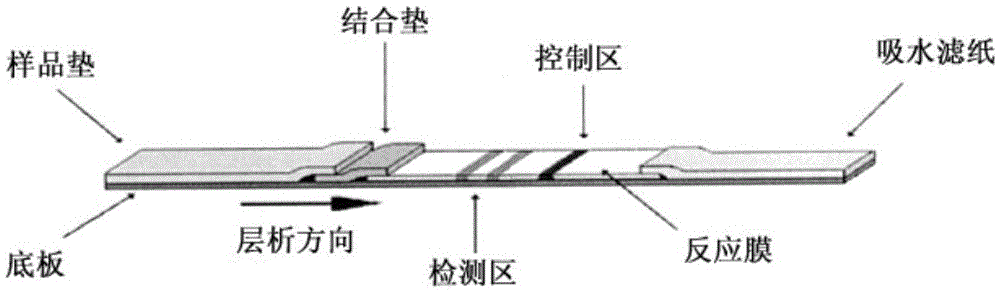
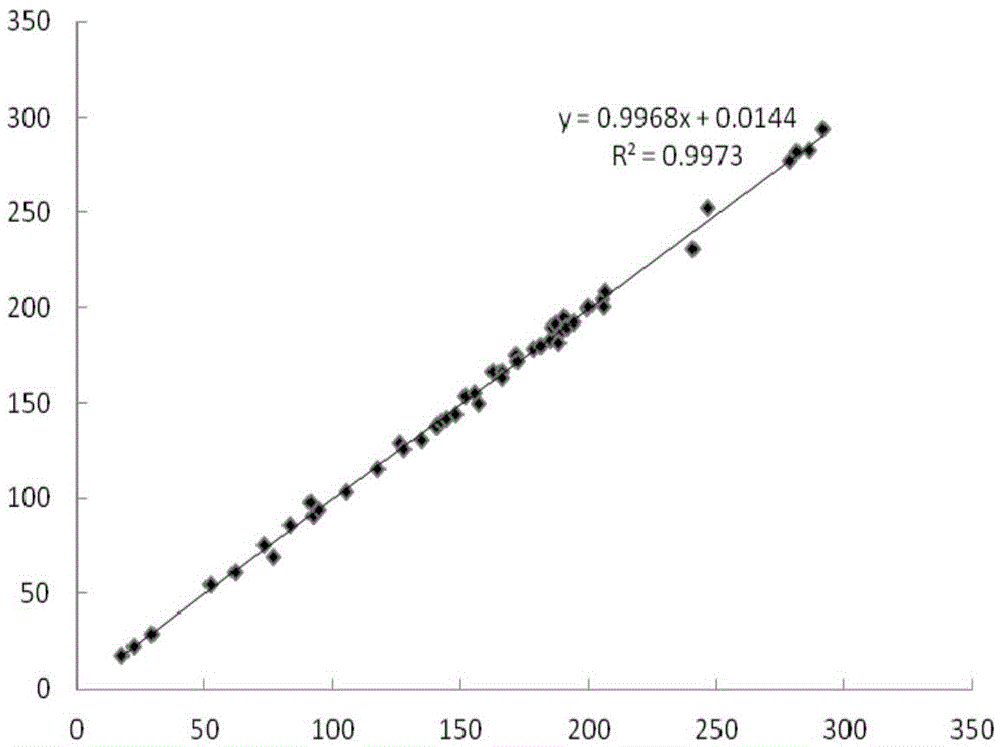
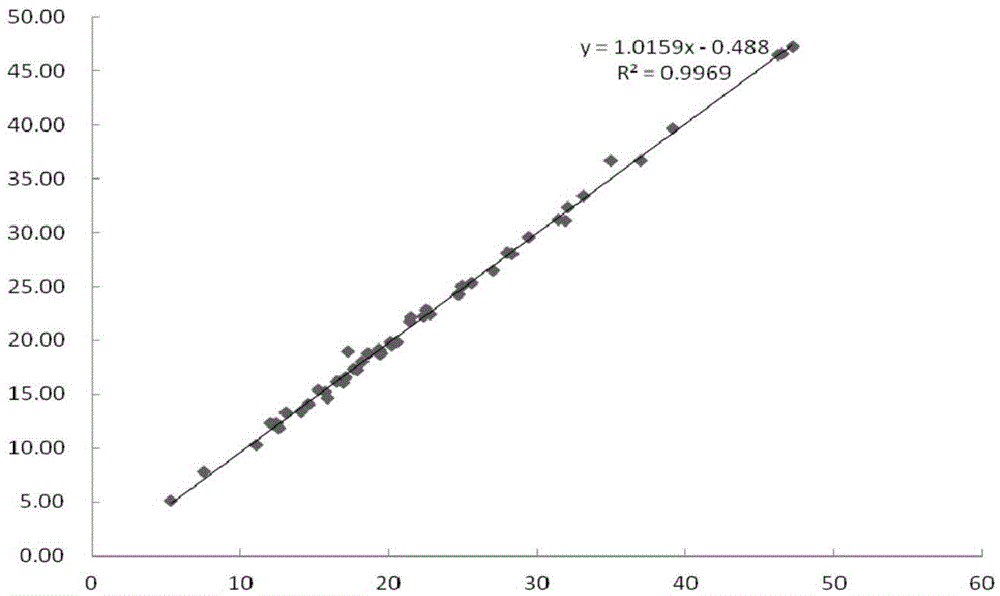
Fluorescent immunochromatographic test paper and preparation method for joint detection of human pgi protein and human pgii protein
A fluorescent immunochromatography, protein technology, applied in biological testing, fluorescence/phosphorescence, measurement devices, etc., can solve the problem of not combined detection of human PGI protein fluorescent immunochromatography test strips, etc., to achieve good antigenicity, wide detection range, Easy to use effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0066] The preparation method of the PGI epitope peptide of the present invention can be a chemical synthesis method: the antigen epitope peptide is synthesized by a solid-phase method using an American ABI431A type polypeptide automatic synthesizer. The molecular weights of the antigenic epitope peptides (1) and (2) of the present invention are 1657.24 and 1616.93 respectively, which can be determined by mass spectrometry, and the synthesized antigenic epitope peptide sequences are identified by polypeptide sequence determination. The purity of the peptides was evaluated by thin-layer chromatography and high-performance liquid chromatography, and the concentration of the epitope peptide was determined.
[0067] 2. PGI antigen
[0068] The present invention also provides a PGI antigen prepared by coupling one of the human PGI epitope peptides (1) and (2) of the present invention with a carrier protein. Specifically, the present invention provides PGI antigens (1) and (2), whe...
Embodiment 1
[0148] Example 1: Preparation of PGI epitope peptides (1) and (2) and PGII epitope peptides (3) and (4).
[0149] The preparation method uses chemical synthesis method: using the American ABI431A automatic peptide synthesizer, the PGI antigenic epitope peptides (1) and (2) and the PGII antigenic epitope peptides (3) and (4) are synthesized respectively by a solid-phase method. The purity of the epitope peptide was assessed by high performance liquid chromatography, and the concentration of the peptide was determined. The molecular weights of the PGI antigenic epitope peptides (1) and (2) of the present invention are 1657.24 and 1616.93 respectively, and the molecular weights of the PGII antigenic epitope peptides (3) and (4) are respectively 1299.66 and 1943.41, which are determined by mass spectrometry. Sequencing identifies the synthesized polypeptide sequence.
[0150] 1. Synthesis of PGI epitope peptides (1) and (2) and PGII epitope peptides (3) and (4)
[0151] The abov...
Embodiment 2
[0241] Embodiment 2: The PGI epitope peptide (1) and (2) and PGII antigen epitope peptide (3) and (4) obtained in Example 1 are connected with carrier protein respectively to prepare PGI antigen (1), PGI antigen (2), PGII antigen (3) and PGII antigen (4), utilize the obtained antigen (1), (2), (3) and (4) to immunize animals respectively, thereby utilize antigen (1), (2) respectively , (3) and (4) prepare specific monoclonal antibodies and polyclonal antibodies.
[0242] 1. Antigen preparation: PGI peptide (1), PGI peptide (2), PGII peptide (3) and PGII peptide (4) were combined with carrier protein KLH (keyhole pore) by BDB (Bis-diazotizedbenzidinedichloride) method Hemocyanin) was connected to prepare PGI antigen (1), PGI antigen (2), PGII antigen (3) and PGII antigen (4).
[0243] Take 10.0mg of PGI peptide (1) or PGI peptide (2) or PGII peptide (3) or PGII peptide (4), dissolve it in 1ml 0.1MPBS buffer (pH7.4); KLH10mg, use 0.2M boron Dissolve 20ml of salt buffer solutio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com