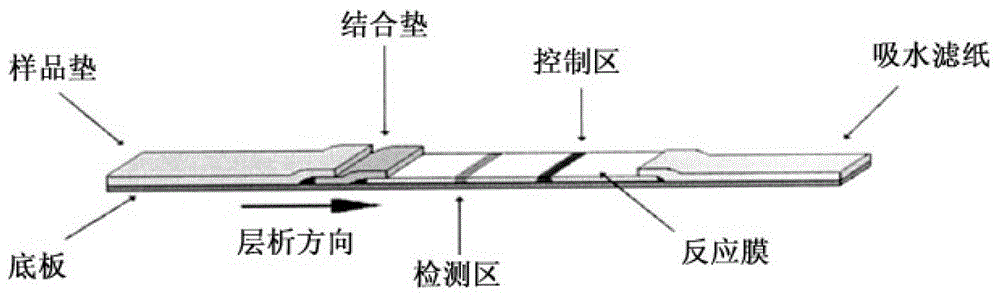
Fluorescent immunochromatographic test paper for detecting human gfap protein and preparation method thereof
A technology of fluorescent immunochromatography and test paper, which is applied in the direction of anti-animal/human immunoglobulin, biological testing, chemical instruments and methods, etc., to achieve the effects of optimizing preparation conditions, wide detection range, and improving fluorescence signal-to-background ratio
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0055] The preparation method of the GFAP epitope peptide of the present invention can be a chemical synthesis method: the antigen epitope peptide is synthesized by a solid-phase method using an American ABI431A type polypeptide automatic synthesizer. The molecular weights of the antigenic epitope peptides (1) and (2) of the present invention are 2690.27 and 1979.35 respectively, which can be determined by mass spectrometry, and the synthesized antigenic epitope peptide sequences are identified by polypeptide sequence determination. The purity of the peptides was evaluated by thin-layer chromatography and high-performance liquid chromatography, and the concentration of the epitope peptide was determined.
[0056] 2. GFAP antigen
[0057] The present invention also provides a GFAP antigen prepared by coupling one of the human GFAP antigen epitope peptides (1) and (2) of the present invention with a carrier protein. Specifically, the present invention provides GFAP antigens (1)...
Embodiment 1
[0107] Example 1: Preparation of GFAP epitope peptides (1) and (2).
[0108] The preparation method uses chemical synthesis method: GFAP antigen epitope peptides (1) and (2) are synthesized respectively by solid-phase method using the American ABI431A automatic peptide synthesizer. The purity of the epitope peptide was assessed by high performance liquid chromatography, and the concentration of the peptide was determined. The molecular weights of the antigenic epitope peptides (1) and (2) of the present invention are 2690.27 and 1979.35 respectively, which are determined by mass spectrometry, and the synthesized polypeptide sequences are identified by polypeptide sequence determination.
[0109] 1. Synthesis of GFAP epitope peptides (1) and (2)
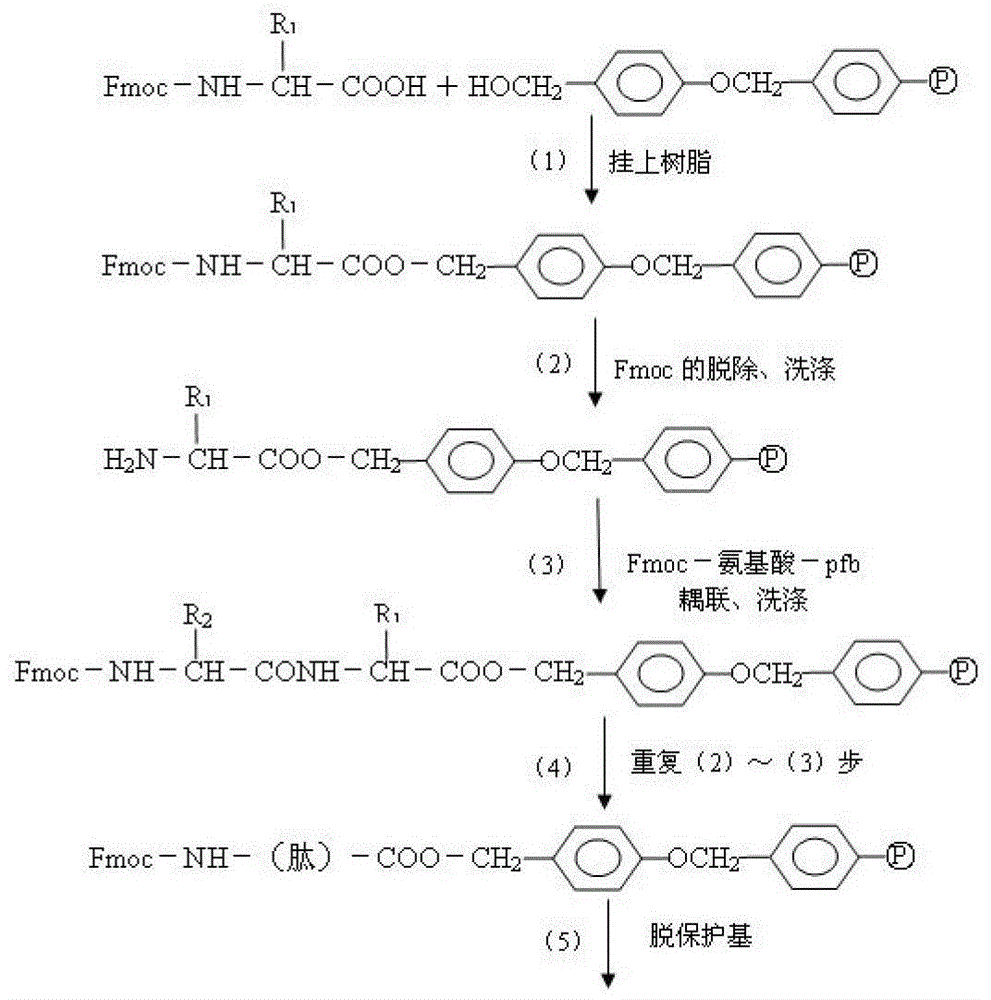
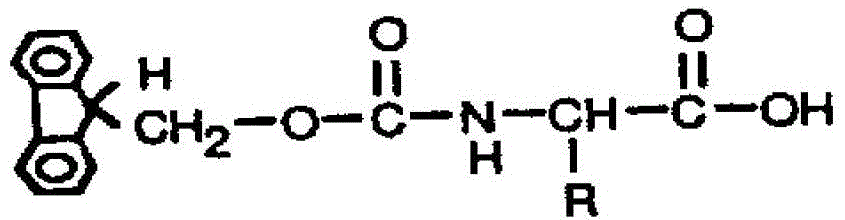
[0110] The above peptides were synthesized by solid-phase method. The main idea of solid-phase peptide synthesis is: first connect the carboxyl group of the carboxyl-terminal amino acid of the peptide chain to be synthesized with ...
Embodiment 2
[0198] Embodiment 2: the GFAP antigen epitope peptide (1) and (2) obtained in Example 1 are connected with carrier protein respectively to prepare GFAP antigen (1) and (2), utilize gained antigen (1) and (2) respectively Animals are immunized to prepare specific monoclonal and polyclonal antibodies using the antigen (1), and specific monoclonal and polyclonal antibodies are prepared using the antigen (2).
[0199] 1. Antigen preparation: GFAP peptides (1) and (2) were respectively linked with carrier protein KLH (keyhole limpet hemocyanin) by BDB (Bis-diazotizedbenzidine dichloride) method to prepare GFAP antigens (1) and (2) .
[0200] Take 10.0mg of GFAP peptide (1) or (2), dissolve it with 1ml 0.1M PBS buffer (pH7.4); dissolve KLH10mg with 20ml of 0.2M borate buffer (pH9.0); then mix the two Mix, cool to 0°C, take BDBCl 2 110 μL, reacted at room temperature for 1.5 h, dialyzed overnight, then aliquoted, and stored at -20 °C.
[0201] In the present embodiment, the formul...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com