has the advantages of being simple and convenient to operate, fast to react, high in sensitivity, strong in specificity, suitable for on-site detection and the like, and is suitable for large-range popularization and application.
Owner:ANHUI IPROCOM BIOTECH CO LTD
Fluorescence immunochromatographic assay and kit for quantitative detection of creatine kinase isoenzyme (CK-MB)
ActiveCN102520173ASolve the backgroundSolve the signal indistinguishableMaterial analysisDiseaseCreatine kinase
The invention discloses a fluorescence immunochromatographic assay and kit for quantitative detection of acute myocardial infarction marker-creatine kinase isoenzyme (CK-MB). The fluorescence immunochromatographic assay for quantitative detection of the CK-MB realizes fluorescent quantitative detection by utilizing excellent fluorescent properties of quantum dots and combining a bicolour marking technology and an immunochromatographic assay on the basis of optimizing each component of a test strip. Compared with the common collaurum immunochromatographic assay, the fluorescence immunochromatographic assay has the advantages of good mark stability, low nonspecificity, high sensitivity, wide linear range and accuracy in quantification. The kit disclosed by the invention is used for carrying out quantification detection on the CK-MB, is suitable for detection of whole blood, blood serum and plasma samples, can provide a reference for cardiovascular and cerebrovascular disease diagnosis, and is widely applied to primary hospitals and clinics.
Owner:SHENZHEN KANGMEI BIOTECH
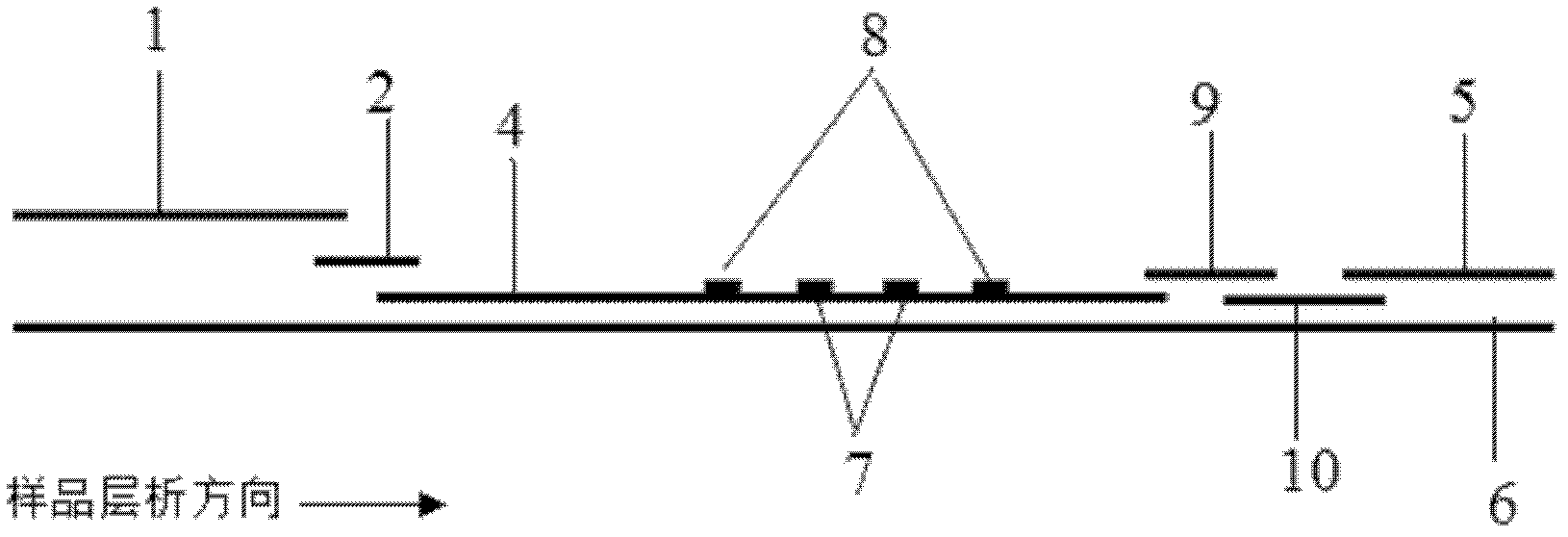
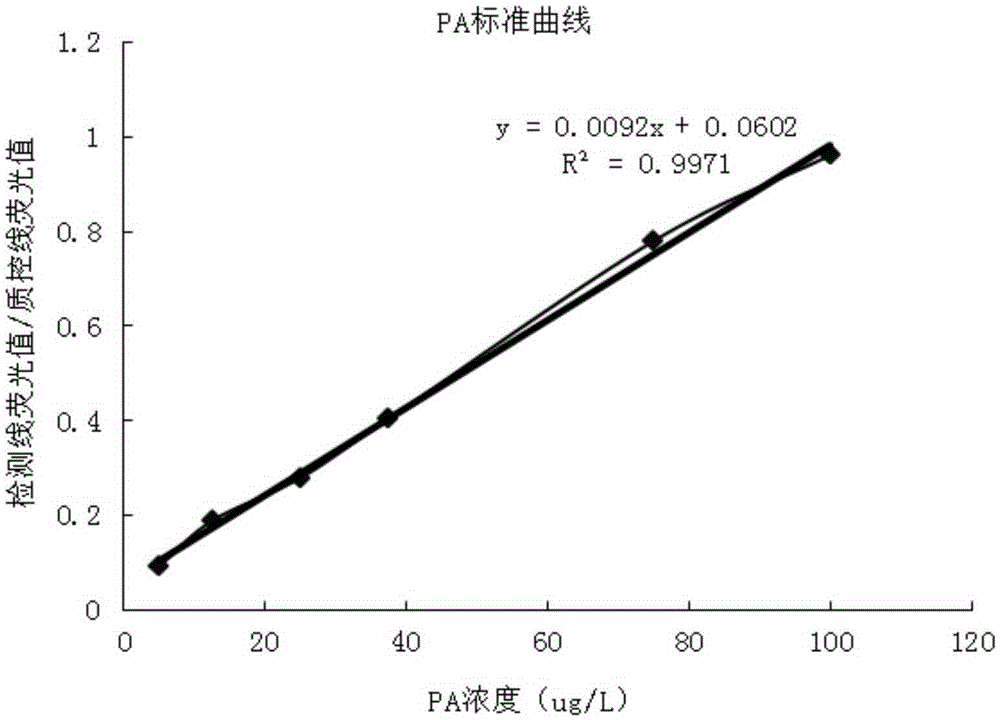
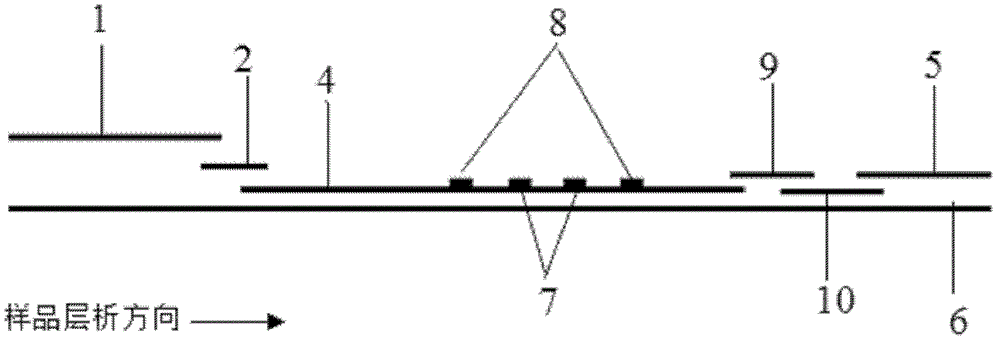
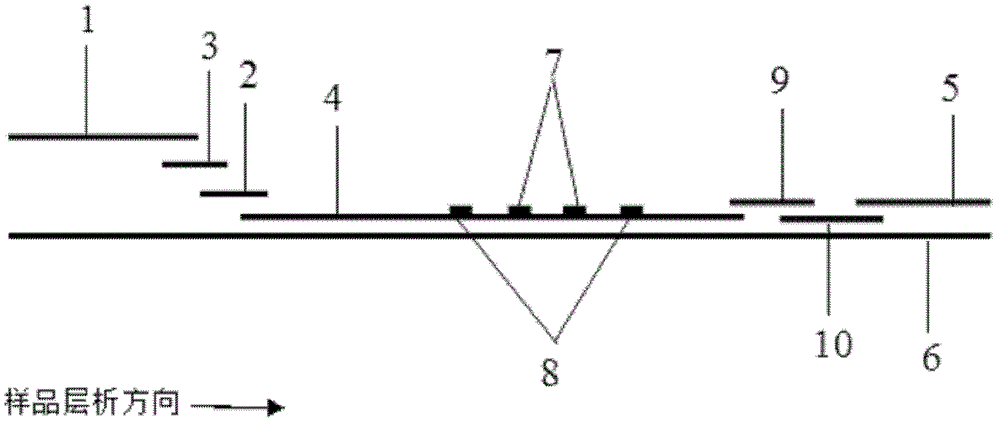
Method and kit for quantitative combined detection of PA (Prealbumin) and CRP (C-reactive Protein), as well as preparation method and application of kit
InactiveCN106680508AHigh sensitivityImprove capture efficiencyBiological testingFluorescence/phosphorescenceQuality controlEngineering
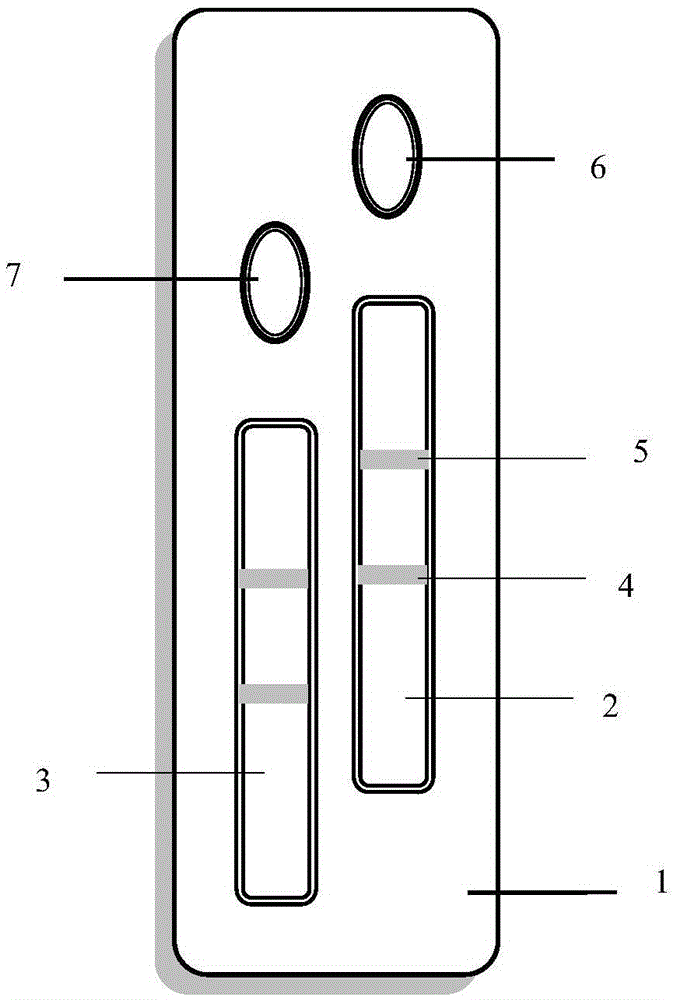
The invention provides a method and a kit for quantitative combined detection of PA (Prealbumin) and CRP (C-reactive Protein), as well as a preparation method and application of the kit. The kit comprises a test paper strip I and a test paper strip II, which are used for detecting PA and CRP, wherein the test paper strip I is composed of a bottom plate I, a sample pad I, a combination pad I, a nitrocellulose membrane I and water absorption paper I; and the test paper strip II is composed of a bottom plate II, a sample pad II, a combination pad II, a nitrocellulose membrane II and water absorption paper II. The detection method comprises the following steps: (1) adding a PA standard object into the sample pad I and adding a CRP standard object into the sample pad II; after reacting, drawing a standard curve according to a specific value of the fluorescence intensity of a detection line and the fluorescence intensity of a quality control line; and (2) adding a solution of an object to be detected into the sample pad I and the sample pad II, and reading PA content and CRP content in the solution of the object to be detected according to the standard curve. The kit and the detection method, provided by the invention, are simple and convenient to operate, high in clinical acceptance degree and good in detection effect.
Owner:北京中生金域诊断技术股份有限公司
Two-photon fluorescence immunochromatography kit for quantitative determination of anti-Mullerian hormone (AMH) and preparation method of kit
InactiveCN107621540ALong fluorescence lifetimeHigh luminous intensityMaterial analysisQuantitative accuracyBlood plasma
The invention discloses a two-photon fluorescence immunochromatography kit for quantitative determination of anti-Mullerian hormone (AMH), which utilizes fluorescent dye as a maker. The two-photon fluorescence immunochromatography kit, realizing fluorescence immunochromatography quantitative determination, has the advantages of good stability, wide linear range, good specificity, high sensitivity,high quantitative accuracy and easy and quick operation, can be applied to detection of whole blood samples, serum samples and plasma samples simultaneously, and is applicable to medical treatment ofhospital at different levels and family practice.
Owner:DEMAIJI BIOTECH BEIJING
PLGF magnetic particle chemiluminiscence kit and detection method thereof
PendingCN110261626AImprove bindingReduce non-specificityChemiluminescene/bioluminescenceBiological testingBiotin-streptavidin complexPlacental growth factor
The present invention discloses a PLGF (Placental Growth Factor) magnetic particle chemiluminiscence kit and a detection method thereof. The kit comprises a placental growth factor antibody with biotin labeling, streptavidin magnetic particle suspension liquid, a placental growth factor antibody with acridinium ester labeling, a calibration product, cleanout fluid, a diluent, chemiluminiscence pre-excitation liquid A, and chemiluminiscence pre-excitation liquid B. The streptavidin and the biotin have a high-specificity combining capacity, the high-purity antibody of the streptavidin and the biotin labeling is subjected to specific binding through a non covalent bond and has a cascade amplification effect. Besides, an acridinium ester chemiluminescent system is adopted, and the acridinium ester does not need a catalyst and can emit light when being put in a H2O2 solution with no need for a catalytic process and an enhancer. The sensitivity is higher, the reaction time is short, the operation is simple, and the anti-interference performance is high.
Owner:NINGBO AUCHEER BIOTECHNOLOGY CO LTD
Method for highly sensitive quantitative detection of quantum dot fluorescence immunochromatographic assay
ActiveCN102520165BHigh luminous intensityWide excitation spectrumMaterial analysisCritical illnessUrine sample
The invention discloses a method for highly sensitive quantitative detection of quantum dot fluorescence immunochromatographic assay. The method includes: building a fluorescence immunochromatographic assay test strip on the basis of optimizing the structure of the test strip and components by the aid of excellent fluorescent characteristics of quantum dots and by means of combining quantum dot fluorescence labeling technology and immunochromatographic assay; detecting fluorescence signal strength of a quantitative belt and a quality control belt by the aid of a fluorescence quantometer and correcting the fluorescence strength of the quantitative belt by the aid of the quality control belt after immunochromatographic assay of the test strip; and further quantitatively detecting analyte according to a standard curve obtained by the fluorescence quantometer. The method is simple, rapid, accurate, low in cost and quite high in sensitivity. Compared with a conventional colloidal gold immunochromatographic assay method, the method has the advantages of fine labeling stability, low non-specificity, high sensitivity, wide linear range and accuracy in quantization. The method is applicable to samples such as blood samples, urine samples, spittle, excrement and the like, and can be applied to detection of critical illness, poison, food safety and the like.
Owner:BEIJING KANGMEI TIANHONG BIOTECH
Magnetic fluorescent microsphere immunochromatography quantitative detection method
ActiveCN102565386BReduce sample matrix varianceHigh detection sensitivityFluorescence/phosphorescenceAdditive ingredientMedical testing
The invention discloses a magnetic fluorescent microsphere immunochromatography quantitative detection method. In the method, respective excellent characteristics of magnetic nano particles and quantum dots are fully utilized, and an immunochromatography technology is combined to realize fluorescent quantitative detection on the basis of optimizing the structure and ingredients of a test strip. The method has a function of amplifying signals; and compared with the conventional colloidal gold immunochromatography method, the method has the advantages of high mark stability, low non-specificity, high sensitivity, wide linear range and accurate quantification. The invention provides a simple, accurate, specific and cheap detection tool for blood samples, urine samples, spittle, excrement and the like, so the method can be widely applied to the fields of medical technology, food safety, veterinary drug residues, environmental monitoring, drug detection and the like.
Owner:BEIJING KANGMEI TIANHONG BIOTECH
Fluorescence immunochromatographic assay and kit for quantitative detection of troponin I/creatine kinase isoenzyme/myohemoglobin
ActiveCN102520192BHigh luminous intensityWide excitation spectrumBiological testingCreatine kinaseFluorescence
The invention discloses a quantum dot multicolor marking method for quantitative detection of various cardiovascular disease markers and a kit of troponin I / creatine kinase isoenzyme / myohemoglobin. The method realizes fluorescent quantitative detection by utilizing excellent fluorescent properties of quantum dots and combining a multicolour marking technology and an immunochromatographic assay on the basis of optimizing each component of a test strip. Compared with the common collaurum immunochromatographic assay, the method has the advantages of good mark stability, low nonspecificity, high sensitivity, wide linear range, small cross interference, and accuracy in quantification. The kit disclosed by the invention is used for carrying out quantification detection on the troponin I, the creatine kinase isoenzyme and the myohemoglobin simultaneously, is suitable for detection of whole blood, blood serum and plasma samples, can provide a reference for cardiovascular and cerebrovascular disease diagnosis, and is widely applied to primary hospitals and clinics.
Owner:SHENZHEN KANGMEI BIOTECH
Fluorescence immunochromatographic assay and kit for quantitative detection of human cardiac troponin I (cTnI)
ActiveCN102520193BHigh luminous intensityWide excitation spectrumBiological testingFluorescenceQuantum dot
The invention discloses a fluorescence immunochromatographic assay and kit for quantitative detection of cardiac troponin I (cTnI). The fluorescence immunochromatographic assay for quantitative detection of the cTnI realizes fluorescent quantitative detection by utilizing excellent fluorescent properties of quantum dots and combining a bicolour marking technology and an immunochromatographic assay on the basis of optimizing each component of a test strip. Compared with the common collaurum immunochromatographic assay, the fluorescence immunochromatographic assay has the advantages of good mark stability, low nonspecificity, high sensitivity, wide linear range and accuracy in quantification. The kit disclosed by the invention is used for carrying out quantification detection on the cTnI, can be used for detecting whole blood, blood serum and plasma samples simultaneously, and is applied to different levels of hospitals and is particularly favored to be widely popularized to primary hospitals and clinics.
Owner:SHENZHEN KANGMEI BIOTECH
Fluorescence immunochromatographic assay kit for quantitatively detecting heart fatty acid binding protein
The invention discloses a fluorescence immunochromatographic assay method for quantitatively detecting hFABP (heart fatty acid binding protein) and a kit for quantitatively detecting the same. The fluorescence immunochromatographic assay method for quantitatively detecting the hFABP realizes quantitative fluorescence detection on the basis of optimizing components of a test strip by the aid of excellent fluorescent characteristics of quantum dots and by means of combining bicolor labeling technique and immunochromatographic assay. Compared with a conventional colloidal gold immunochromatographic assay method, the fluorescence immunochromatographic assay method has the advantages of fine labeling stability, low non-specificity, high sensitivity, wide linear range and accuracy in quantization. The kit is used for quantitatively detecting the hFABP, can be used for simultaneously detecting whole blood, blood serum and plasma samples, serves as a simple, accurate, specific and inexpensive detecting tool for early screening and prognosis evaluation of acute myocardial infarction, is applicable to hospitals at all levels, and is particularly beneficial to wide popularization in primary hospitals and clinics.
Owner:SHENZHEN KANGMEI BIOTECH
Immunofiltration assay fluorescent quantitative detection method based on high-sensitivity quantum dot
ActiveCN102539771BHigh luminous intensityWide excitation spectrumFluorescence/phosphorescenceQuantum dotQuality control
The invention discloses an immunofiltration assay fluorescent quantitative detection method based on a high-sensitivity quantum dot; the immunofiltration assay fluorescent quantitative detection method comprises the following steps of: constructing a fluorescence immunofiltration array device by using the excellent fluorescent characteristic of the quantum dot in combination of a quantum dot fluorescence labeling technology and an immunofiltration array technology on the basis of optimizing constituent parts for the immunofiltration assay; and after immunofiltration array, detecting the strenght of fluorescent signals of the quantum dot and a quality control dot by using a fluorescence quantometer, correcting the fluorescence strenght of the quantum pot by using the quality control dot, and further realizing the quantitative detection of a tested object according to a standard curve obtained by using the fluorescence quantometer. The method is simple, rapid, accurate, low in cost and high in sensitiveness. Comapred with the conventional collodial gold immunofiltration array method, the immunofiltration assay fluorescent quantitative detection method has the advantages of good labeling stability, low non-specificity, high sensitivity, wide linear range and accurate quantification. The method is suitable for samples such as serums, urine, spittle, excrement and the like and can be applicable to the detection of serious illness, poisons, food safety and the like.
Owner:BEIJING KANGMEI TIANHONG BIOTECH
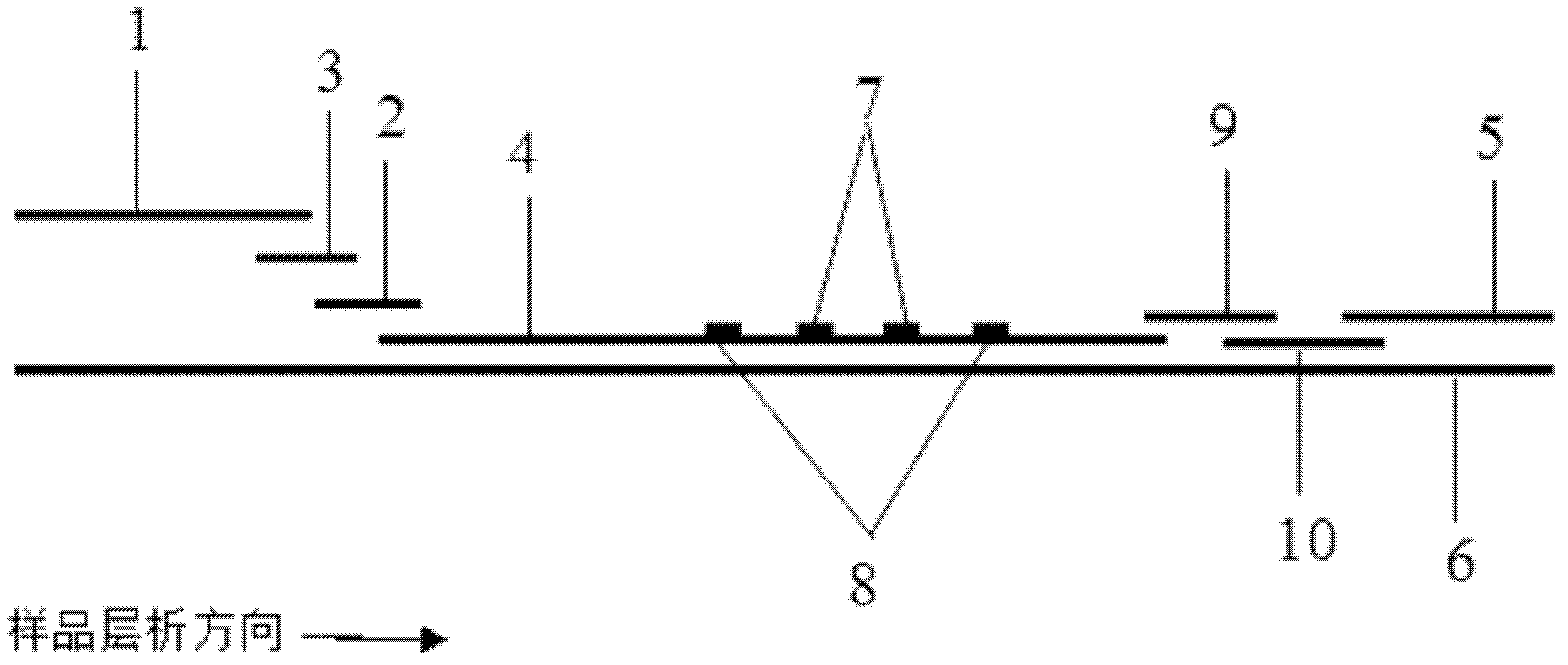
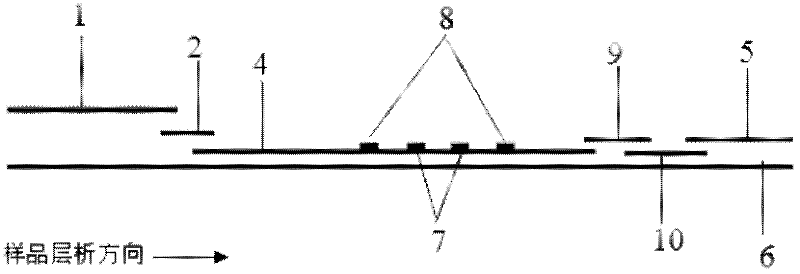
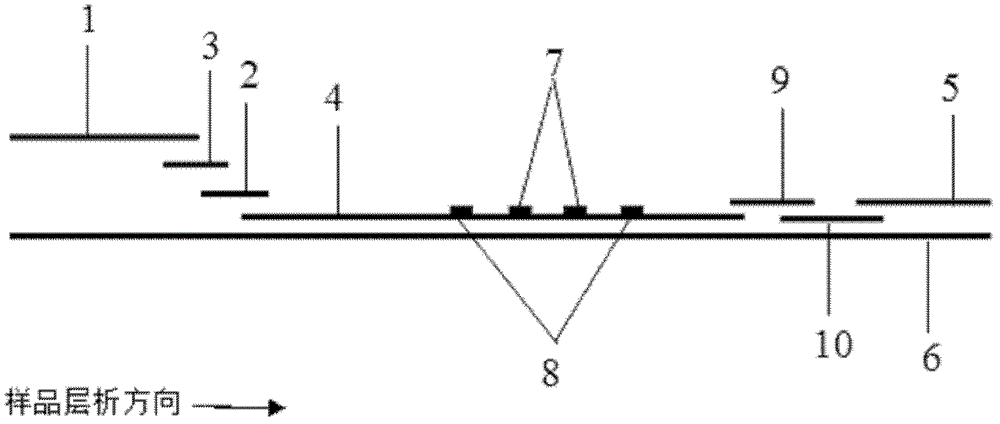
Immunochromatographic test strip for full quantitative detection of C-reactive protein and preparation method thereof
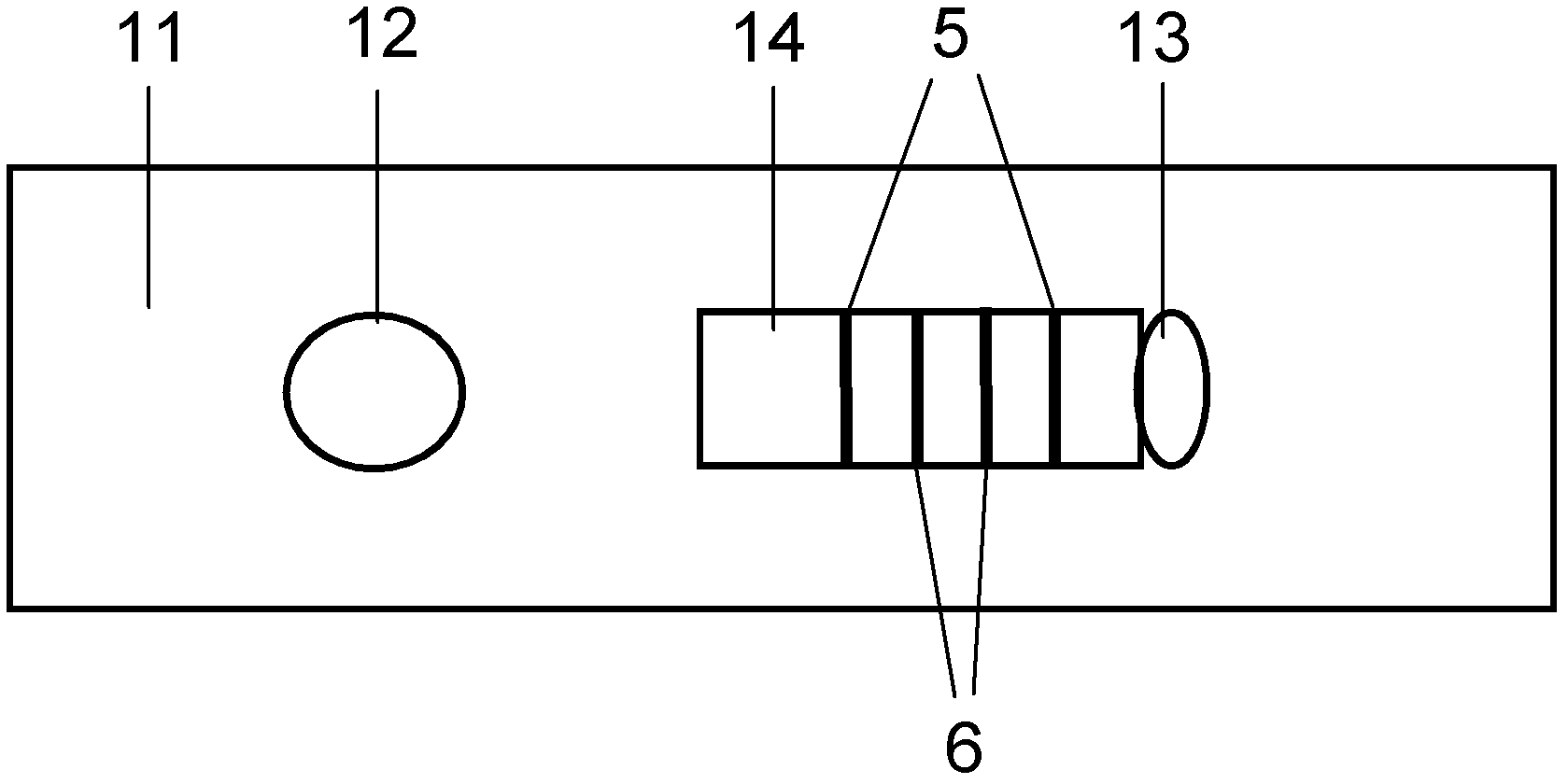
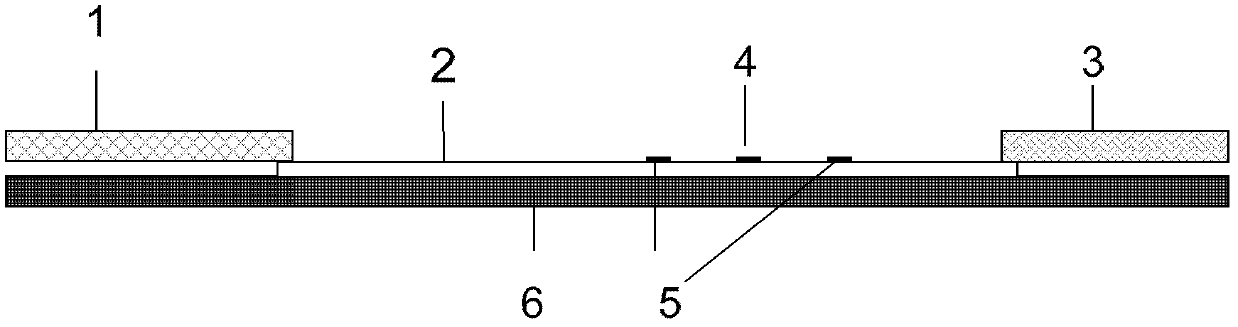
The invention discloses an immunochromatographic test strip for full quantitative detection of C-reactive protein and a preparation method thereof. The test strip is composed of a sample pad, a mark pad, a coating film and absorbent paper which are successively overlapped and adhered on a baseplate, wherein the mark pad is coated with C-reactive protein (CRP) monoclonal antibody labelled by fluorescent latex particles and rabbit IgG labelled by fluorescent latex particles; and the coating film is composed of a detection region and a quality control region, and the detection region is coated with another CRP monoclonal antibody which is at different epitope with the CRP monoclonal antibody labelled by fluorescent latex particles. The C-reactive protein immunochromatographic test strip can perform sensitive quantitative detection on the full CRP in 10 seconds, can diagnose diseases and identify infection more rapidly and accurately, and can detect infectious illness state and determine the curative effect of antibiotics; and the full CRP has two detection results, namely hs-CRP and conventional CRP, comprehensive linear range and good detection sensitivity and less required sample amount, and is very convenient to operate.
Owner:GUANGZHOU WONDFO BIOTECH
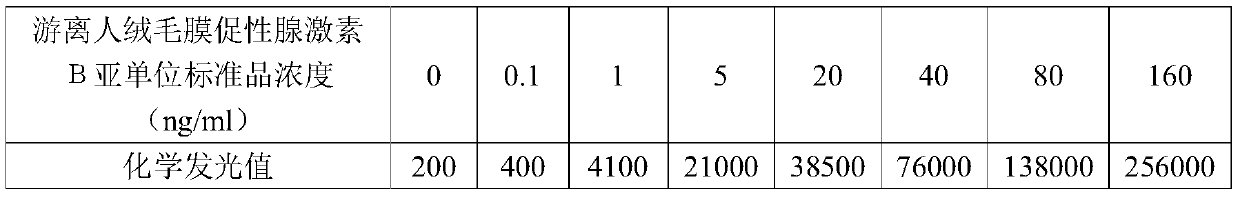
Detection kit for free human chorionic gonadotropin beta subunit
PendingCN110887969AReduce interferenceImprove luminous efficiencyChemiluminescene/bioluminescenceBiological testingAcridineHuman Chorionic Gonadotropin Beta Subunit
The invention discloses a detection kit for a free human chorionic gonadotropin beta subunit. The detection kit comprises a biotin-labeled free human chorionic gonadotropin beta subunit antibody, a streptavidin magnetic particle suspension, an acridinium ester-labeled free human chorionic gonadotropin beta subunit antibody, a standard substance, a cleaning solution, a diluent, a chemiluminescent substrate solution A and a chemiluminescent substrate solution B. The streptavidin and the biotin have high-specificity binding capacity, streptavidin and biotin-labeled high-purity antibody non-covalent bonds are specifically bound, the cascade amplification effect is achieved and the reaction is highly specific. The high specificity of antibody-antigen reaction and high sensitivity of acridiniumester luminescence are combined, photons generated by reaction are captured by acridinium ester to detect the concentration of the product, and the detection kit has the characteristics of higher sensitivity, short reaction time, simple operation and high anti-interference performance.
Owner:NINGBO AUCHEER BIOTECHNOLOGY CO LTD
Fluorescence immunochromatographic assay and kit for quantitatively detecting cardiac troponin T
ActiveCN102565422BHigh luminous intensityWide excitation spectrumBiological testingFluorescenceBlood plasma
The invention discloses a fluorescence immunochromatographic assay and kit for quantitatively detecting cardiac troponin T (cTnT). The fluorescence immunochromatographic assay for quantitatively detecting cTnT realizes fluorescence quantitative detection on the basis of optimizing various constituent parts of test paper by using excellent fluorescence characteristic of quantum dots and combining a bicolor marking technology and an immunochromatographic technology. Compared with the conventional colloidal gold immunochromatographic assay, the fluorescence immunochromatographic assay disclosed by the invention has the advantages of good marking stability, low non-specificity, high sensitivity, wide linear range and quantifying accuracy. The fluorescence immunochromatographic kit disclosed by the invention is used for carrying out quantitative detection on the cTnT and detecting whole blood, blood serum and blood plasma samples and is suitable for different levels of hospitals and particularly good for wide popularization in primary hospitals and clinics.
Owner:SHENZHEN KANGMEI BIOTECH
A kit for rapid quantitative detection of troponin and creatine kinase isozymes
ActiveCN106198997BSimple and fast operationRapid responseDisease diagnosisBiological testingCreatine kinaseMagnetic bead
Owner:ANHUI IPROCOM BIOTECH CO LTD
Fluorescent immunochromatography method for whole quantitative detection of C-reactive protein and reagent kit thereof
ActiveCN102539785BReduce sensitivityHigh luminous intensityBiological testingFluorescence/phosphorescenceFluorescenceAntibiotic effect
The invention discloses a fluorescent immunochromatography method for whole quantitative detection of C-reactive protein and a reagent kit thereof. The fluorescent immunochromatography method for the whole quantitative detection of the C-reactive protein (CRP) utilizes excellent fluorescent characteristics of quantum dots, and combines double-color marking technology and immunochromatography technology to achieve fluorescent quantitative detection on the basis of optimizing each constituent elements of test paper. Compared with a conventional colloidal gold immunochromatography method, the fluorescent immunochromatography method for the whole quantitative detection of the CRP has the advantages of being good in stability, low in non-specificity, high in flexibility, wide in linear range and accurate in quantifying. The reagent kit of the fluorescent immunochromatography method can perform the whole quantifying and can simultaneously predict and evaluate infectious diseases, antibiotic effects and cardiovascular and cerebrovascular diseases. The fluorescent immunochromatography method for the whole quantitative detection of the CRP and the reagent kit of the fluorescent immunochromatography method are suitable for various-level hospitals, and particularly contribute to wide popularization in basic-level hospitals and clinics.
Owner:SHENZHEN KANGMEI BIOTECH
Fluorescence immunochromatographic assay and kit for quantitatively detecting N-terminal pro brain natriuretic peptide
ActiveCN102565423BHigh luminous intensityWide excitation spectrumBiological testingFluorescence/phosphorescenceDiseaseN-terminal pro-Brain Natriuretic Peptide
The invention discloses a fluorescence immunochromatographic assay and kit for quantitatively detecting acute myocardial infarction marker, namely, N-terminal pro brain natriuretic peptide (NT-proBNP). The fluorescence immunochromatographic assay for quantitatively detecting the NT-proBNP realizes fluorescence quantitative detection on the basis of optimizing various constituent parts of test paper by using excellent fluorescence characteristic of quantum dots and combining a bicolor marking technology and an immunochromatographic technology. Compared with the conventional colloidal gold immunochromatographic assay, the fluorescence immunochromatographic assay disclosed by the invention has the advantages of good marking stability, low non-specificity, high sensitivity, wide linear range and quantifying accuracy. The fluorescence immunochromatographic kit disclosed by the invention is used for carrying out quantitative detection on the NT-proBNP and detecting whole blood, blood serum and blood plasma samples, can provide reference for diagnosis of cardiovascular and cerebrovascular diseases and can be widely applied to primary hospitals and clinics.
Owner:SHENZHEN KANGMEI BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com