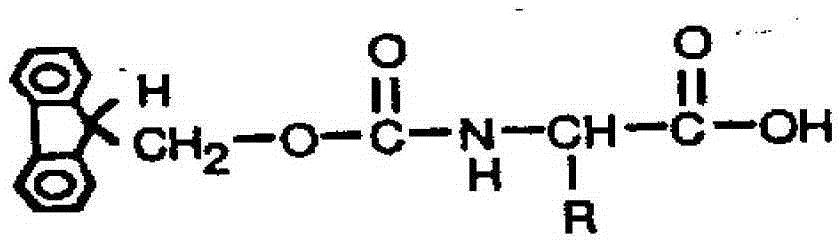Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
33results about How to "Fluorescent signal-to-background ratio improvement" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
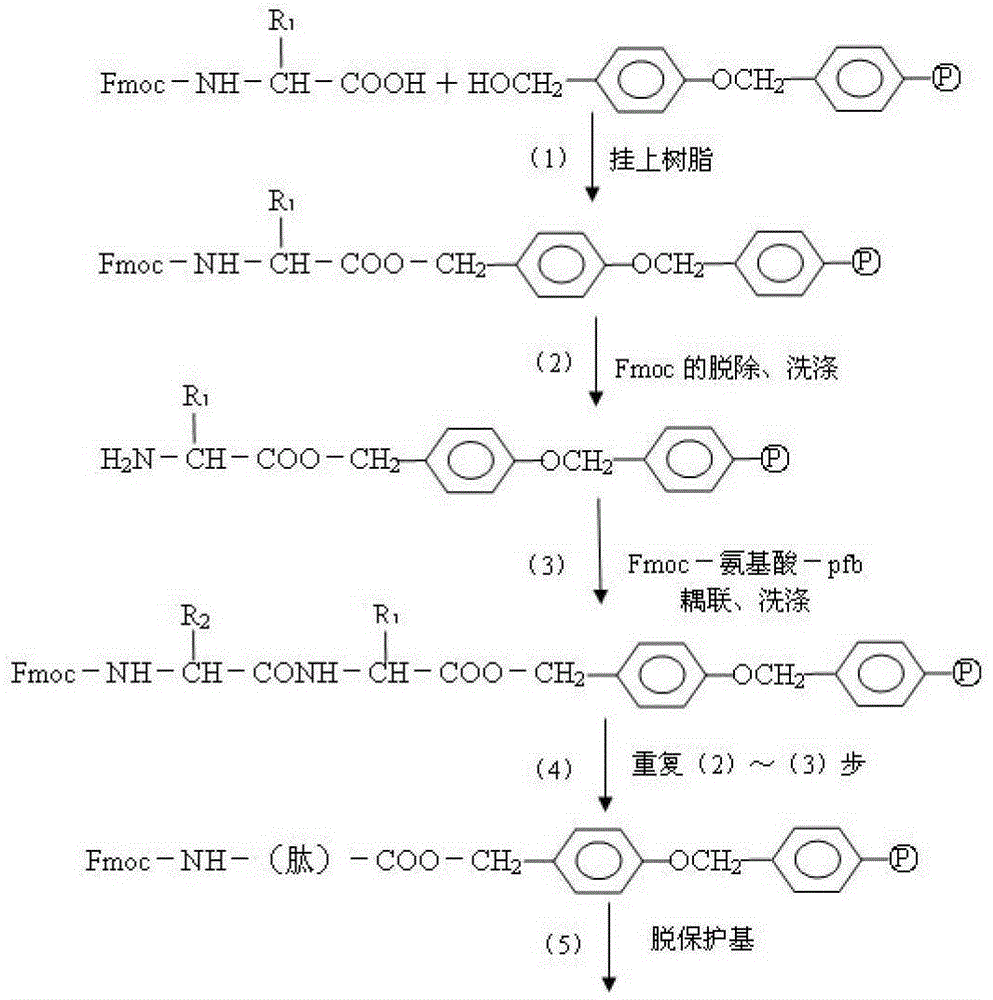
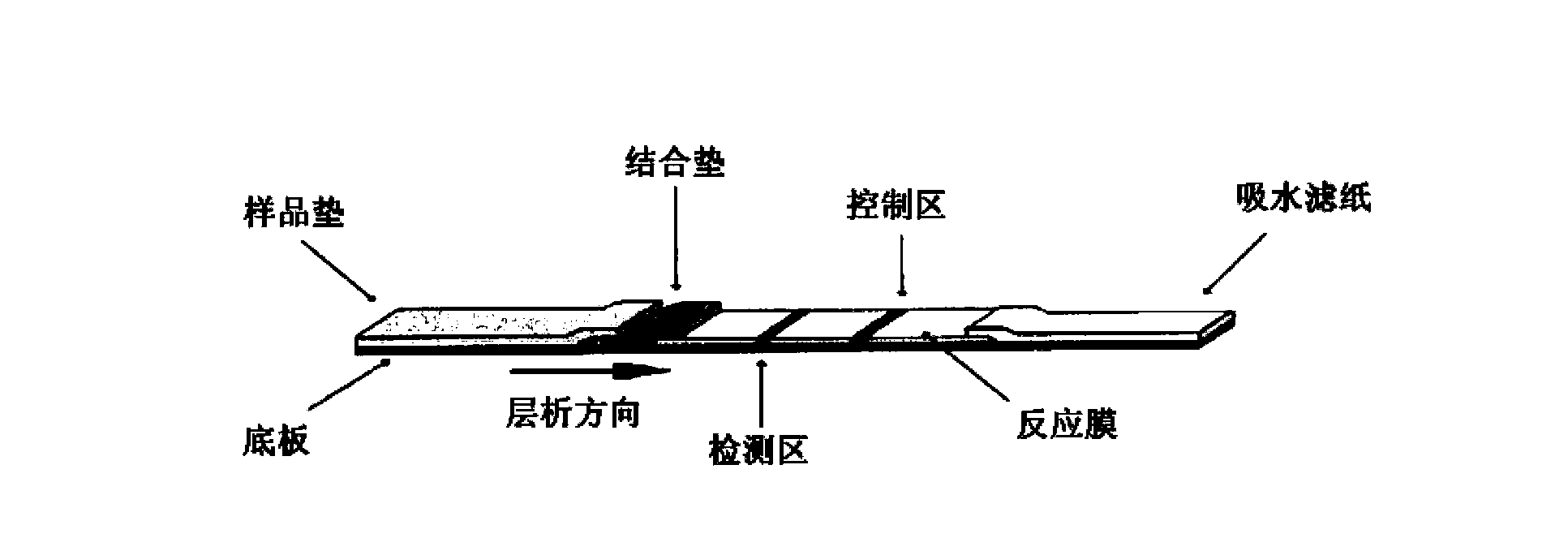
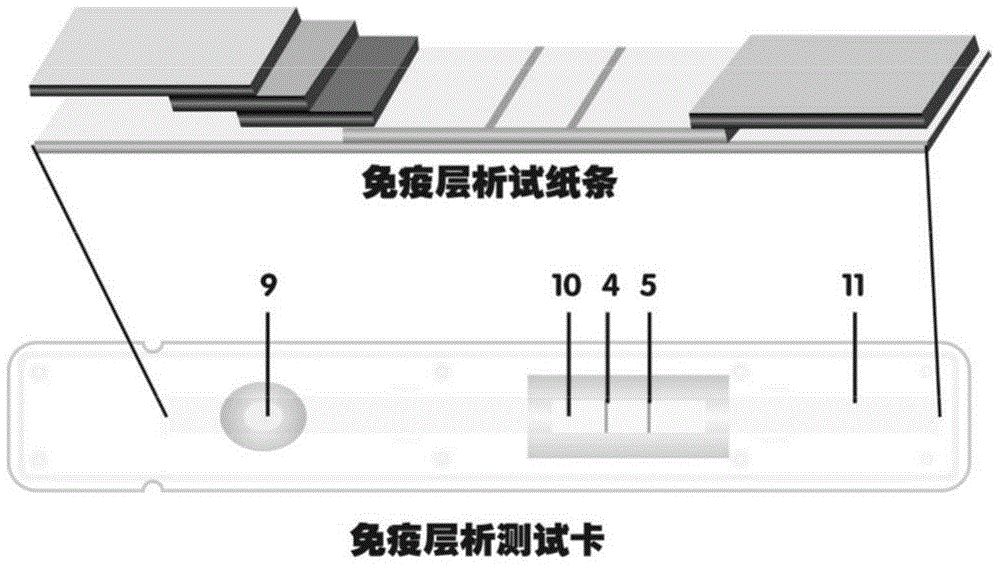
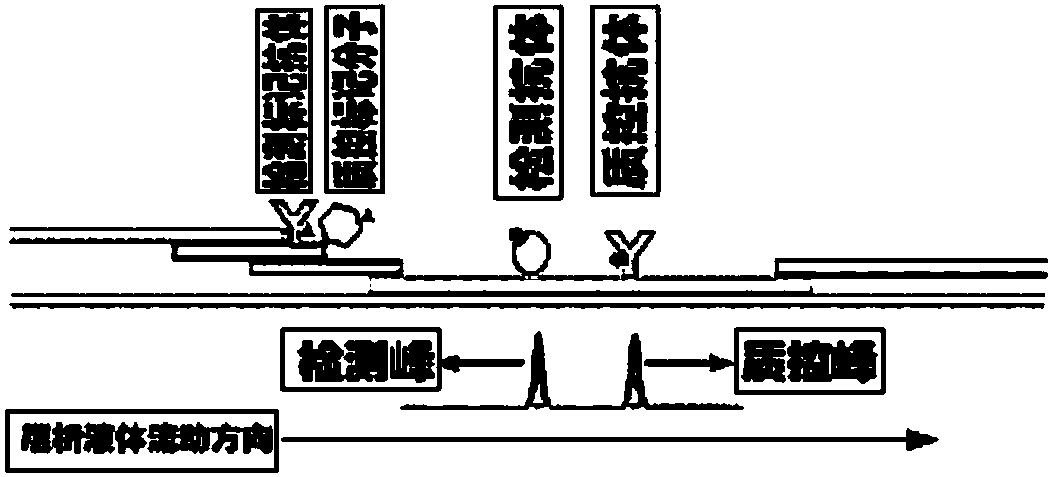
Method for highly sensitive quantitative detection of quantum dot fluorescence immunochromatographic assay
ActiveCN102520165ASensitive quantitative detection fastRealize detectionMaterial analysisCritical illnessLinear range
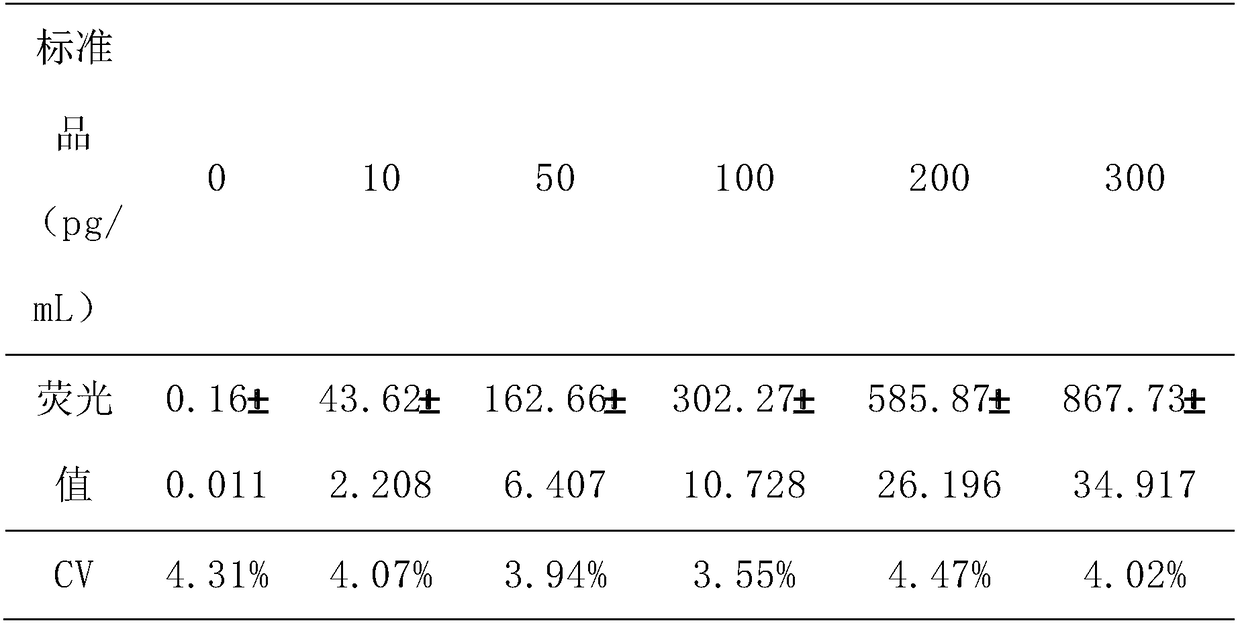
The invention discloses a method for highly sensitive quantitative detection of quantum dot fluorescence immunochromatographic assay. The method includes: building a fluorescence immunochromatographic assay test strip on the basis of optimizing the structure of the test strip and components by the aid of excellent fluorescent characteristics of quantum dots and by means of combining quantum dot fluorescence labeling technology and immunochromatographic assay; detecting fluorescence signal strength of a quantitative belt and a quality control belt by the aid of a fluorescence quantometer and correcting the fluorescence strength of the quantitative belt by the aid of the quality control belt after immunochromatographic assay of the test strip; and further quantitatively detecting analyte according to a standard curve obtained by the fluorescence quantometer. The method is simple, rapid, accurate, low in cost and quite high in sensitivity. Compared with a conventional colloidal gold immunochromatographic assay method, the method has the advantages of fine labeling stability, low non-specificity, high sensitivity, wide linear range and accuracy in quantization. The method is applicable to samples such as blood samples, urine samples, spittle, excrement and the like, and can be applied to detection of critical illness, poison, food safety and the like.
Owner:BEIJING KANGMEI TIANHONG BIOTECH
Fluorescence immunochromatographic assay and kit for quantitative detection of troponin I/creatine kinase isoenzyme/myohemoglobin
ActiveCN102520192AHigh sensitivityHigh detection sensitivityBiological testingNon specificImmunochromatographic Assays
The invention discloses a quantum dot multicolor marking method for quantitative detection of various cardiovascular disease markers and a kit of troponin I / creatine kinase isoenzyme / myohemoglobin. The method realizes fluorescent quantitative detection by utilizing excellent fluorescent properties of quantum dots and combining a multicolour marking technology and an immunochromatographic assay on the basis of optimizing each component of a test strip. Compared with the common collaurum immunochromatographic assay, the method has the advantages of good mark stability, low nonspecificity, high sensitivity, wide linear range, small cross interference, and accuracy in quantification. The kit disclosed by the invention is used for carrying out quantification detection on the troponin I, the creatine kinase isoenzyme and the myohemoglobin simultaneously, is suitable for detection of whole blood, blood serum and plasma samples, can provide a reference for cardiovascular and cerebrovascular disease diagnosis, and is widely applied to primary hospitals and clinics.
Owner:SHENZHEN KANGMEI BIOTECH
Immunofiltration assay fluorescent quantitative detection method based on high-sensitivity quantum dot
ActiveCN102539771AHigh luminous intensityWide excitation spectrumFluorescence/phosphorescenceQuantum dotQuality control
The invention discloses an immunofiltration assay fluorescent quantitative detection method based on a high-sensitivity quantum dot; the immunofiltration assay fluorescent quantitative detection method comprises the following steps of: constructing a fluorescence immunofiltration array device by using the excellent fluorescent characteristic of the quantum dot in combination of a quantum dot fluorescence labeling technology and an immunofiltration array technology on the basis of optimizing constituent parts for the immunofiltration assay; and after immunofiltration array, detecting the strenght of fluorescent signals of the quantum dot and a quality control dot by using a fluorescence quantometer, correcting the fluorescence strenght of the quantum pot by using the quality control dot, and further realizing the quantitative detection of a tested object according to a standard curve obtained by using the fluorescence quantometer. The method is simple, rapid, accurate, low in cost and high in sensitiveness. Comapred with the conventional collodial gold immunofiltration array method, the immunofiltration assay fluorescent quantitative detection method has the advantages of good labeling stability, low non-specificity, high sensitivity, wide linear range and accurate quantification. The method is suitable for samples such as serums, urine, spittle, excrement and the like and can be applicable to the detection of serious illness, poisons, food safety and the like.
Owner:BEIJING KANGMEI TIANHONG BIOTECH
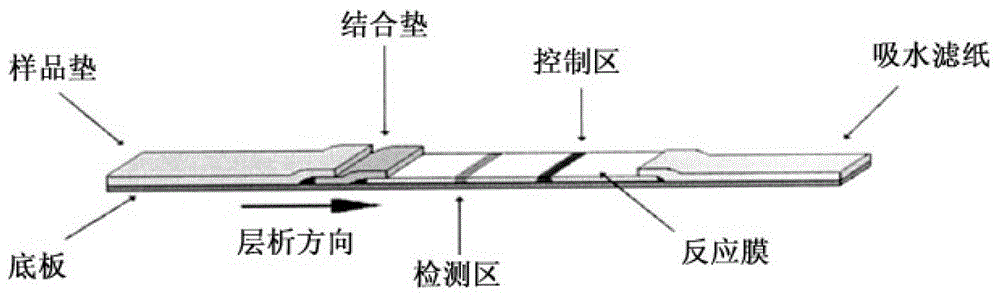
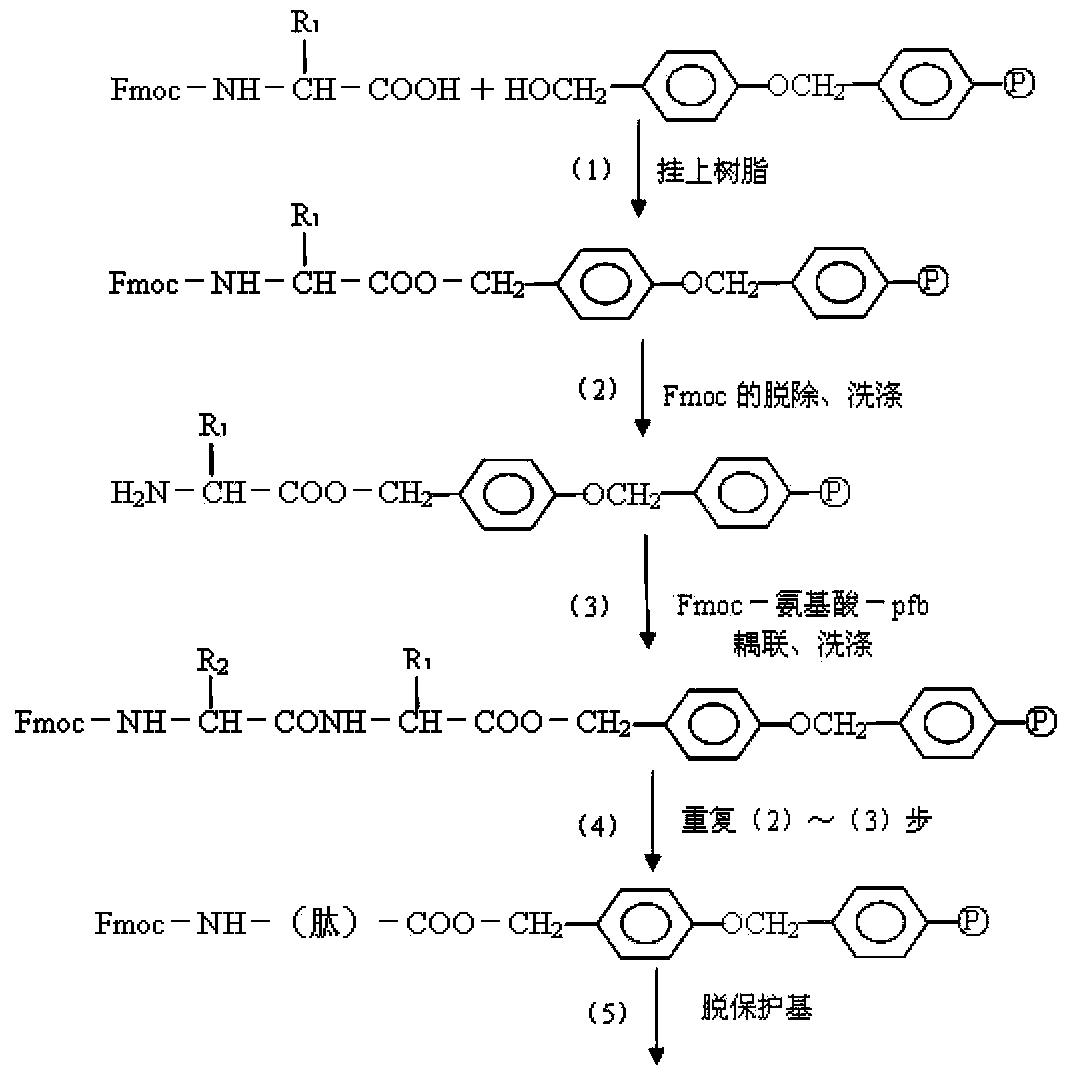
Fluorescence immunochromatography test paper for detecting human Lp-PLA2 proteins and preparation method of fluorescence immunochromatography test paper
The invention relates to fluorescence immunochromatography test paper for detecting human Lp-PLA2 proteins and a preparation method of the fluorescence immunochromatography test paper. The test paper is used for detecting the human Lp-PLA2 proteins by virtue of a two-antibody sandwich method, and in the two-antibody sandwich method, a first Lp-PLA2 monoclonal antibody which is labeled with fluorescence microspheres serves as a trapping antibody, and is sourced from one of sequences indicated in sequence tables of SEQ ID NO.1 and SEQ ID NO.2; a second Lp-PLA2 monoclonal antibody serves as a detection antibody, and is sourced from the other one of the sequences indicated in the sequence tables of SEQ ID NO.1 and SEQ ID NO.2. The fluorescence immunochromatography test paper is simple and rapid to operate, wide in detection range, high in specificity and good in sensitivity.
Owner:深圳市安群生物工程有限公司
Fluorescence immunochromatography kit for quantitatively detecting human epididymis secretory protein-4 and preparation method for fluorescence immunochromatography kit
InactiveCN104655858AHigh luminous intensityWide excitation spectrumDisease diagnosisBiological testingPlasma samplesFluorescence
The invention discloses a fluorescence immunochromatography kit for quantitatively detecting human epididymis secretory protein-4 by taking fluorescent dye as a marker. The fluorescence immunochromatography kit disclosed by the invention realizes fluorescence quantitative detection for the human epididymis secretory protein-4, has the advantages of being good in stability, wide in linear range, good in specificity, accurate to quantify, simple and quick, can be used for simultaneously detecting whole blood, blood serum and plasma samples, and is suitable for hospitals of various levels.
Owner:DEMAIJI BIOTECH BEIJING
Two-photon fluorescence immunochromatography kit for quantitative determination of anti-Mullerian hormone (AMH) and preparation method of kit
InactiveCN107621540ALong fluorescence lifetimeHigh luminous intensityMaterial analysisQuantitative accuracyBlood plasma
The invention discloses a two-photon fluorescence immunochromatography kit for quantitative determination of anti-Mullerian hormone (AMH), which utilizes fluorescent dye as a maker. The two-photon fluorescence immunochromatography kit, realizing fluorescence immunochromatography quantitative determination, has the advantages of good stability, wide linear range, good specificity, high sensitivity,high quantitative accuracy and easy and quick operation, can be applied to detection of whole blood samples, serum samples and plasma samples simultaneously, and is applicable to medical treatment ofhospital at different levels and family practice.
Owner:DEMAIJI BIOTECH BEIJING
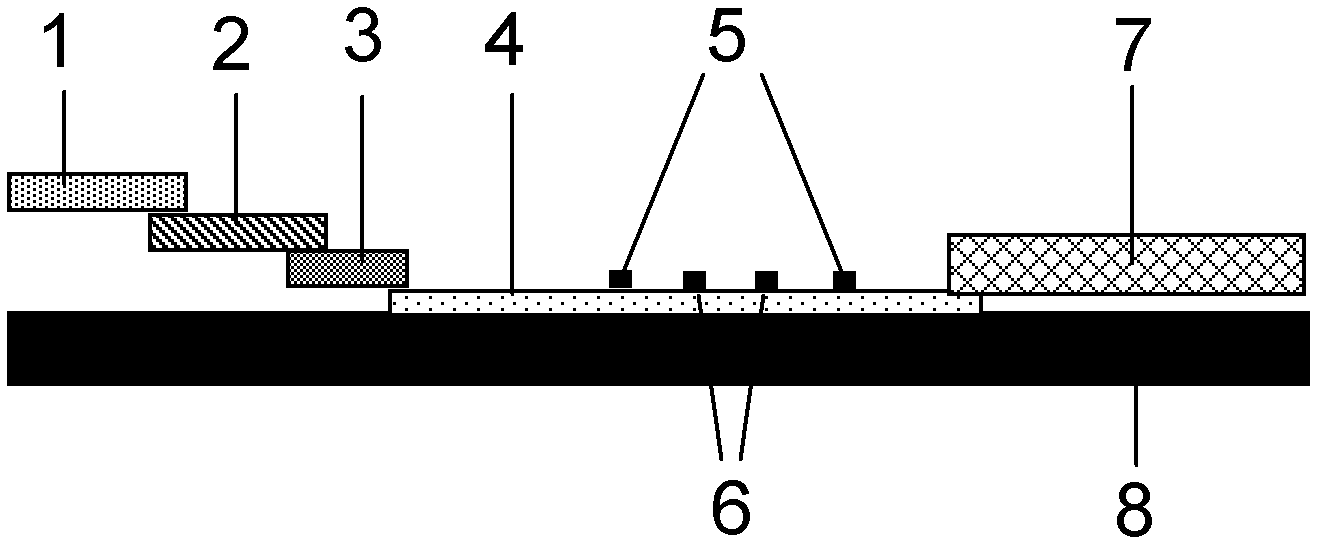
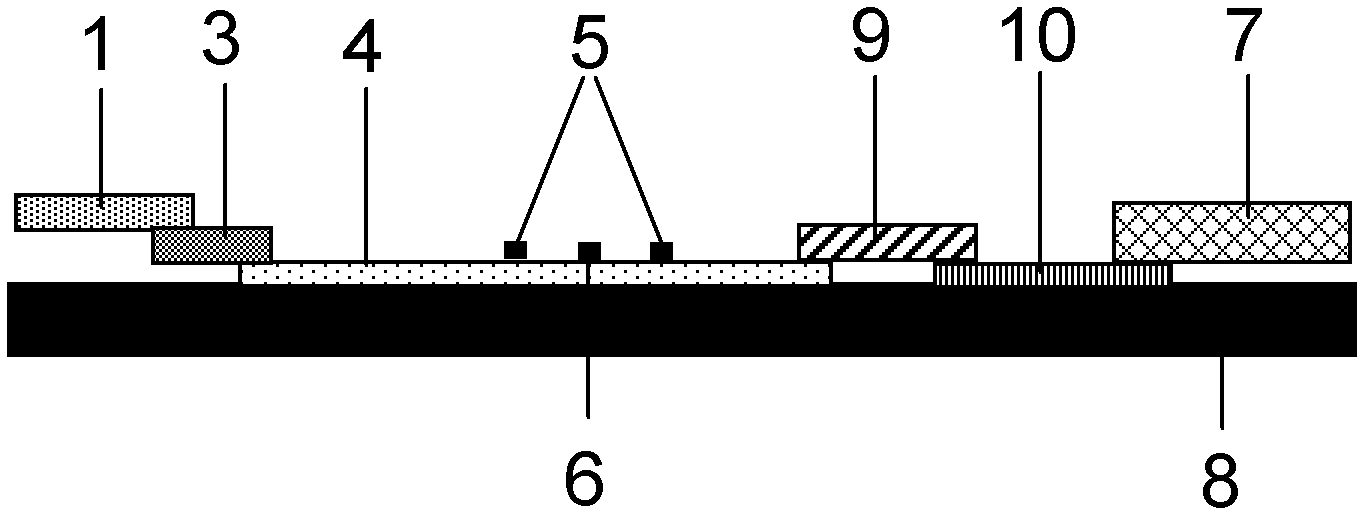
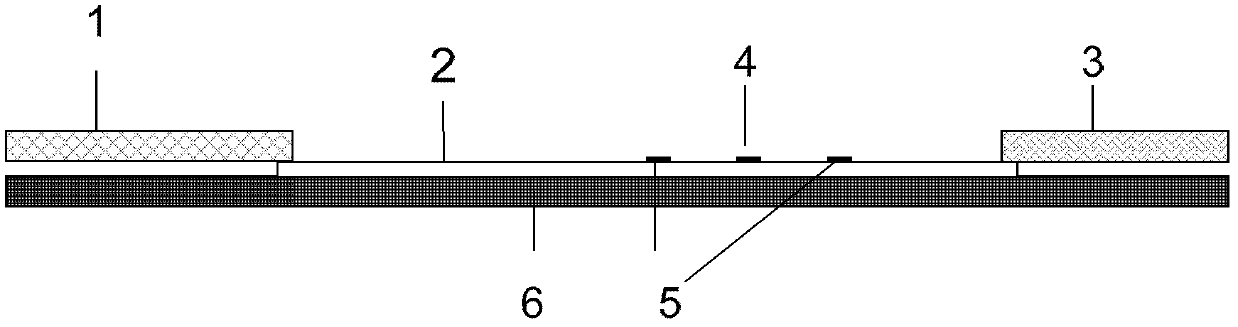
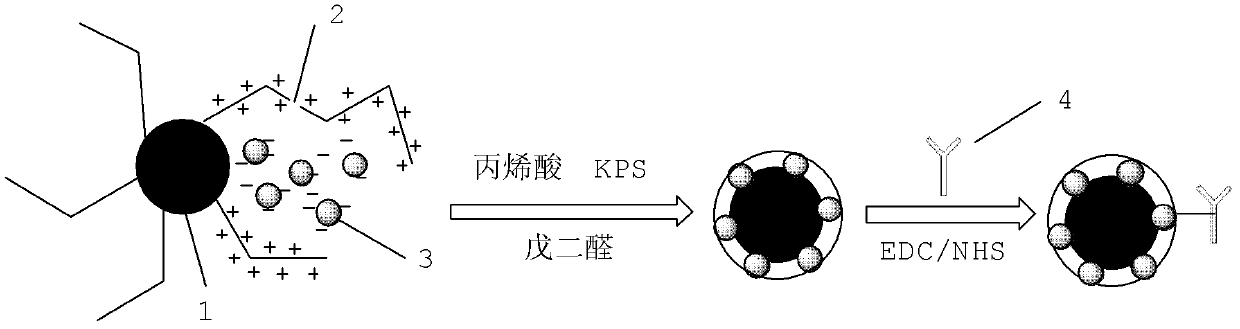
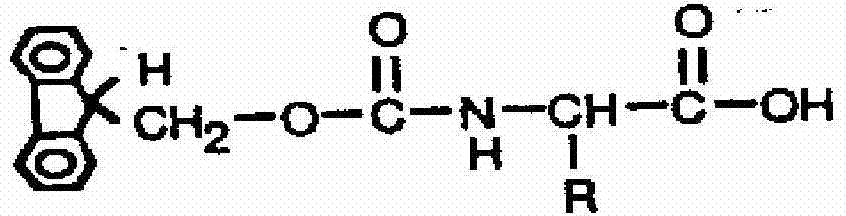
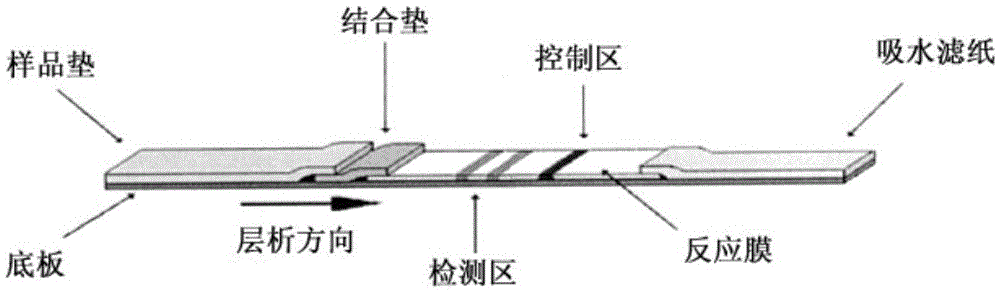
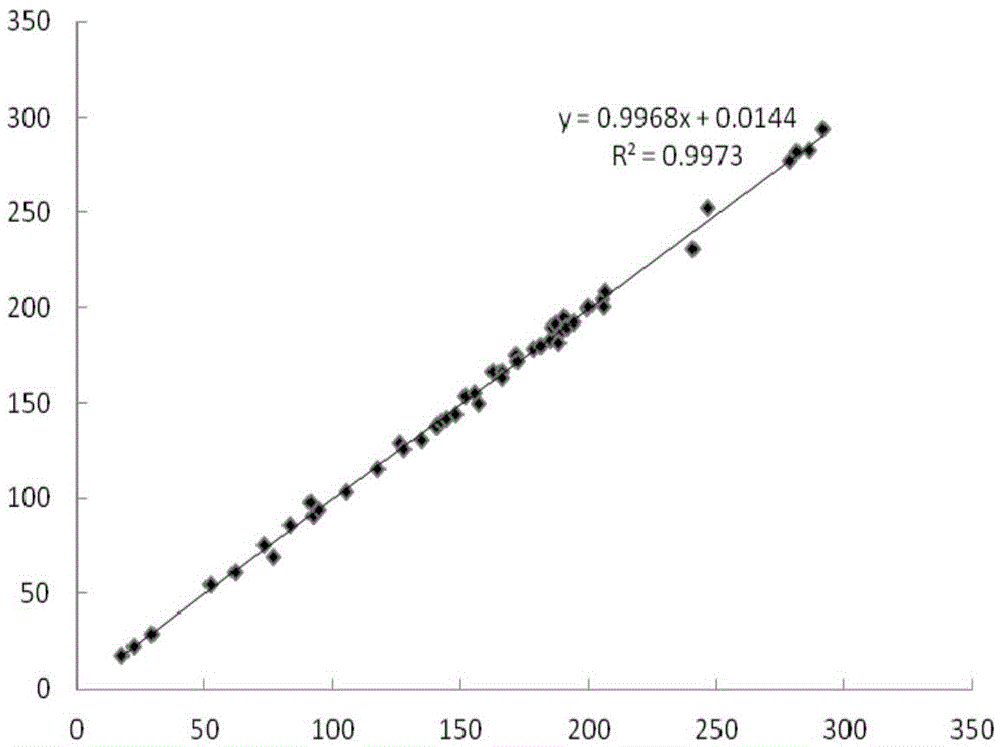
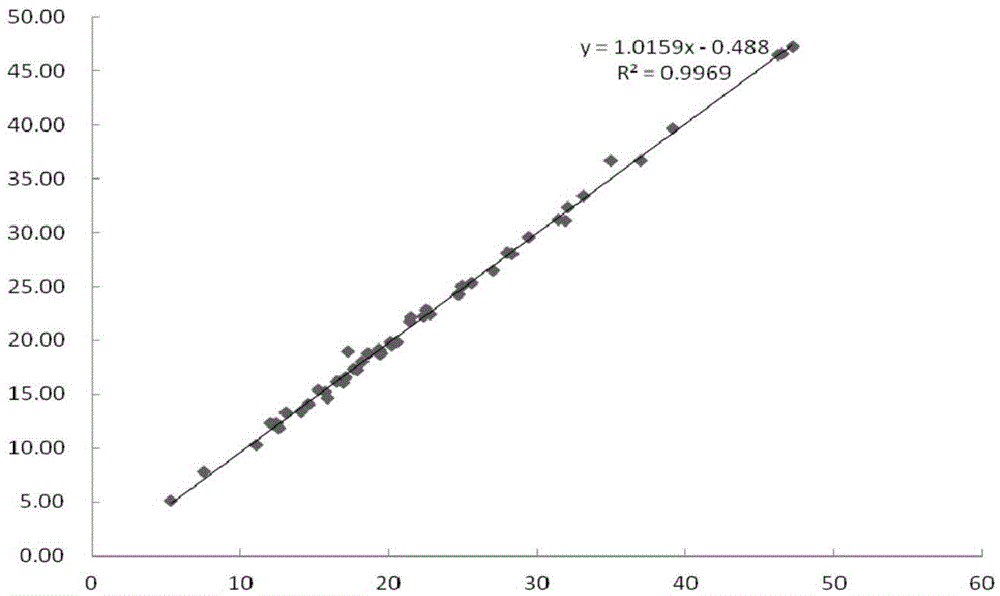
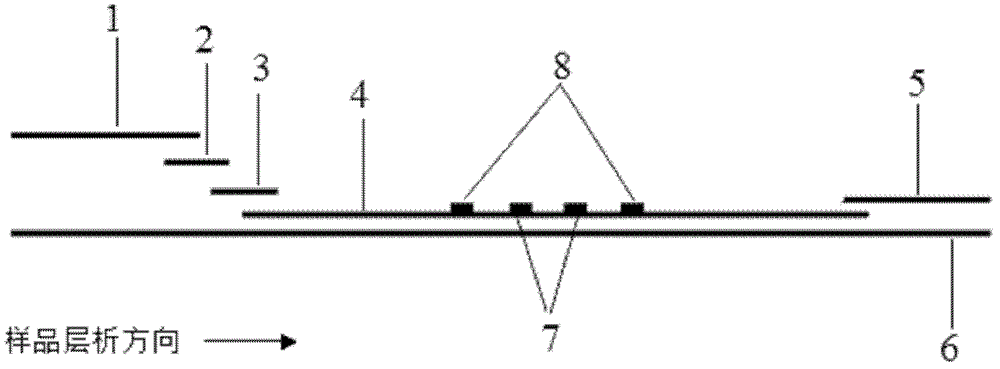
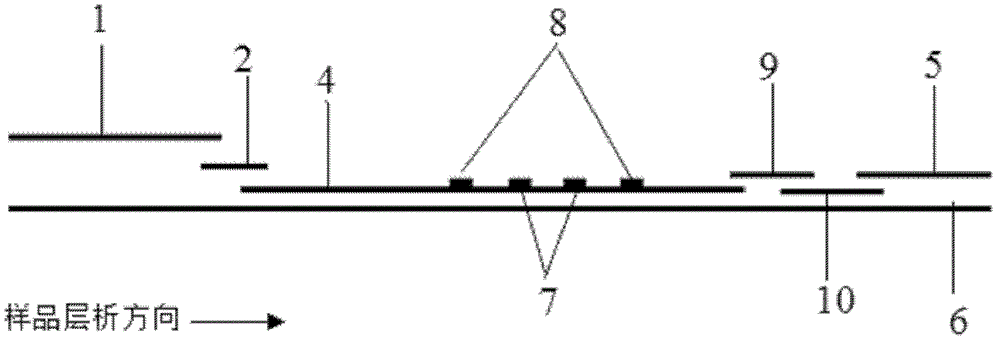
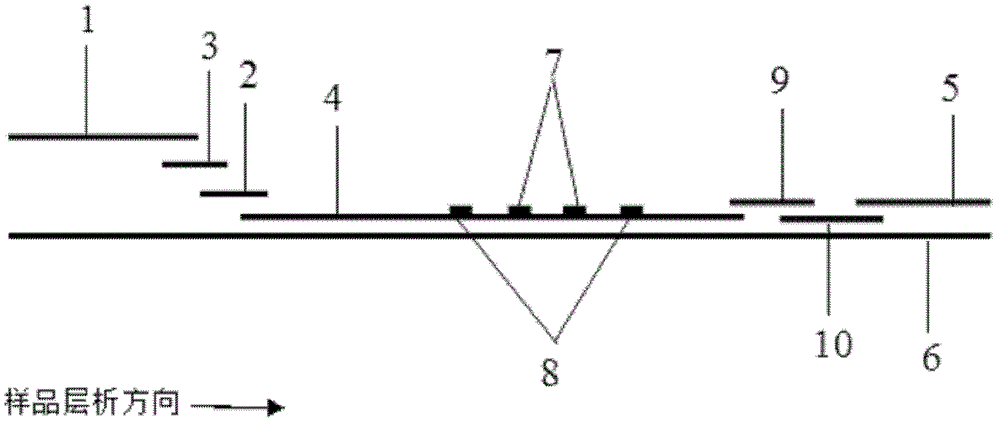
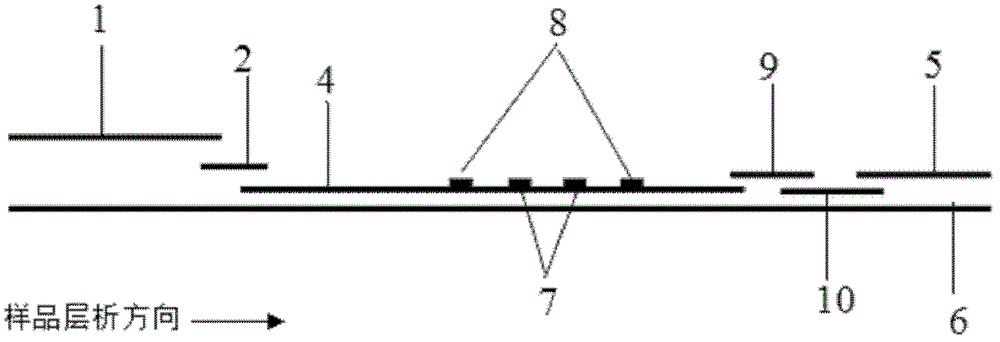
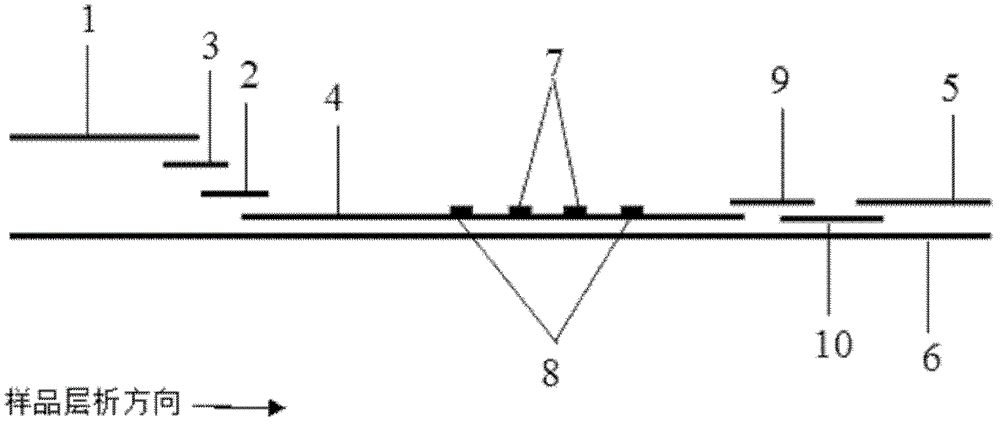
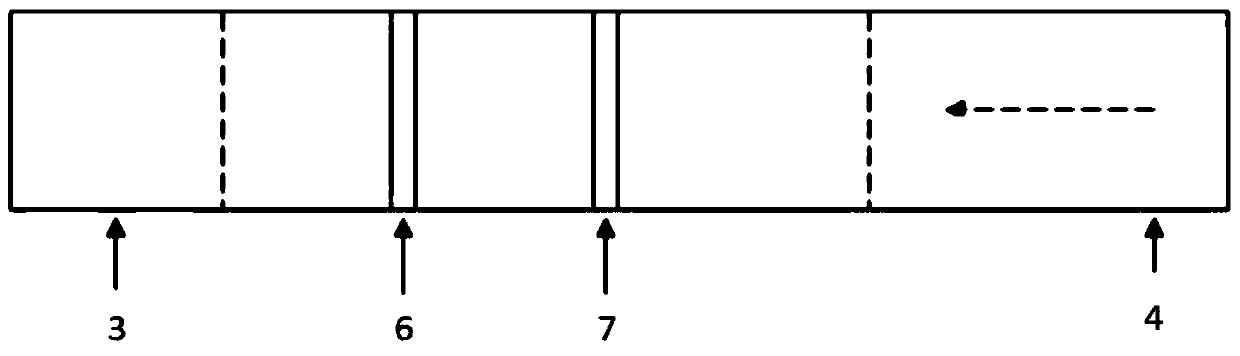
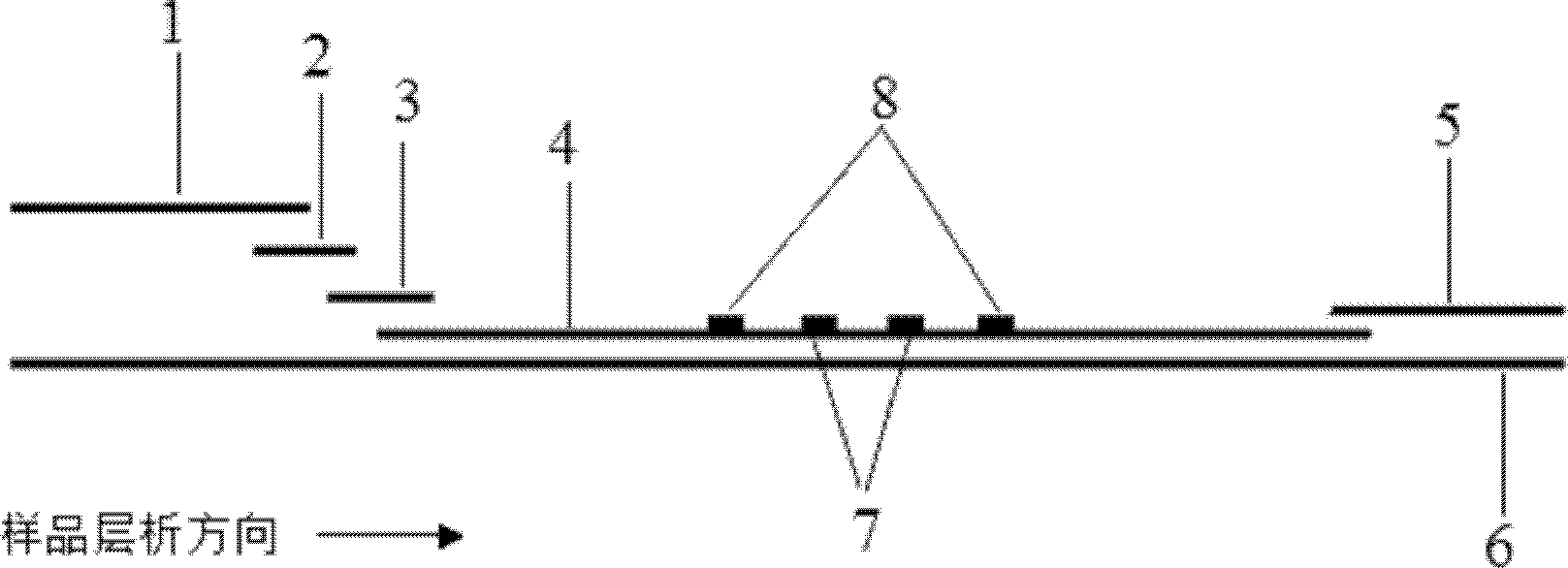
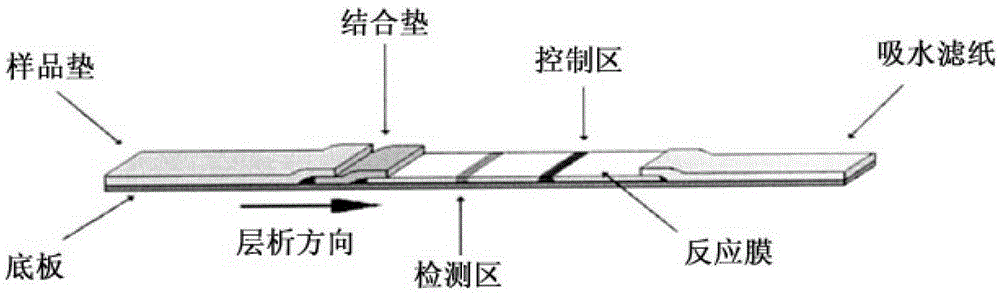
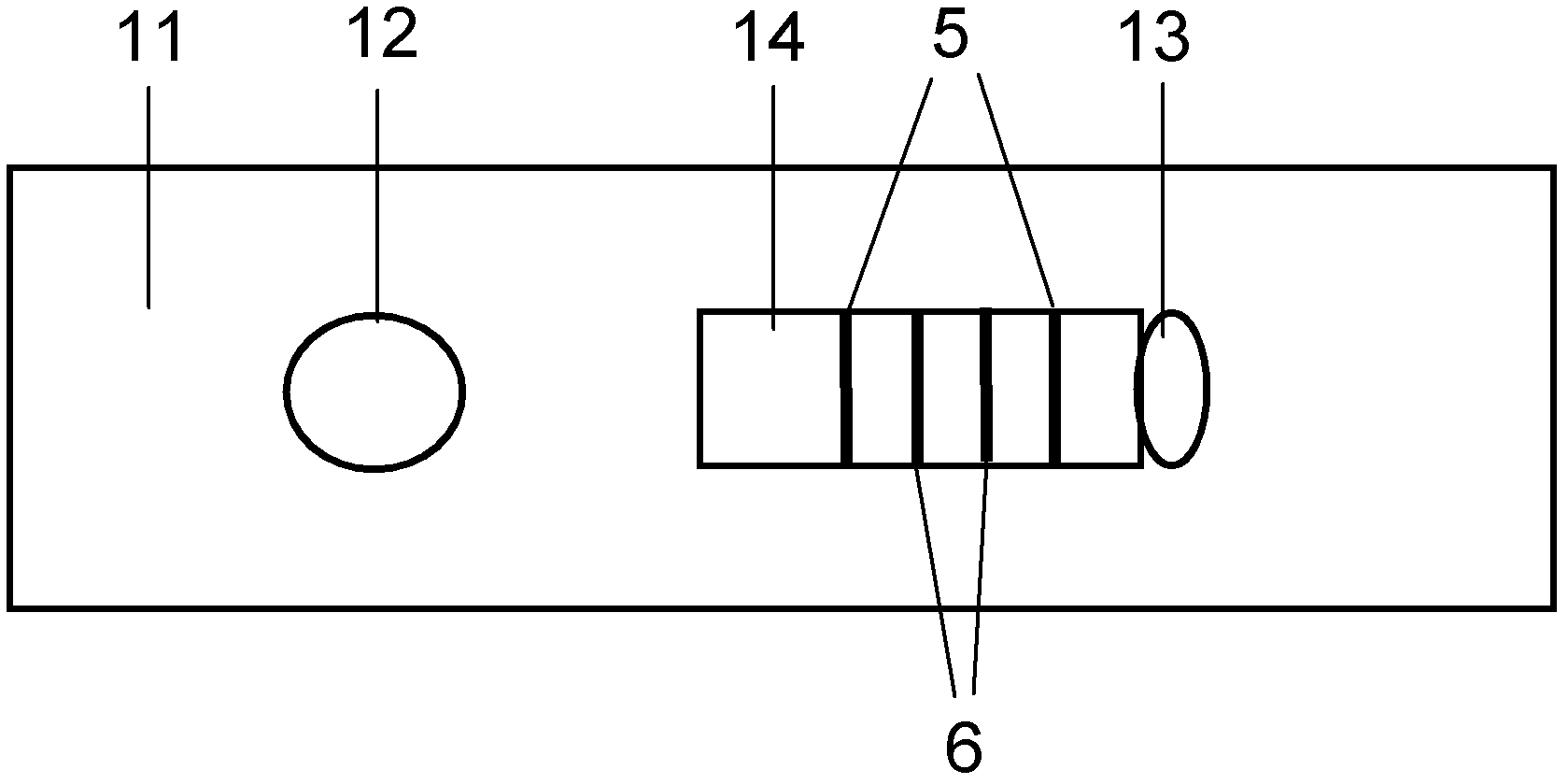
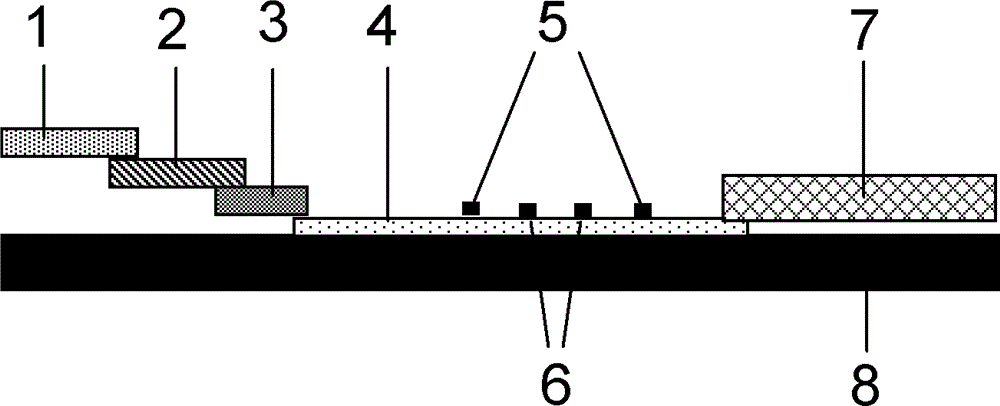
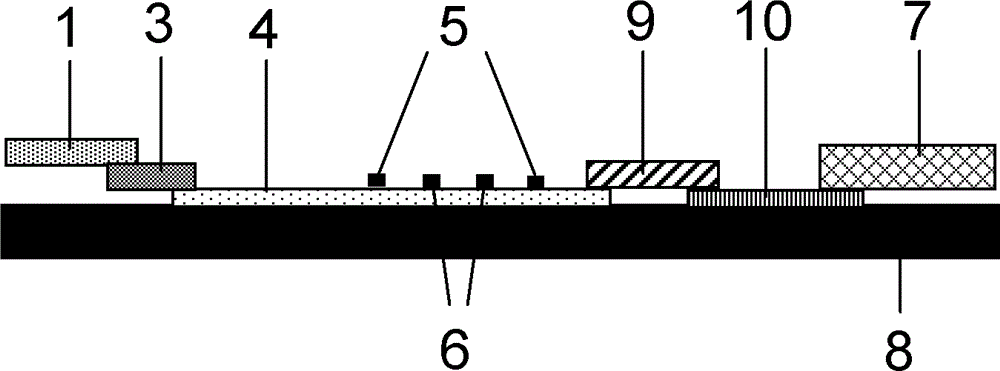
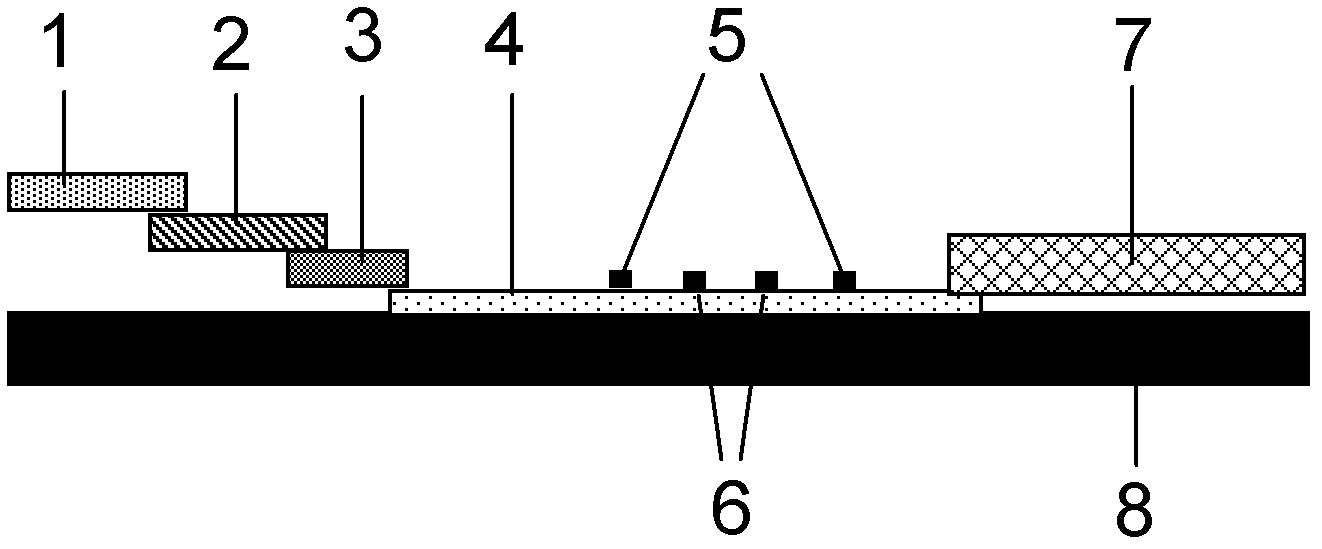
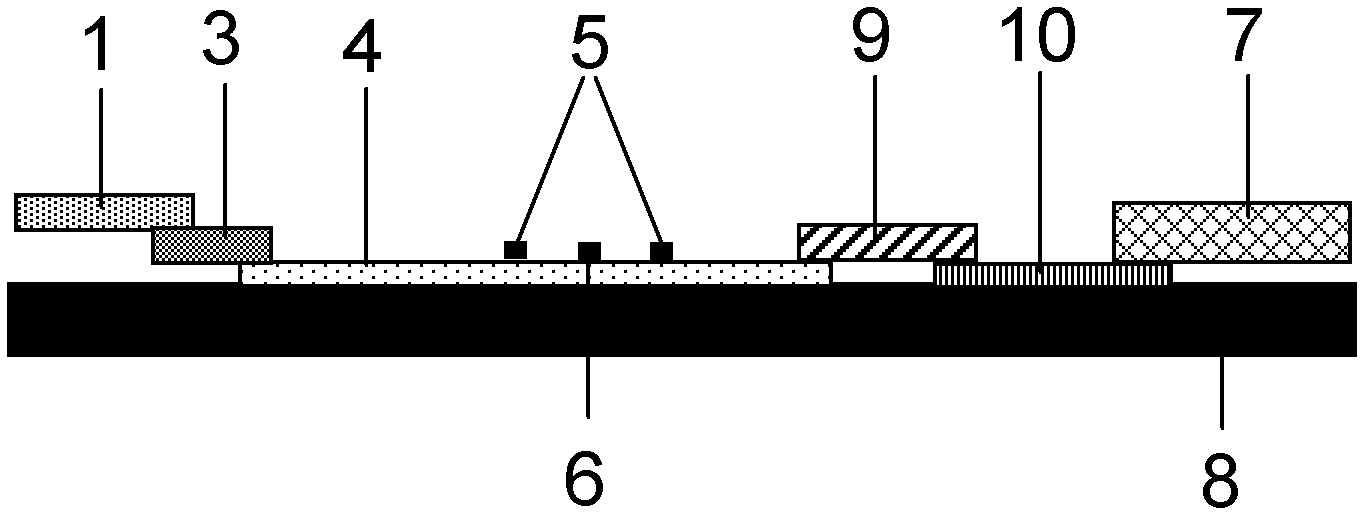
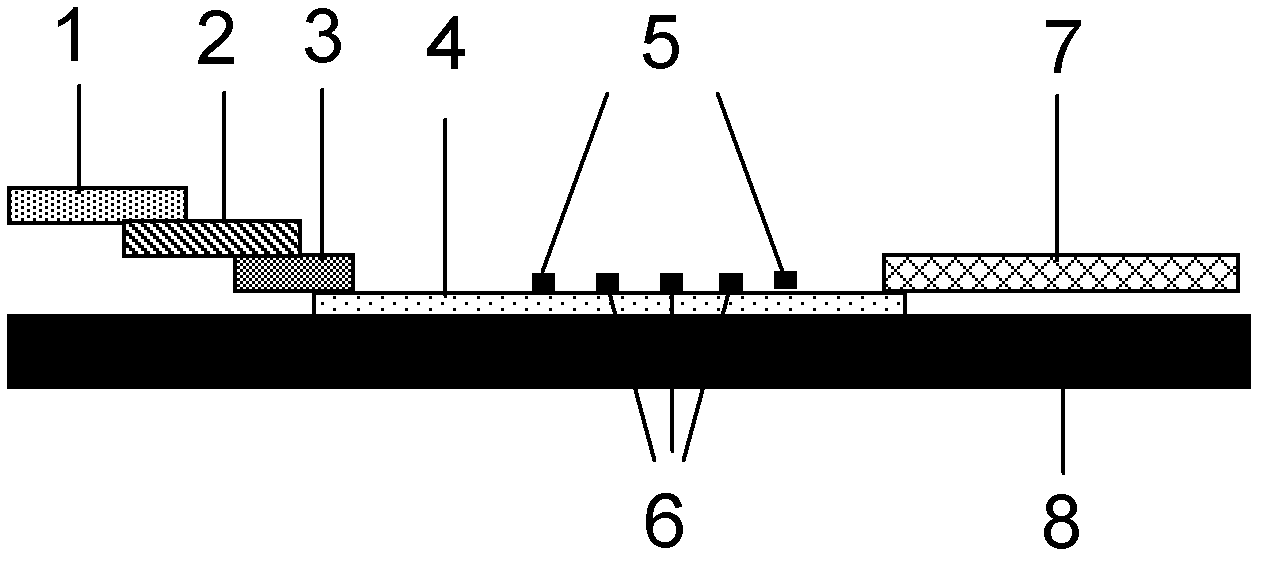
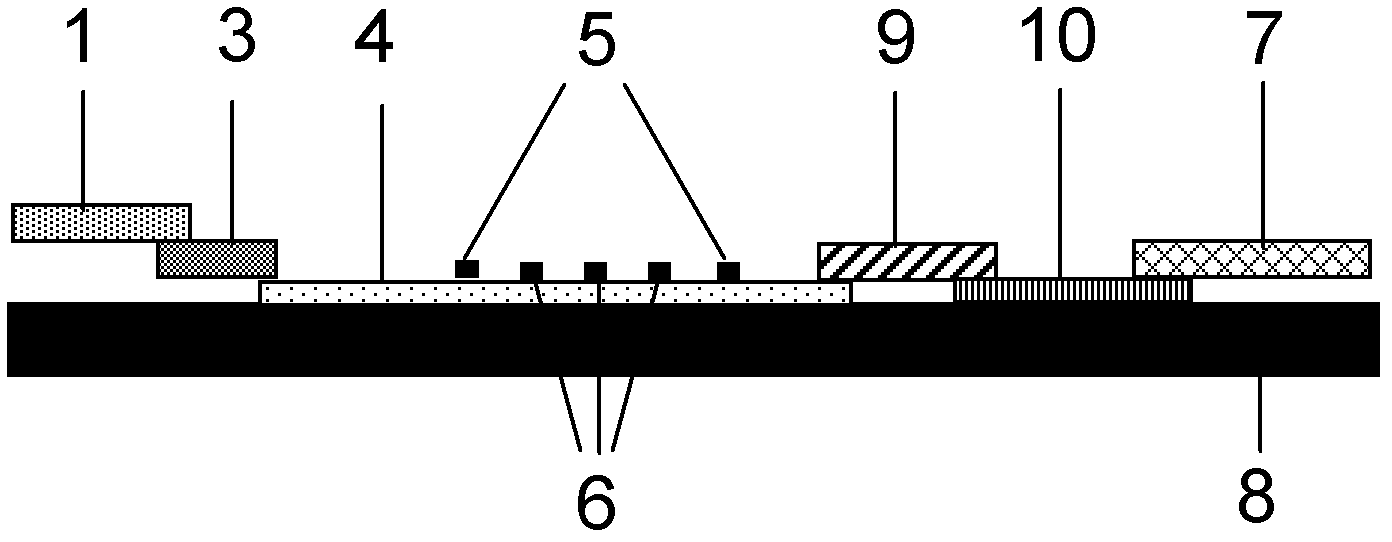
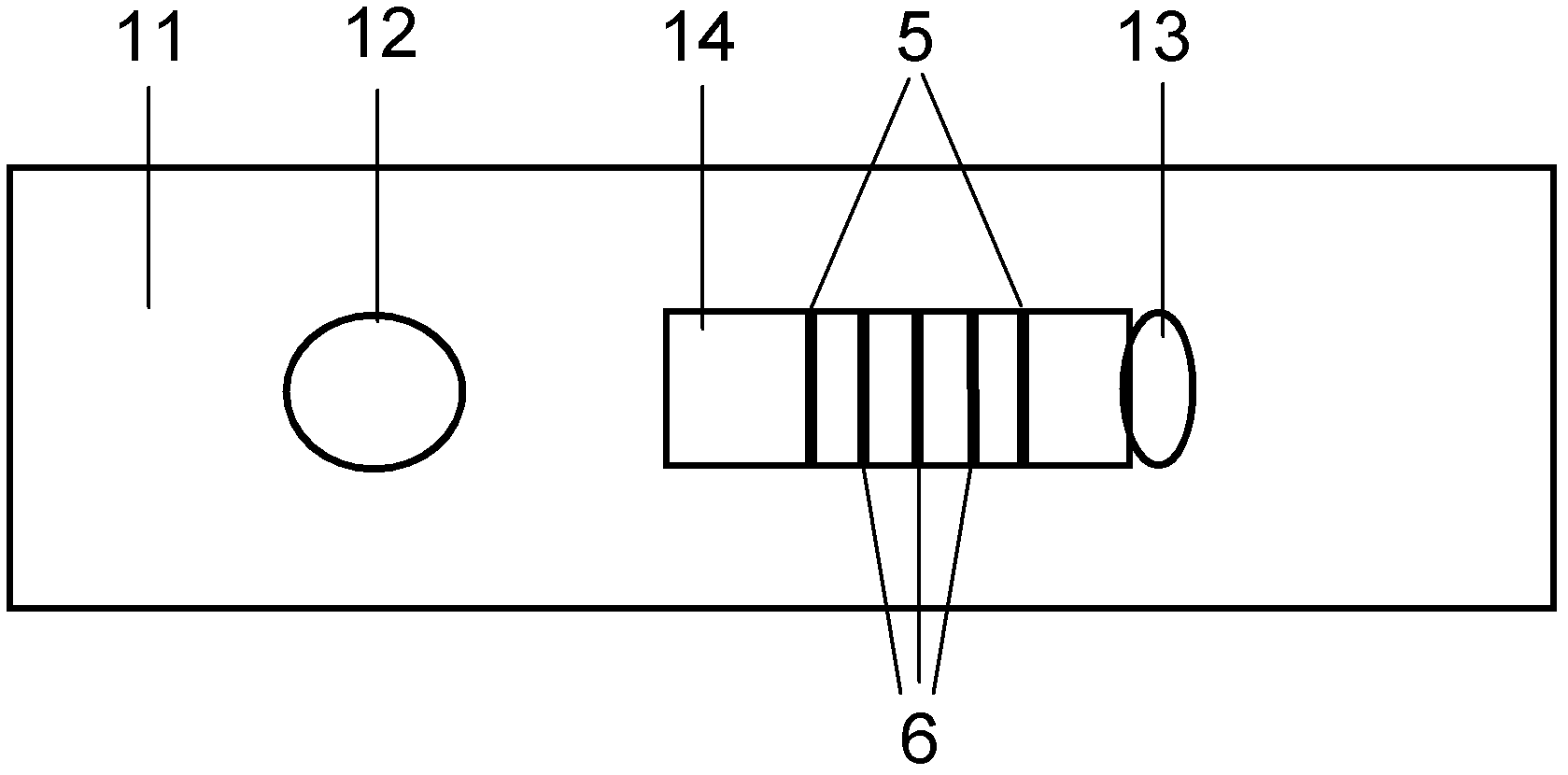
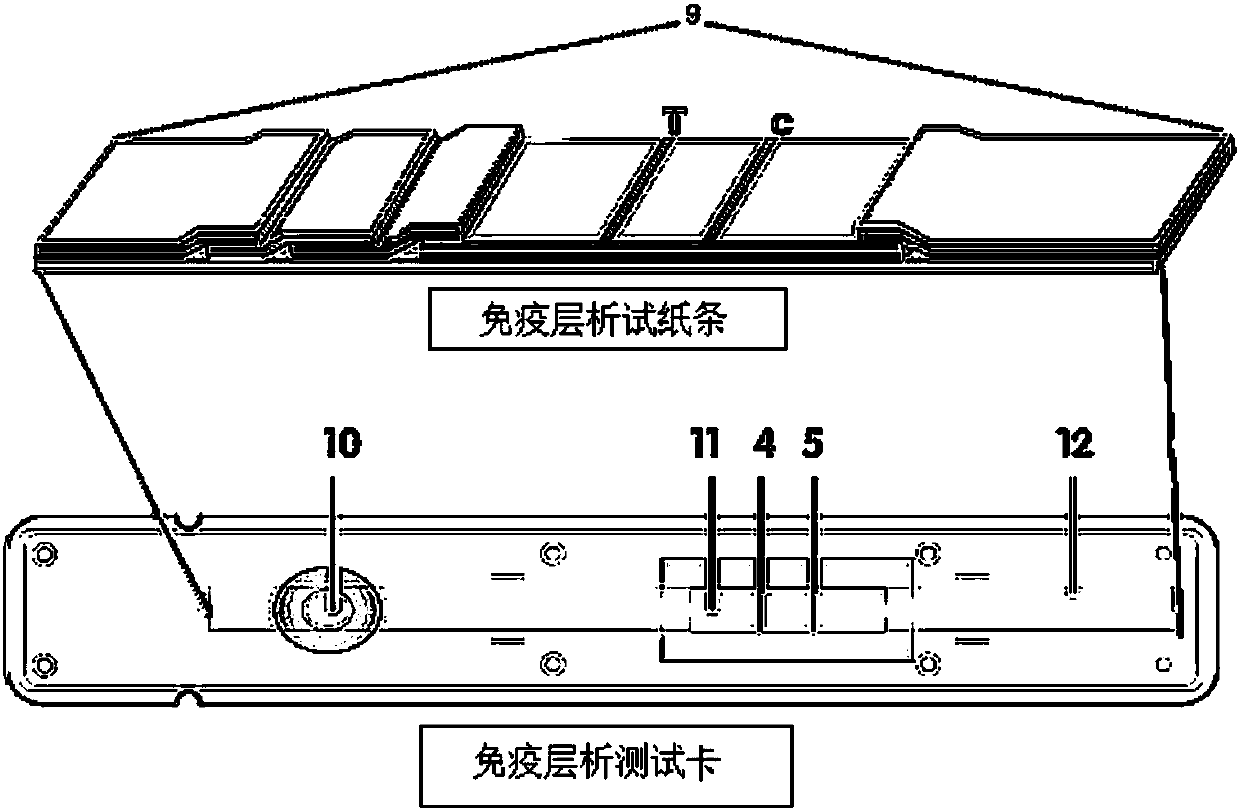
Fluorescence immune chromatography test paper for detecting human ApoE-[epsilon]4 protein and preparation method thereof
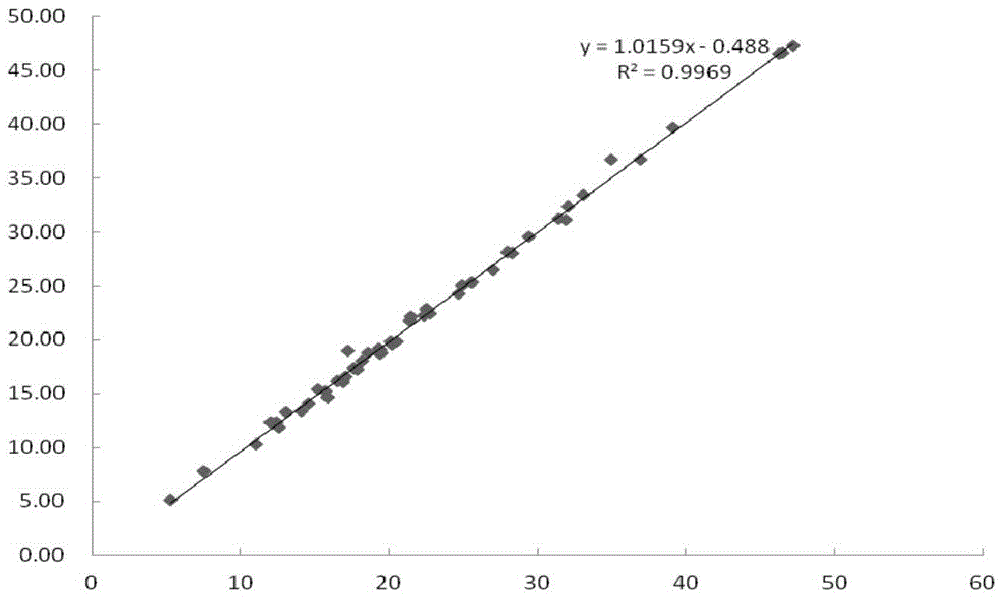
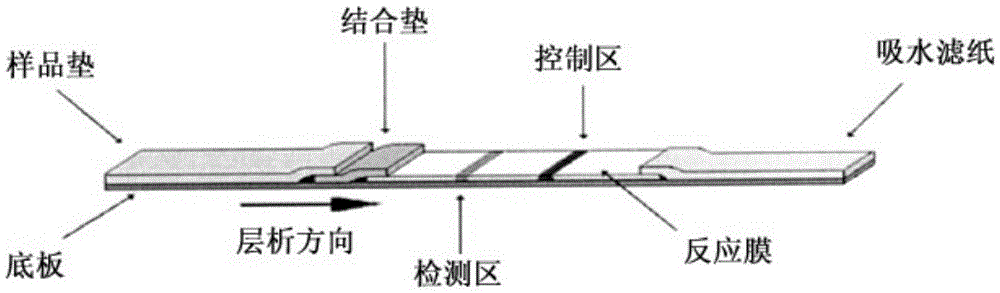
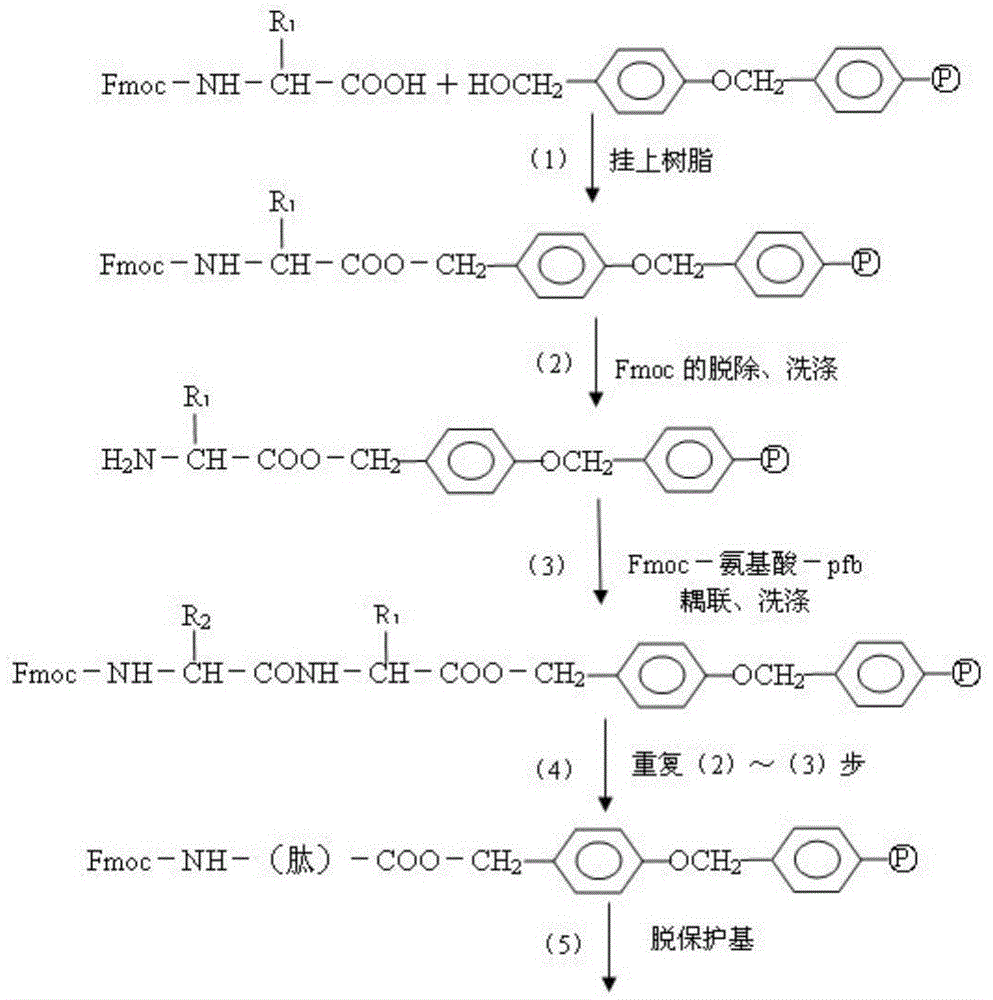
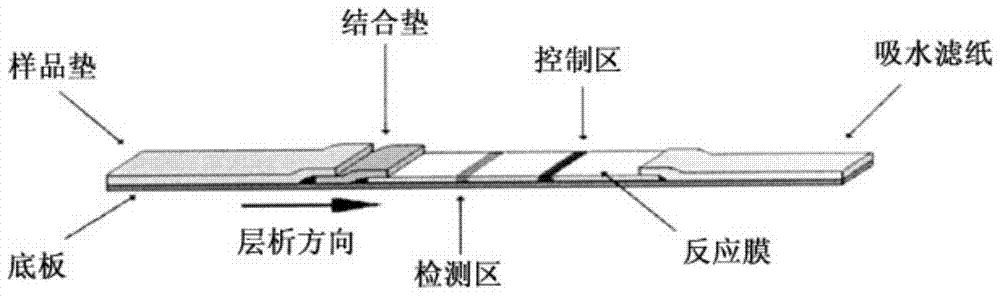
The invention relates to a fluorescence immune chromatography test paper for detecting human ApoE-[epsilon]4 protein and a preparation method thereof. The test paper is used for detecting the human ApoE-[epsilon]4 protein by virtue of a double-antibody sandwich method, and in the double-antibody sandwich method, a first ApoE-[epsilon]4 monoclonal antibody is labeled with fluorescence microspheres serves as a trapping antibody and is sourced from one of sequences indicated in sequence tables of SEQ ID NO.1 and SEQ ID NO.2; a second ApoE-[epsilon]4 monoclonal antibody serves as a detection antibody, and is sourced from the other one of the sequences indicated in the sequence tables of SEQ ID NO.1 and SEQ ID NO.2. The fluorescence immune chromatography test paper is simple and rapid to operate, wide in detection range, high in specificity and good in sensitivity.
Owner:深圳市安群生物工程有限公司
Immunochromatography test paper for detecting human PGI protein and preparation method thereof
ActiveCN104422773AGood antigenicityStrong specificityBiological testingFluorescence/phosphorescenceCapture antibodyTest strips
The invention relates to a Immunochromatography test paper for detecting a human PGI protein and a preparation method thereof. The test paper employs a double antibody sandwich method for detection of the human PGI protein. The double antibody sandwich method uses a first PGI monoclonal antibody labeled with fluorescent microspheres as a capture antibody, and the first PGI monoclonal antibody is one of the sequences from the SEQ ID NO.1 and SEQ ID NO.2 in the sequence table; and the double antibody sandwich method uses a second PGI monoclonal antibody as a detection antibody, and the second PGI monoclonal antibody is another one from SEQ ID NO.1 and SEQ ID NO.2 in the sequence table. The invention of the immunochromatography test paper has the advantages of simple operation, rapidness, wide detection range, high specificity and good sensitivity.
Owner:朱建安
Fluorescent immunochromatographic test paper for quantitative detection of human parathyroid hormone and preparation method thereof
The invention discloses fluorescence immunochromatography test paper for achieving intraoperative judgment of parathyroid tissues through quantitatively detecting a human parathyroid hormone (PTH) and a preparation method of the fluorescence immunochromatography test paper. The test paper capable of detecting the human PTH through a double-antibody sandwich method and a fluorescence immunochromatography; the antibody is prepared by using a specific antigen peptide. The human PTH in a to-be-detected matter can be quickly and accurately detected, so that the fluorescence immunochromatography test paper is simple, convenient and fast to operate, wide in detection range, high in specificity and good in sensitivity.
Owner:JIANGSU INST OF NUCLEAR MEDICINE +1
Fluorescent immunochromatographic test paper for detecting human gfap protein and preparation method thereof
ActiveCN104142404BGood antigenicityStrong specificityImmunoglobulins against animals/humansBiological testingCapture antibodyImmunochromatographic test
The invention relates to a fluorescent immunochromatographic test paper for detecting a human GFAP protein, and a preparation method thereof. The test paper detects the human GFAP protein through a double antibody sandwich technology, the double antibody sandwich technology adopts a fluorescent microsphere-labeled first GFAP monoclonal antibody as a capture antibody, and the first GFAP monoclonal antibody is from one of a sequence represented by SEQ ID NO.1 in a sequence table and a sequence represented by SEQ ID NO.2 in the sequence table; and the double antibody sandwich technology adopts a second GFAP monoclonal antibody as a detection antibody, and the second GFAP monoclonal antibody is from the other one of the sequence represented by SEQ ID NO.1 in the sequence table and the sequence represented by SEQ ID NO.2 in the sequence table. The fluorescent immunochromatographic test paper has the advantages of simple operation, rapidness, wide detection range, high specificity and good sensitivity.
Owner:深圳市安群生物工程有限公司
Method for highly sensitive quantitative detection of quantum dot fluorescence immunochromatographic assay
ActiveCN102520165BHigh luminous intensityWide excitation spectrumMaterial analysisCritical illnessUrine sample
The invention discloses a method for highly sensitive quantitative detection of quantum dot fluorescence immunochromatographic assay. The method includes: building a fluorescence immunochromatographic assay test strip on the basis of optimizing the structure of the test strip and components by the aid of excellent fluorescent characteristics of quantum dots and by means of combining quantum dot fluorescence labeling technology and immunochromatographic assay; detecting fluorescence signal strength of a quantitative belt and a quality control belt by the aid of a fluorescence quantometer and correcting the fluorescence strength of the quantitative belt by the aid of the quality control belt after immunochromatographic assay of the test strip; and further quantitatively detecting analyte according to a standard curve obtained by the fluorescence quantometer. The method is simple, rapid, accurate, low in cost and quite high in sensitivity. Compared with a conventional colloidal gold immunochromatographic assay method, the method has the advantages of fine labeling stability, low non-specificity, high sensitivity, wide linear range and accuracy in quantization. The method is applicable to samples such as blood samples, urine samples, spittle, excrement and the like, and can be applied to detection of critical illness, poison, food safety and the like.
Owner:BEIJING KANGMEI TIANHONG BIOTECH
Magnetic fluorescent microsphere immunochromatography quantitative detection method
ActiveCN102565386BReduce sample matrix varianceHigh detection sensitivityFluorescence/phosphorescenceAdditive ingredientMedical testing
The invention discloses a magnetic fluorescent microsphere immunochromatography quantitative detection method. In the method, respective excellent characteristics of magnetic nano particles and quantum dots are fully utilized, and an immunochromatography technology is combined to realize fluorescent quantitative detection on the basis of optimizing the structure and ingredients of a test strip. The method has a function of amplifying signals; and compared with the conventional colloidal gold immunochromatography method, the method has the advantages of high mark stability, low non-specificity, high sensitivity, wide linear range and accurate quantification. The invention provides a simple, accurate, specific and cheap detection tool for blood samples, urine samples, spittle, excrement and the like, so the method can be widely applied to the fields of medical technology, food safety, veterinary drug residues, environmental monitoring, drug detection and the like.
Owner:BEIJING KANGMEI TIANHONG BIOTECH
Fluorescent immunochromatographic test paper for detecting human pgii protein and preparation method thereof
ActiveCN104422774BGood antigenicityStrong specificityBiological testingCapture antibodyImmunochromatographic test
Owner:朱建安
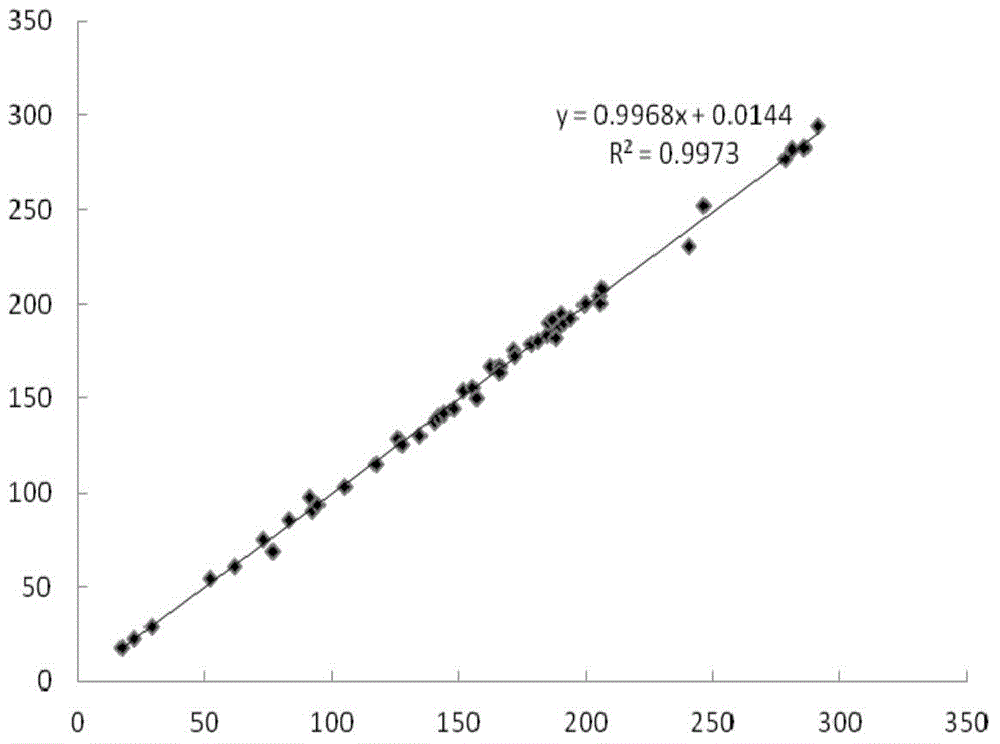
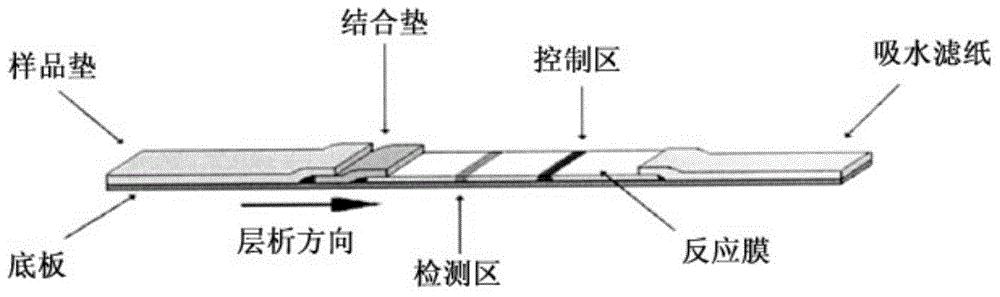
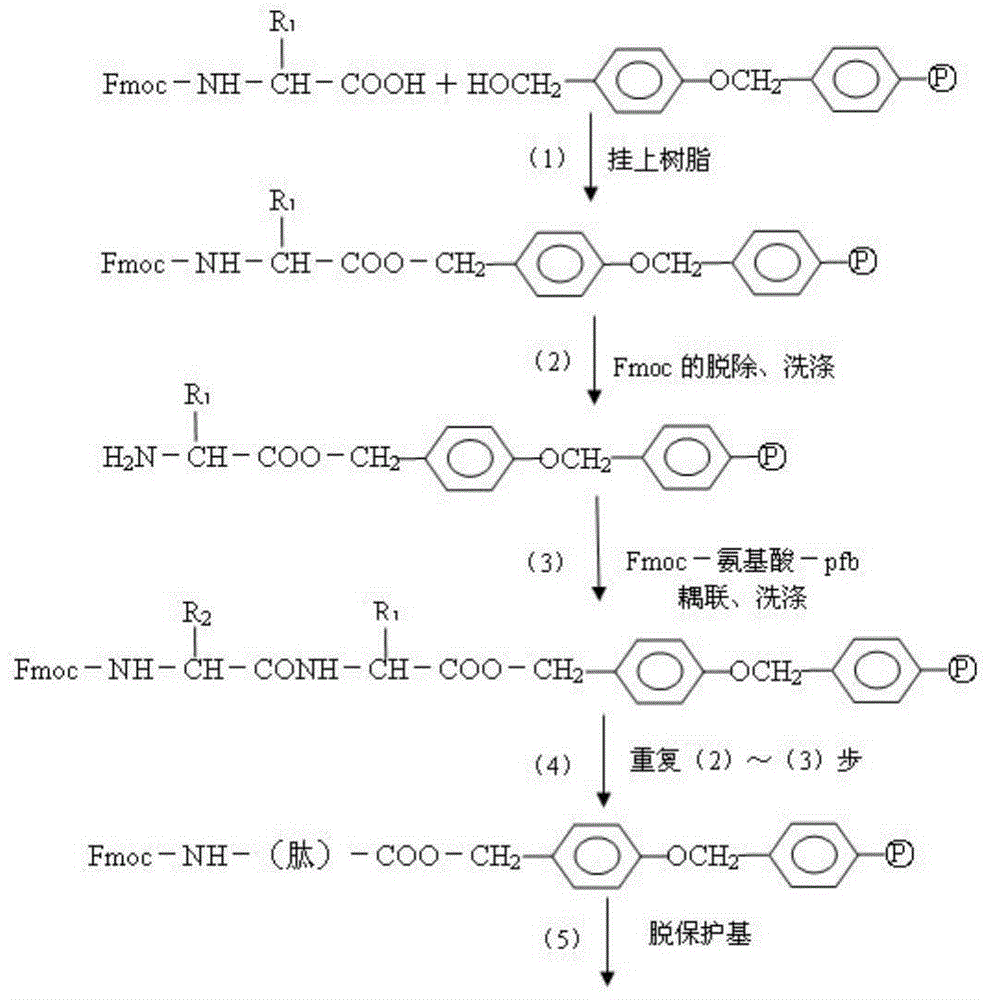
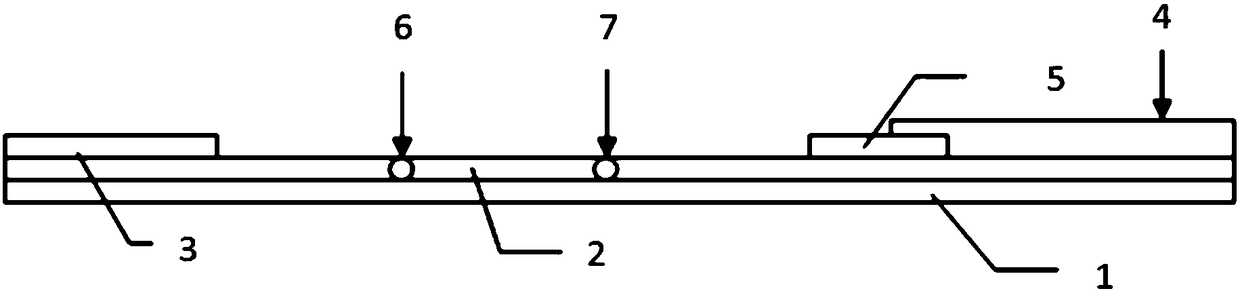
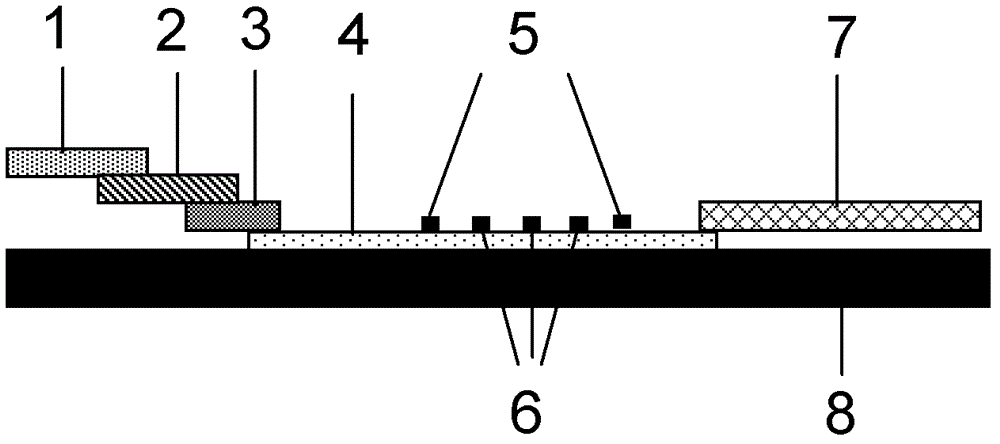
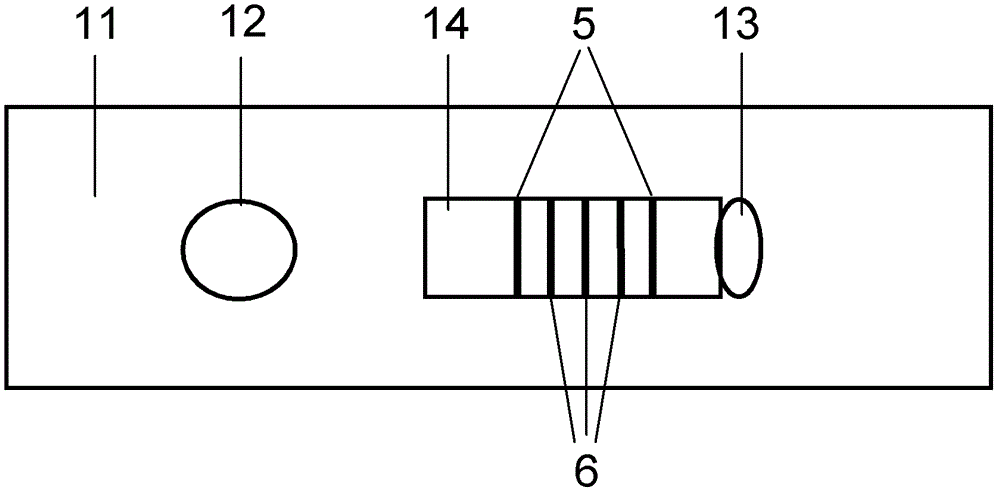
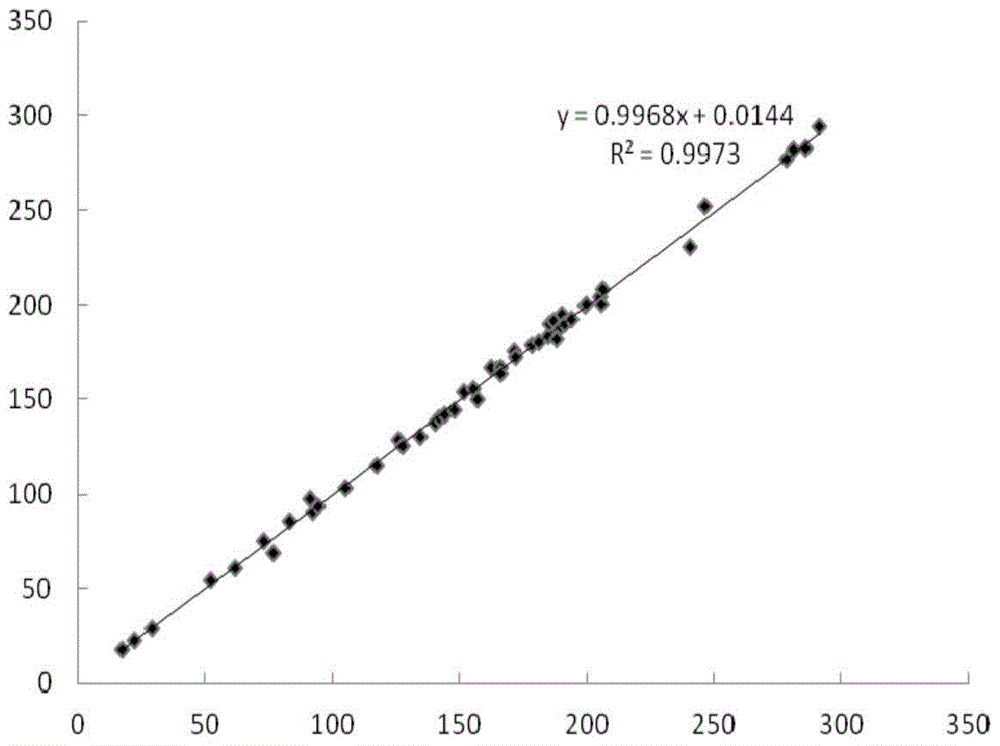
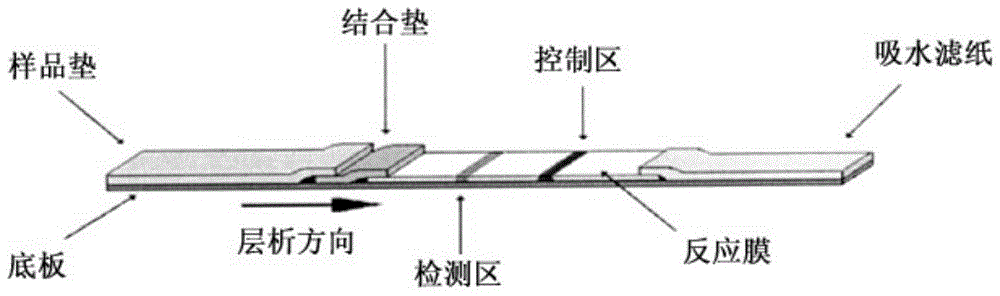
Fluorescent immunochromatographic test paper for detecting human apoe-ε4 protein and preparation method thereof
The invention relates to a fluorescence immune chromatography test paper for detecting human ApoE-[epsilon]4 protein and a preparation method thereof. The test paper is used for detecting the human ApoE-[epsilon]4 protein by virtue of a double-antibody sandwich method, and in the double-antibody sandwich method, a first ApoE-[epsilon]4 monoclonal antibody is labeled with fluorescence microspheres serves as a trapping antibody and is sourced from one of sequences indicated in sequence tables of SEQ ID NO.1 and SEQ ID NO.2; a second ApoE-[epsilon]4 monoclonal antibody serves as a detection antibody, and is sourced from the other one of the sequences indicated in the sequence tables of SEQ ID NO.1 and SEQ ID NO.2. The fluorescence immune chromatography test paper is simple and rapid to operate, wide in detection range, high in specificity and good in sensitivity.
Owner:深圳市安群生物工程有限公司
Fluorescent immunochromatographic test paper for detecting human hsp90α-1 protein and preparation method thereof
ActiveCN105652010BGood antigenicityStrong specificityBiological testingCapture antibodyHSP90 Heat-Shock Proteins
Owner:深圳市安群生物工程有限公司
Fluorescent immunochromatographic test paper and preparation method for joint detection of human pgi protein and human pgii protein
ActiveCN104422775BGood antigenicityStrong specificityBiological testingFluorescence/phosphorescenceCapture antibodyImmunochromatographic test
The present invention relates to fluorescent immunochromatography test paper for combined detection of human PGI protein and human PGII protein, and a production method. According to the test paper of the present invention, human PGI protein and human PGII protein are detected through a double-antibody sandwich method, wherein the capture antibodies adopted by the double-antibody sandwich method respectively are first PGI monoclonal antibody derived from one of the sequence 1 and the sequence 2 in the sequence table and first PGII monoclonal antibody derived from one of the sequence 3 and the sequence 4 in the sequence table, and the detection antibodies adopted by the double-antibody sandwich method respectively are second PGI monoclonal antibody derived from the other one of the sequence 1 and the sequence 2 in the sequence table and second PGII monoclonal antibody derived from the other one of the sequence 3 and the sequence 4 in the sequence table; and the fluorescent immunochromatography test paper of the present invention ha characteristics of convenient and rapid operation, wide detection range, high specificity and good sensitivity.
Owner:朱建安
Fluorescence immunochromatographic assay and kit for quantitative detection of troponin I/creatine kinase isoenzyme/myohemoglobin
ActiveCN102520192BHigh luminous intensityWide excitation spectrumBiological testingCreatine kinaseFluorescence
The invention discloses a quantum dot multicolor marking method for quantitative detection of various cardiovascular disease markers and a kit of troponin I / creatine kinase isoenzyme / myohemoglobin. The method realizes fluorescent quantitative detection by utilizing excellent fluorescent properties of quantum dots and combining a multicolour marking technology and an immunochromatographic assay on the basis of optimizing each component of a test strip. Compared with the common collaurum immunochromatographic assay, the method has the advantages of good mark stability, low nonspecificity, high sensitivity, wide linear range, small cross interference, and accuracy in quantification. The kit disclosed by the invention is used for carrying out quantification detection on the troponin I, the creatine kinase isoenzyme and the myohemoglobin simultaneously, is suitable for detection of whole blood, blood serum and plasma samples, can provide a reference for cardiovascular and cerebrovascular disease diagnosis, and is widely applied to primary hospitals and clinics.
Owner:SHENZHEN KANGMEI BIOTECH
Fluorescent immunochromatographic test paper for detecting human pgi protein and preparation method thereof
ActiveCN104422773BGood antigenicityStrong specificityBiological testingFluorescence/phosphorescenceCapture antibodyImmunochromatographic test
Owner:朱建安
Fluorescent immunochromatographic test paper for detecting human hsp90α-2 protein and preparation method thereof
The invention relates to fluorescence immunochromatographic test paper for detection of a human heat shock protein 90 alpha-2 (HSP90 alpha-2) and a preparation method thereof. The test paper detects the human HSP90 alpha-2 by a double-antibody sandwich method, and the double-antibody sandwich method adopts a fluorescent microsphere-labeled first HSP90 alpha-2 monoclonal antibody as a capture antibody, wherein the first HSP90 alpha-2 monoclonal antibody is sourced from one of sequences SEQ ID NO.1 and SEQ ID NO.2 shown in a sequence table; moreover, the double-antibody sandwich method adopts a second HSP90 alpha-2 monoclonal antibody as a detection antibody, wherein the second HSP90 alpha-2 monoclonal antibody is sourced from the other one of the sequences SEQ ID NO.1 and SEQ ID NO.2 shown in the sequence table. The fluorescence immunochromatographic test paper has the advantages of simple and rapid operation, wide detection range, high specificity and good sensitivity.
Owner:深圳市安群生物工程有限公司
Fluorescence immunochromatographic assay and kit for quantitative detection of human cardiac troponin I (cTnI)
ActiveCN102520193BHigh luminous intensityWide excitation spectrumBiological testingFluorescenceQuantum dot
The invention discloses a fluorescence immunochromatographic assay and kit for quantitative detection of cardiac troponin I (cTnI). The fluorescence immunochromatographic assay for quantitative detection of the cTnI realizes fluorescent quantitative detection by utilizing excellent fluorescent properties of quantum dots and combining a bicolour marking technology and an immunochromatographic assay on the basis of optimizing each component of a test strip. Compared with the common collaurum immunochromatographic assay, the fluorescence immunochromatographic assay has the advantages of good mark stability, low nonspecificity, high sensitivity, wide linear range and accuracy in quantification. The kit disclosed by the invention is used for carrying out quantification detection on the cTnI, can be used for detecting whole blood, blood serum and plasma samples simultaneously, and is applied to different levels of hospitals and is particularly favored to be widely popularized to primary hospitals and clinics.
Owner:SHENZHEN KANGMEI BIOTECH
Fluorescence immunochromatographic assay kit for quantitatively detecting heart fatty acid binding protein
The invention discloses a fluorescence immunochromatographic assay method for quantitatively detecting hFABP (heart fatty acid binding protein) and a kit for quantitatively detecting the same. The fluorescence immunochromatographic assay method for quantitatively detecting the hFABP realizes quantitative fluorescence detection on the basis of optimizing components of a test strip by the aid of excellent fluorescent characteristics of quantum dots and by means of combining bicolor labeling technique and immunochromatographic assay. Compared with a conventional colloidal gold immunochromatographic assay method, the fluorescence immunochromatographic assay method has the advantages of fine labeling stability, low non-specificity, high sensitivity, wide linear range and accuracy in quantization. The kit is used for quantitatively detecting the hFABP, can be used for simultaneously detecting whole blood, blood serum and plasma samples, serves as a simple, accurate, specific and inexpensive detecting tool for early screening and prognosis evaluation of acute myocardial infarction, is applicable to hospitals at all levels, and is particularly beneficial to wide popularization in primary hospitals and clinics.
Owner:SHENZHEN KANGMEI BIOTECH
Immunofiltration assay fluorescent quantitative detection method based on high-sensitivity quantum dot
ActiveCN102539771BHigh luminous intensityWide excitation spectrumFluorescence/phosphorescenceQuantum dotQuality control
The invention discloses an immunofiltration assay fluorescent quantitative detection method based on a high-sensitivity quantum dot; the immunofiltration assay fluorescent quantitative detection method comprises the following steps of: constructing a fluorescence immunofiltration array device by using the excellent fluorescent characteristic of the quantum dot in combination of a quantum dot fluorescence labeling technology and an immunofiltration array technology on the basis of optimizing constituent parts for the immunofiltration assay; and after immunofiltration array, detecting the strenght of fluorescent signals of the quantum dot and a quality control dot by using a fluorescence quantometer, correcting the fluorescence strenght of the quantum pot by using the quality control dot, and further realizing the quantitative detection of a tested object according to a standard curve obtained by using the fluorescence quantometer. The method is simple, rapid, accurate, low in cost and high in sensitiveness. Comapred with the conventional collodial gold immunofiltration array method, the immunofiltration assay fluorescent quantitative detection method has the advantages of good labeling stability, low non-specificity, high sensitivity, wide linear range and accurate quantification. The method is suitable for samples such as serums, urine, spittle, excrement and the like and can be applicable to the detection of serious illness, poisons, food safety and the like.
Owner:BEIJING KANGMEI TIANHONG BIOTECH
Method for rapidly identifying thyroid papillary carcinoma lymphonodi cervicales metastasis in operation
The invention discloses fluorescence immunochromatographic test paper for rapidly detecting human thyroglobulin (Tg) so as to rapidly identify thyroid papillary carcinoma lymphonodi cervicales metastasis. According to the test paper, a double-antibody sandwich method and a fluorescence immunochromatography technology are adopted to detect human Tg; and antibodies are prepared through specific antigen epitope peptides. With the method adopted, human Tg in an object to be detected can be rapidly and accurately detected. The test paper has the advantages of simple, convenient and rapid operation, wide detection range, high specificity and high sensitivity.
Owner:JIANGSU INST OF NUCLEAR MEDICINE
Fluorescence immunochromatographic assay and kit for quantitatively detecting cardiac troponin T
ActiveCN102565422BHigh luminous intensityWide excitation spectrumBiological testingFluorescenceBlood plasma
The invention discloses a fluorescence immunochromatographic assay and kit for quantitatively detecting cardiac troponin T (cTnT). The fluorescence immunochromatographic assay for quantitatively detecting cTnT realizes fluorescence quantitative detection on the basis of optimizing various constituent parts of test paper by using excellent fluorescence characteristic of quantum dots and combining a bicolor marking technology and an immunochromatographic technology. Compared with the conventional colloidal gold immunochromatographic assay, the fluorescence immunochromatographic assay disclosed by the invention has the advantages of good marking stability, low non-specificity, high sensitivity, wide linear range and quantifying accuracy. The fluorescence immunochromatographic kit disclosed by the invention is used for carrying out quantitative detection on the cTnT and detecting whole blood, blood serum and blood plasma samples and is suitable for different levels of hospitals and particularly good for wide popularization in primary hospitals and clinics.
Owner:SHENZHEN KANGMEI BIOTECH
Fluorescence immunochromatographic test paper for detecting human lp-pla2 protein and preparation method thereof
The invention relates to fluorescence immunochromatography test paper for detecting human Lp-PLA2 proteins and a preparation method of the fluorescence immunochromatography test paper. The test paper is used for detecting the human Lp-PLA2 proteins by virtue of a two-antibody sandwich method, and in the two-antibody sandwich method, a first Lp-PLA2 monoclonal antibody which is labeled with fluorescence microspheres serves as a trapping antibody, and is sourced from one of sequences indicated in sequence tables of SEQ ID NO.1 and SEQ ID NO.2; a second Lp-PLA2 monoclonal antibody serves as a detection antibody, and is sourced from the other one of the sequences indicated in the sequence tables of SEQ ID NO.1 and SEQ ID NO.2. The fluorescence immunochromatography test paper is simple and rapid to operate, wide in detection range, high in specificity and good in sensitivity.
Owner:深圳市安群生物工程有限公司
Fluorescent immunochromatography method for whole quantitative detection of C-reactive protein and reagent kit thereof
ActiveCN102539785BReduce sensitivityHigh luminous intensityBiological testingFluorescence/phosphorescenceFluorescenceAntibiotic effect
The invention discloses a fluorescent immunochromatography method for whole quantitative detection of C-reactive protein and a reagent kit thereof. The fluorescent immunochromatography method for the whole quantitative detection of the C-reactive protein (CRP) utilizes excellent fluorescent characteristics of quantum dots, and combines double-color marking technology and immunochromatography technology to achieve fluorescent quantitative detection on the basis of optimizing each constituent elements of test paper. Compared with a conventional colloidal gold immunochromatography method, the fluorescent immunochromatography method for the whole quantitative detection of the CRP has the advantages of being good in stability, low in non-specificity, high in flexibility, wide in linear range and accurate in quantifying. The reagent kit of the fluorescent immunochromatography method can perform the whole quantifying and can simultaneously predict and evaluate infectious diseases, antibiotic effects and cardiovascular and cerebrovascular diseases. The fluorescent immunochromatography method for the whole quantitative detection of the CRP and the reagent kit of the fluorescent immunochromatography method are suitable for various-level hospitals, and particularly contribute to wide popularization in basic-level hospitals and clinics.
Owner:SHENZHEN KANGMEI BIOTECH
A rapid method for identifying human parathyroid glands
The invention discloses a method for rapidly identifying human parathyroid glands. The method is realized by using parathyroid hormone (PTH) fluorescence immunochromatography test paper to determine the content of PTH in liquid to be detected; the test paper is used for detecting human PTH by using a double-antibody sandwich method and a fluorescence immunochromatography; the antibodies are prepared by using specific antigenic epitope peptide. The method can be used for accurately detecting the human PTH in a matter to be detected, and is simple and convenient to operate, wide in detection range, high in specificity and good in sensitivity.
Owner:无锡市江原实业技贸有限公司
Fluorescence immunochromatographic assay and kit for quantitatively detecting N-terminal pro brain natriuretic peptide
ActiveCN102565423BHigh luminous intensityWide excitation spectrumBiological testingFluorescence/phosphorescenceDiseaseN-terminal pro-Brain Natriuretic Peptide
The invention discloses a fluorescence immunochromatographic assay and kit for quantitatively detecting acute myocardial infarction marker, namely, N-terminal pro brain natriuretic peptide (NT-proBNP). The fluorescence immunochromatographic assay for quantitatively detecting the NT-proBNP realizes fluorescence quantitative detection on the basis of optimizing various constituent parts of test paper by using excellent fluorescence characteristic of quantum dots and combining a bicolor marking technology and an immunochromatographic technology. Compared with the conventional colloidal gold immunochromatographic assay, the fluorescence immunochromatographic assay disclosed by the invention has the advantages of good marking stability, low non-specificity, high sensitivity, wide linear range and quantifying accuracy. The fluorescence immunochromatographic kit disclosed by the invention is used for carrying out quantitative detection on the NT-proBNP and detecting whole blood, blood serum and blood plasma samples, can provide reference for diagnosis of cardiovascular and cerebrovascular diseases and can be widely applied to primary hospitals and clinics.
Owner:SHENZHEN KANGMEI BIOTECH
Fluorescence immunochromatographic assay and kit for quantitative detection of creatine kinase isoenzyme (CK-MB)
ActiveCN102520173BHigh luminous intensityWide excitation spectrumMaterial analysisDiseaseCreatine kinase
Owner:SHENZHEN KANGMEI BIOTECH
Preparation method of FTO demethylase detection kit and detection kit
PendingCN114107483AAvoid the effects of inadvertent mutationsStrong correlation for normal functioningMicrobiological testing/measurementDisease diagnosisCelluloseFreeze-drying
The invention discloses a preparation method of an FTO demethylase detection kit, the detection kit and application. The preparation method comprises the following steps: preparing a sample pad: soaking a nitrocellulose membrane in a phosphate buffer solution containing BSA (Bovine Serum Albumin) with the mass concentration of 2%, 0.1 M NaCl and a surfactant at 4 DEG C for 8 hours, and drying to obtain the sample pad; treating the filtering membrane; preparing a combination pad: respectively labeling fluorescent microspheres on antibodies containing four sites of FTO demethylase, adding a microsphere diluent, and uniformly mixing to obtain an antibody mixed solution; uniformly laying the antibody mixed solution on a treated sample pad, carrying out vacuum freeze drying, and sealing to obtain a conjugate pad; preparing a chromatographic membrane: respectively preparing a detection band and a quality control band on the nitrocellulose membrane to obtain the chromatographic membrane; preparing the absorbent paper; and assembling. The FTO demethylase kit disclosed by the invention is helpful for obese high-risk persons to know own genetic conditions in advance, and obesity and overweight are avoided by adjusting dietary structures and lifestyles in advance.
Owner:余朴芬
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com




![Fluorescence immune chromatography test paper for detecting human ApoE-[epsilon]4 protein and preparation method thereof Fluorescence immune chromatography test paper for detecting human ApoE-[epsilon]4 protein and preparation method thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/6d5d9677-766c-48a5-b2c7-a9440602c36d/HDA0000612572780000011.PNG)
![Fluorescence immune chromatography test paper for detecting human ApoE-[epsilon]4 protein and preparation method thereof Fluorescence immune chromatography test paper for detecting human ApoE-[epsilon]4 protein and preparation method thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/6d5d9677-766c-48a5-b2c7-a9440602c36d/BDA0000612572770000131.PNG)
![Fluorescence immune chromatography test paper for detecting human ApoE-[epsilon]4 protein and preparation method thereof Fluorescence immune chromatography test paper for detecting human ApoE-[epsilon]4 protein and preparation method thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/6d5d9677-766c-48a5-b2c7-a9440602c36d/BDA0000612572770000151.PNG)