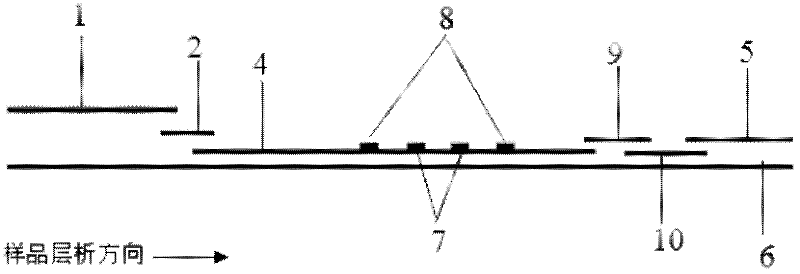
Fluorescence immunochromatographic assay and kit for quantitatively detecting cardiac troponin T
A technology of fluorescence immunochromatography and cardiac troponin, which is applied in the field of medical testing, can solve the problems of low sensitivity and inaccurate quantification of cardiac troponin T, and achieve the effects of expanding the detection range, increasing the sensitivity, and improving the detection sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Example 1: Quantitative detection of cTnT by modifying antibodies to quantum dots by covalent cross-linking and using direct pre-wetting immunochromatography
[0070] (1) Modification of quantum dots and antibodies
[0071] Quantum dots with an emission wavelength of 650nm were mixed with 1mg / mL cTnT monoclonal antibody, and mixed with freshly prepared N-hydroxysuccinimide (NHS, 1mg / mL) and carbodiimide hydrochloride (EDC, 1mg / mL) under the action of room temperature for 4 to 5 hours, adding 1mol / L glycine to block, and using a chromatographic column or chromatographic column to separate and purify to obtain cTnT monoclonal antibody modified quantum dots. Similarly, rabbit IgG-modified quantum dots were obtained. The fluorescence emission wavelength of the cTnT antibody-modified quantum dot is 650nm, and the fluorescence emission wavelength of the rabbit IgG-modified quantum dot is 570nm.
[0072] (2) Construction of the kit
[0073] Mix the two kinds of quantum dot...
Embodiment 2
[0085] Example 2: Quantitative detection of cTnT by modifying antibody to quantum dots with biotin-avidin system and using indirect pre-wetting immunochromatography
[0086] (1) Modification of quantum dots and antibodies
[0087] First, 1 mL of cTnT monoclonal antibody (1 mg / mL) was fully dialyzed with pH 9.0 sodium bicarbonate buffer, and 20-120 μl of N-hydroxysuccinimide biotin ester freshly prepared in dimethyl sulfoxide (DMSO) was added (NHSB, 1mg / mL), react at room temperature in the dark for 4h. Add 1mol / L NH 4 Cl, shaking reaction at room temperature for 10 min, then place the solution in a dialysis bag, dialysis and purification overnight, and concentrate with an ultrafiltration centrifuge tube to prepare the required concentration, and the obtained biotinylated cTnT monoclonal antibody.
[0088] Streptavidin-modified quantum dots with an emission wavelength of 900nm and biotinylated cTnT monoclonal antibody were mixed and reacted at a ratio of 1:3-1:12 for 30-60 mi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Emission wavelength | aaaaa | aaaaa |
| Emission wavelength | aaaaa | aaaaa |
| Emission wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com