Fluorescence immunochromatographic assay and kit for quantitative detection of human cardiac troponin I (cTnI)
A technology of fluorescence immunochromatography and cardiac troponin, which is applied in the field of medical testing, can solve the problems of inaccurate quantification and low sensitivity of detecting cardiac troponin I, and achieve the purpose of expanding the detection range, improving detection sensitivity, and improving quantitative accuracy Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
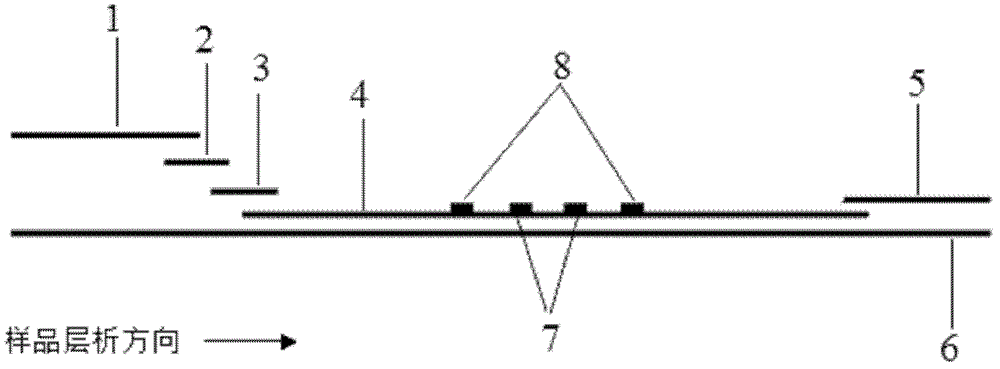
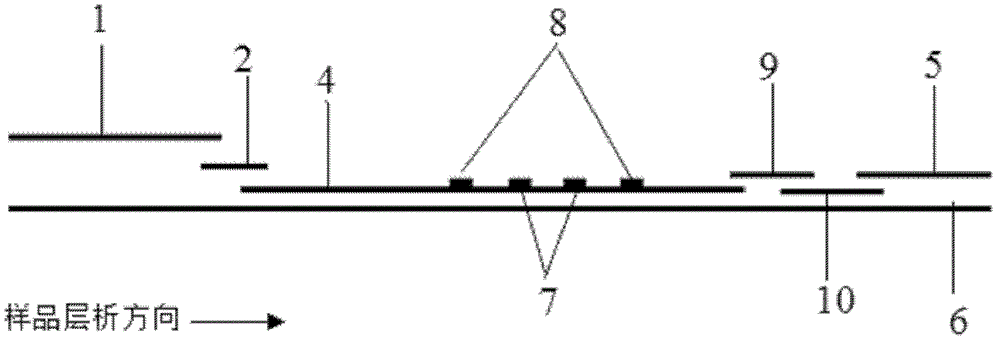
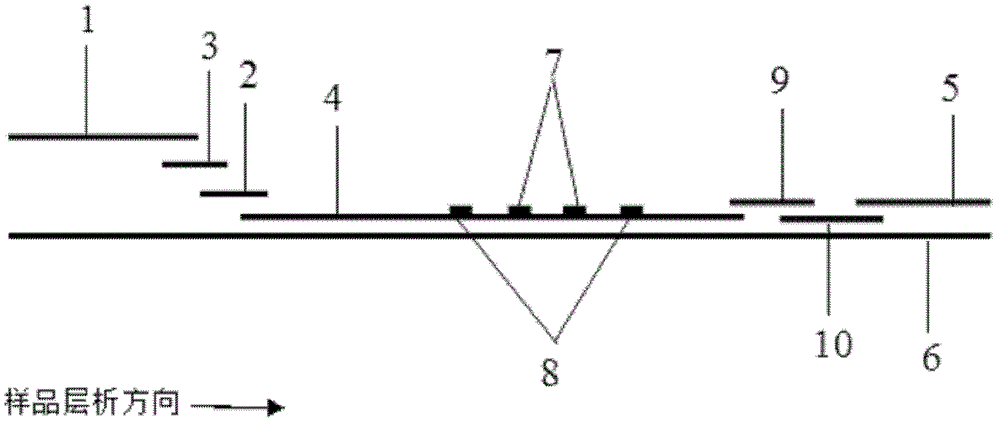
Image
Examples
Embodiment 1
[0070] Example 1: Quantitative detection of cTnI by modifying antibodies to quantum dots by covalent cross-linking and using direct pre-wetting immunochromatography
[0071] (1) Modification of quantum dots and antibodies
[0072] Quantum dots with an emission wavelength of 650 nm were mixed with cTnI monoclonal antibody at 1 mg / mL, and mixed with freshly prepared N-hydroxysuccinimide (NHS, 1 mg / mL) and carbodiimide hydrochloride (EDC, 1 mg / mL) at room temperature for 4-5 hours, add 1 mol / L glycine to block, and separate and purify with chromatographic column or chromatographic column to obtain cTnI monoclonal antibody-modified quantum dots. Similarly, rabbit IgG-modified quantum dots were obtained. The fluorescence emission wavelength of the cTnI antibody-modified quantum dot is 650nm, and the fluorescence emission wavelength of the rabbit IgG-modified quantum dot is 570nm.
[0073] (2) Construction of the kit
[0074] Mix the two kinds of quantum dot markers in a ratio o...
Embodiment 2
[0086] Example 2: Quantitative detection of cTnI by modifying antibody to quantum dots with biotin-avidin system and using indirect pre-wetting immunochromatography
[0087] (1) Modification of quantum dots and antibodies
[0088] First, 1 mL of cTnI monoclonal antibody (1 mg / mL) was fully dialyzed with pH 9.0 sodium bicarbonate buffer, and 20-120 μl of N-hydroxysuccinimide biotin ester freshly prepared in dimethyl sulfoxide (DMSO) was added (NHSB, 1mg / mL), react at room temperature in the dark for 4h. Add 1mol / L NH 4 Cl, shaking reaction at room temperature for 10 minutes, then put the solution in a dialysis bag, dialysis and purification overnight, and concentrated with an ultrafiltration centrifuge tube to prepare the required concentration, and the obtained biotinylated cTnI monoclonal antibody.
[0089] Streptavidin-modified quantum dots with an emission wavelength of 900nm and biotinylated cTnI monoclonal antibody were mixed and reacted at a ratio of 1:3-1:12 for 30-60...
PUM
| Property | Measurement | Unit |
|---|---|---|
| emission peak | aaaaa | aaaaa |
| emission peak | aaaaa | aaaaa |
| emission peak | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com