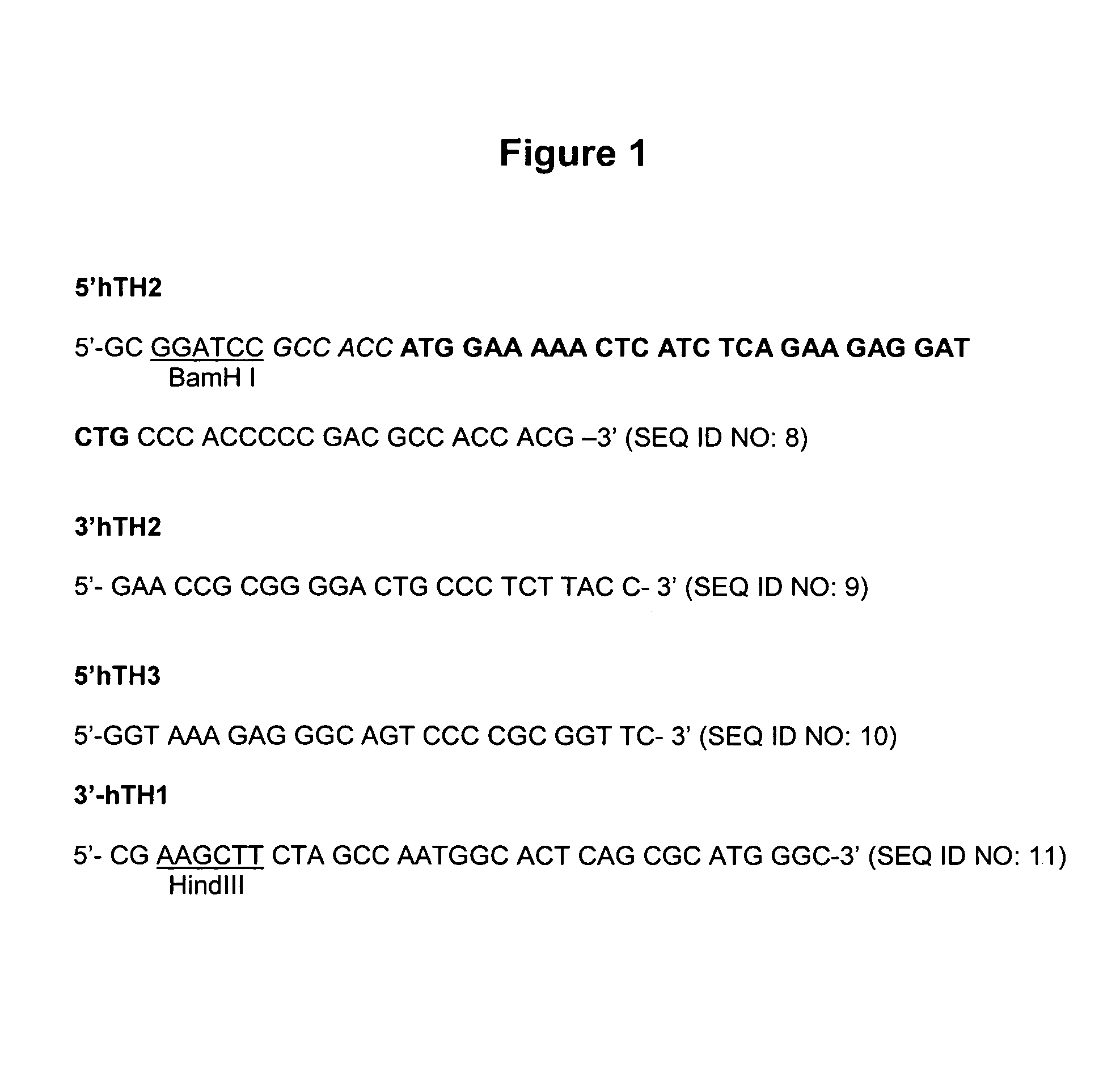
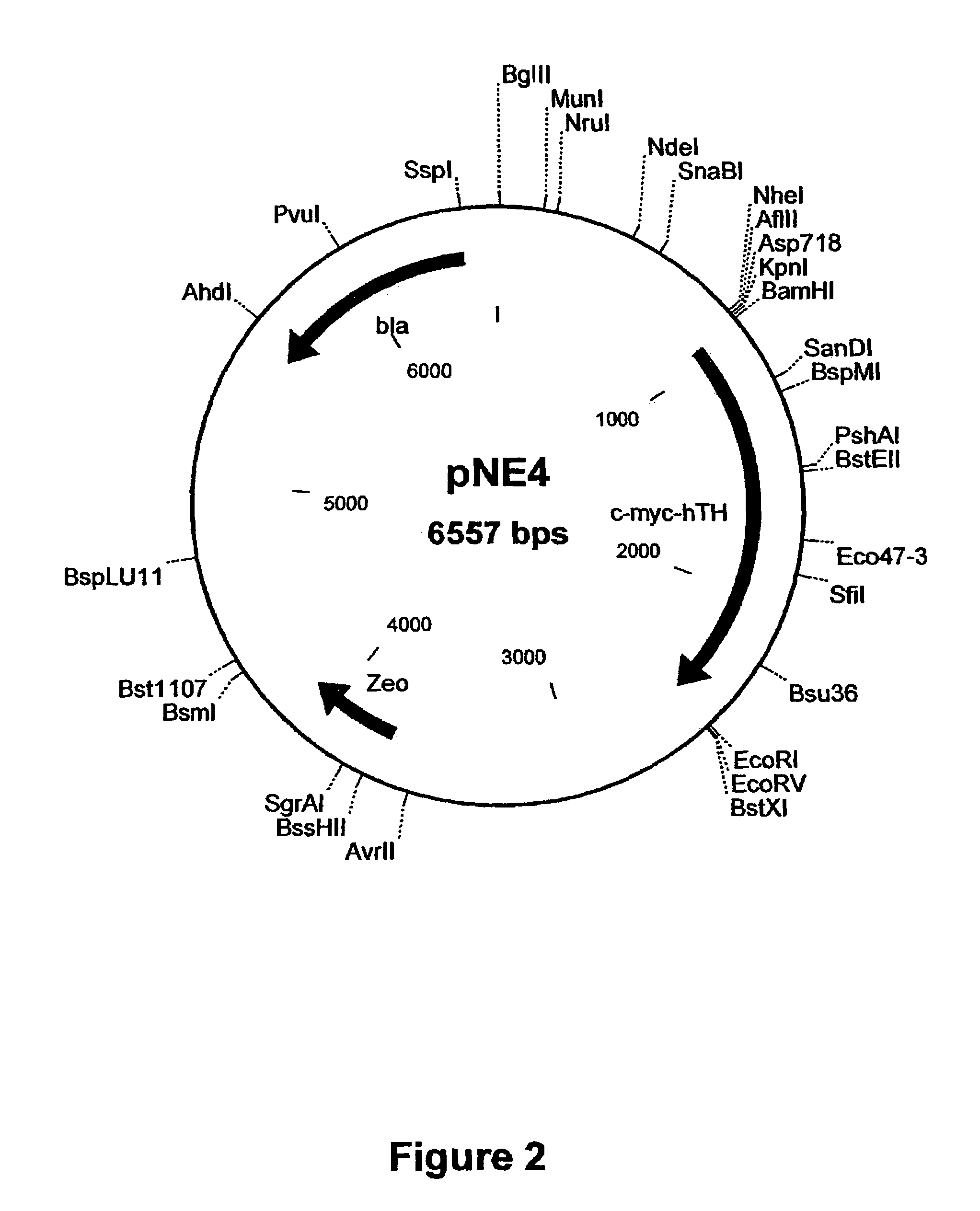
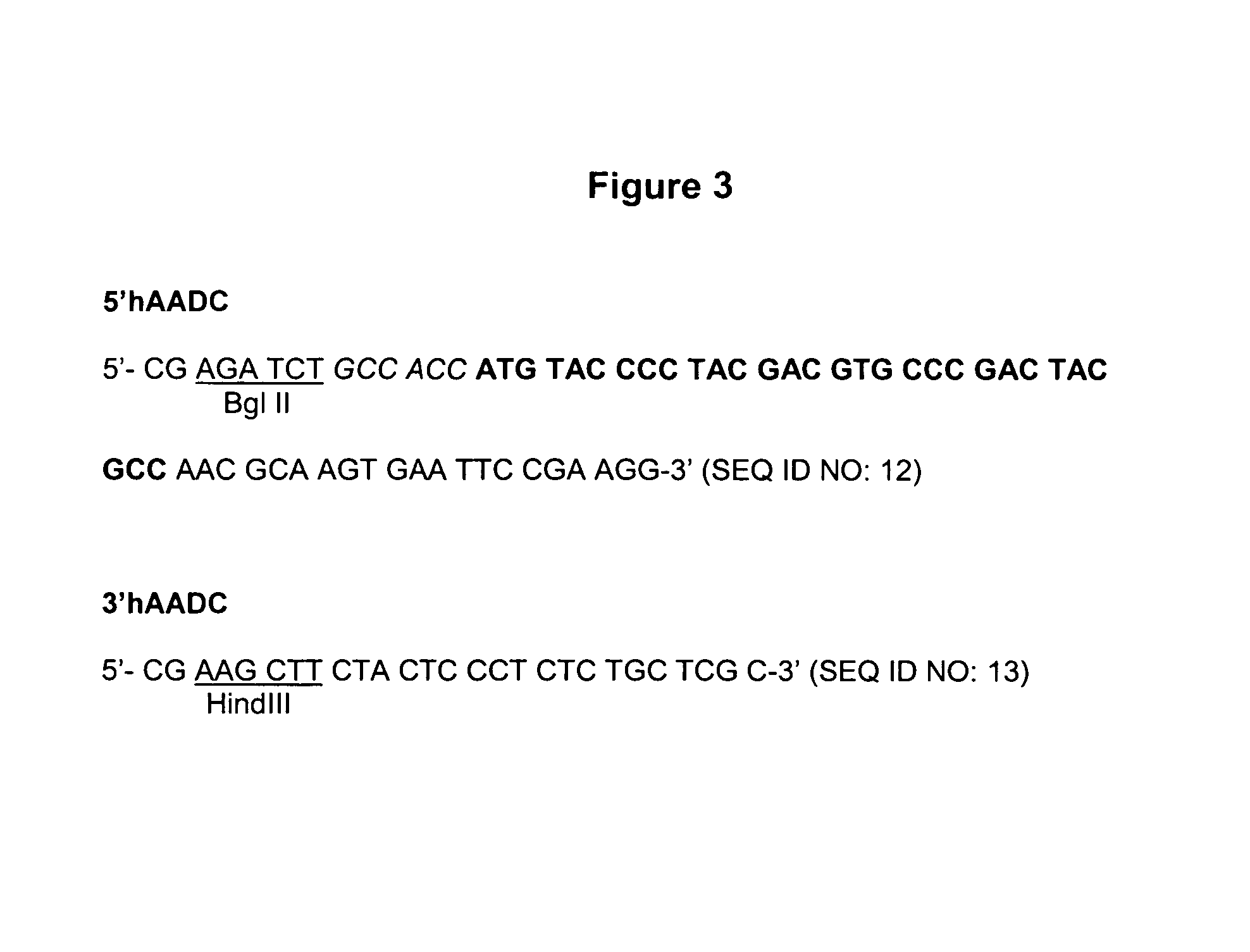
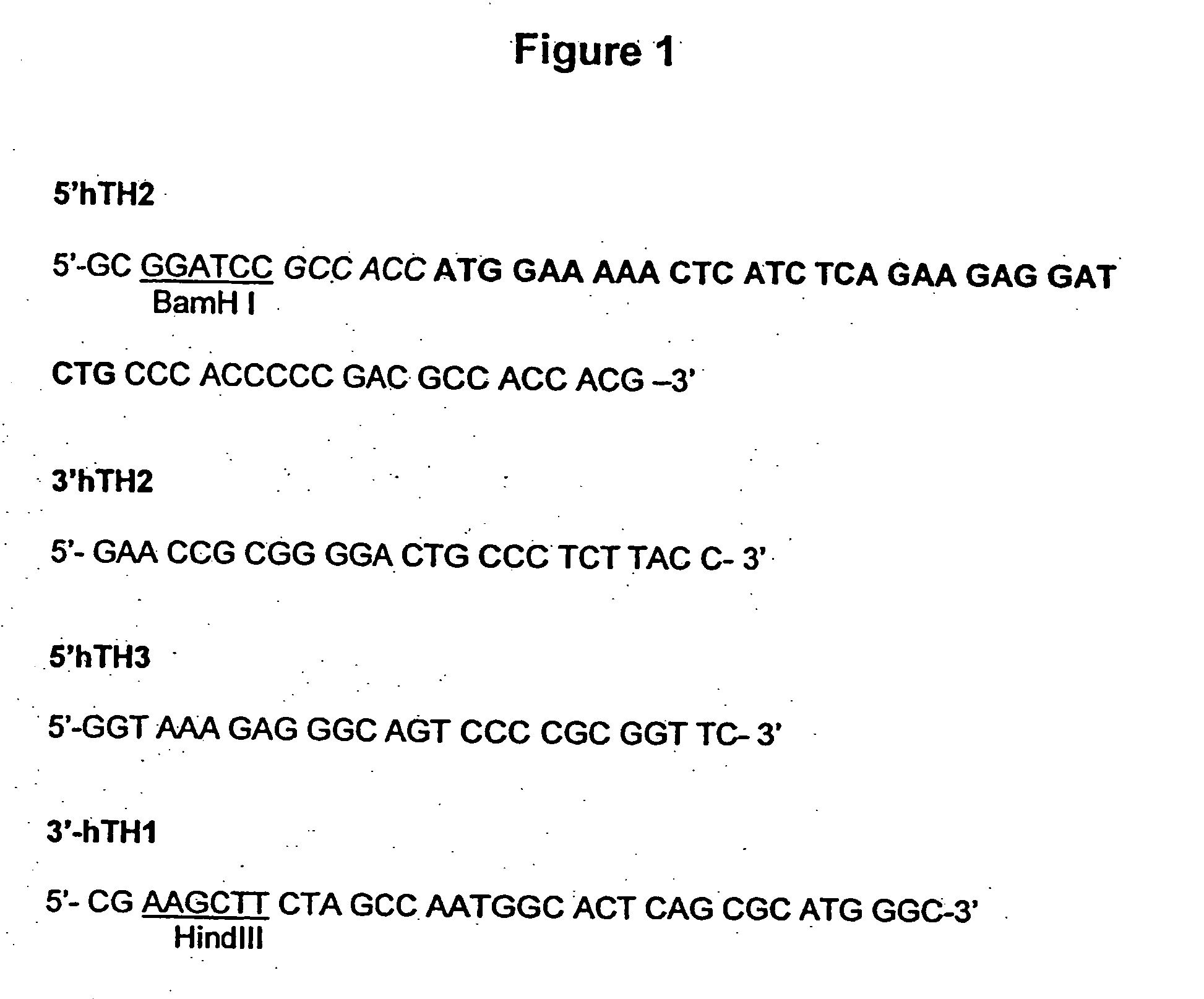
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
83 results about "Tyrosine hydroxylase" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
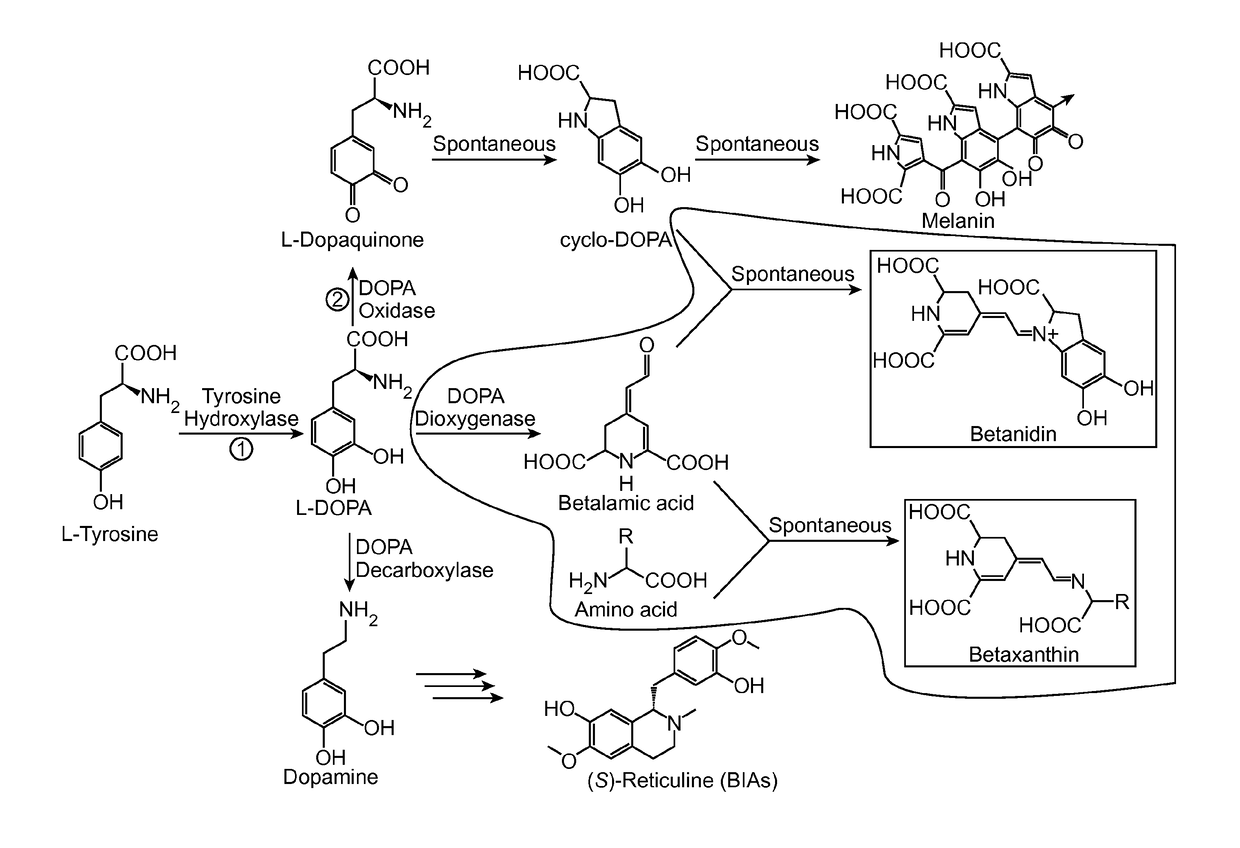
Tyrosine hydroxylase or tyrosine 3-monooxygenase is the enzyme responsible for catalyzing the conversion of the amino acid L-tyrosine to L-3,4-dihydroxyphenylalanine (L-DOPA). It does so using molecular oxygen (O₂), as well as iron (Fe²⁺) and tetrahydrobiopterin as cofactors. L-DOPA is a precursor for dopamine, which, in turn, is a precursor for the important neurotransmitters norepinephrine (noradrenaline) and epinephrine (adrenaline). Tyrosine hydroxylase catalyzes the rate limiting step in this synthesis of catecholamines. In humans, tyrosine hydroxylase is encoded by the TH gene, and the enzyme is present in the central nervous system (CNS), peripheral sympathetic neurons and the adrenal medulla. Tyrosine hydroxylase, phenylalanine hydroxylase and tryptophan hydroxylase together make up the family of aromatic amino acid hydroxylases (AAAHs).
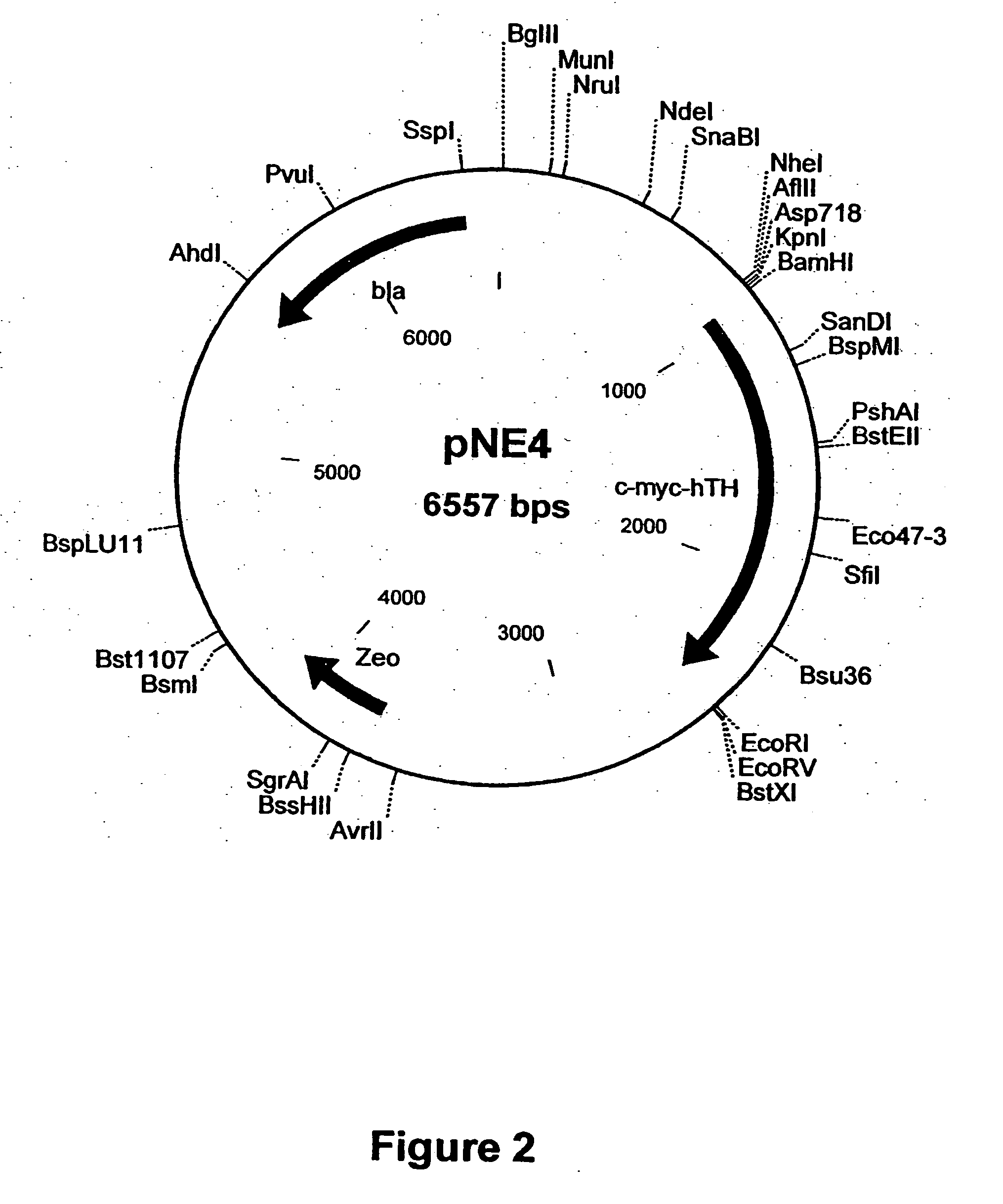
Vector system
InactiveUS7259015B2Eliminate the effects ofInhibition of activationFungiNervous disorderVector systemTyrosine
Provided are retroviral vector genomes and vector systems comprising the genomes. In particular, a retroviral vector genome comprising two or more NOIs, operably linked by one or more Internal Ribosome Entry Site(s); a lentiviral vector genome comprising two or more NOIs suitable for treating a neurodegenerative disorder; and a lentiviral vector genome which encodes tyrosine hydroxylase, GTP-cyclohydrolase I and, optionally, Aromatic Amino Acid Dopa Decarboxylase are provided.
Owner:OXFORD BIOMEDICA (UK) LTD
Vector system
InactiveUS20070025970A1Eliminate the effects ofInhibition of activationBiocideFungiVector systemTyrosine hydroxylase
The present invention relates to retroviral vector genomes and to vector systems comprising such genomes. In particular the present invention relates to a retroviral vector genome comprising two or more NOIs operably linked by one or more Internal Ribosome Entry Site(s); a lentiviral vector genome comprising two or more NOIs suitable for treating a neurodegenerative disorder; and a lentiviral vector genome which encodes tyrosine hydroxylase, GTP-cyclohydrolase I and optionally Aromatic Amino Acid Dopa Decarboxylase.
Owner:OXFORD BIOMEDICA (UK) LTD
Bone marrow cells as a source of neurons for brain and spinal cord repair
Owner:SOUTH FLORIDA UNIVESITY OF
Isolation and Cultivation of Stem/Progenitor Cells From the Amniotic Membrane of Umbilical Cord and Uses of Cells Differentiated Therefrom
The present invention relates to a skin equivalent and a method for producing the same, wherein the skin equivalent comprises a scaffold and stem / progenitor cells isolated from the amniotic membrane of umbilical cord. These stem / progenitor cells may be mesenchymal (UCMC) and / or epithelial (UCEC) stem cells, which may then be further differentiated to fibroblast and keratinocytes. Further described is a method for isolating stem / progenitor cells from the amniotic membrane of umbilical cord, wherein the method comprises separating the amniotic membrane from the other components of the umbilical cord in vitro, culturing the amniotic membrane tissue under conditions allowing cell proliferation, and isolating the stem / progenitor cells from the tissue cultures. The invention also refers to therapeutic uses of these skin equivalents. Another aspect of the invention relates to the generation of a mucin-producing cell using stem / progenitor cells obtained from the amniotic membrane of umbilical cord and therapeutic uses of such mucin-producing cells. In yet another aspect, the invention relates to a method for generating an insulin-producing cell using stem / progenitor cells isolated from the amniotic membrane of umbilical cord and therapeutic uses thereof. The invention further refers to a method of treating a bone or cartilage disorder using UCMC. Furthermore, the invention refers to a method of generating a dopamin and tyrosin hydroxylase as well as a HLA-G and hepatocytes using UCMC and / or UCEC. The present invention also refers to a method of inducing proliferation of aged keratinocytes using UCMC.
Owner:CELLRESEARCH CORP PTE LTD
Bone marrow cells as a source of neurons for brain and spinal cord repair
Bone marrow stromal cells (BMSC) differentiate into neuron-like phenotypes in vitro and in vivo, engrafted into normal or denervated rat striatum. The BMSC did not remain localized to the site of the graft, but migrated throughout the brain and integrated into specific brain regions in various architectonic patterns. The most orderly integration of BMSC was in the laminar distribution of cerebellar Purkinje cells, where the BMSC-derived cells took on the Purkinje phenotype. The BMSC exhibited site-dependent differentiation and expressed several neuronal markers including neuron-specific nuclear protein, tyrosine hydroxylase and calbindin. BMSC can be used to target specific brain nuclei in strategies of neural repair and gene therapy.
Owner:SOUTH FLORIDA UNIVESITY OF
Derivation of terminally differentiated dopaminergic neurons from human embryonic stem cells
InactiveUS20060211109A1Simple methodIncrease percentageNervous disorderCulture processNervous systemNeural cell
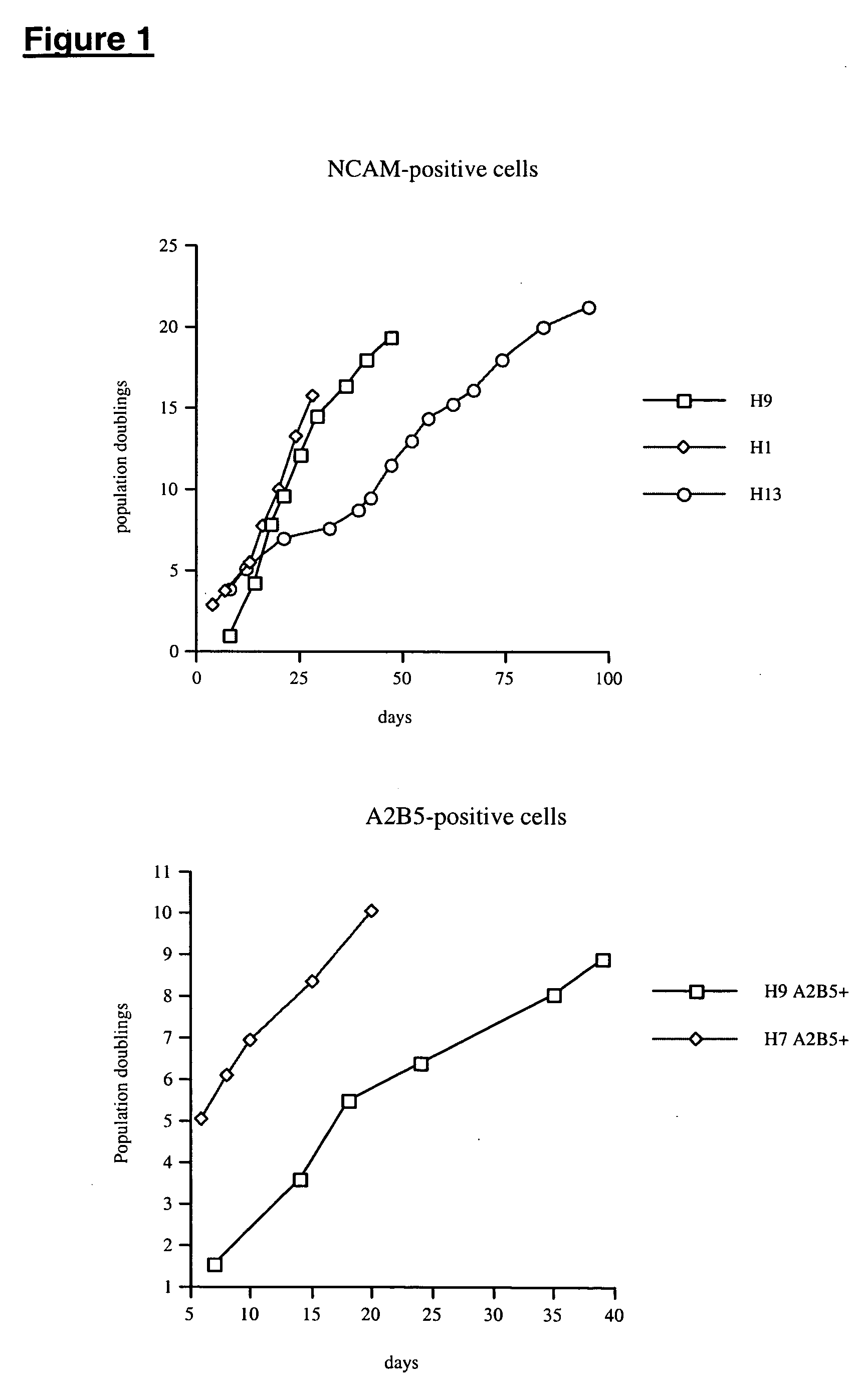
The present disclosure is directed to improved methods for efficiently producing neuroprogenitor cells and differentiated neural cells such as dopaminergic neurons and serotonergic neurons from pluripotent stem cells, for example human embryonic stem cells. Using the disclosed methods, cell populations containing a high proportion of cells positive for tyrosine hydroxylase, a specific marker for dopaminergic neurons, have been isolated. The neuroprogenitor cells and terminally differentiated cells of the present disclosure can be generated in large quantities, and therefore may serve as an excellent source for cell replacement therapy in neurological disorders such as Parkinson's disease.
Owner:RELIANCE LIFE SCI PVT
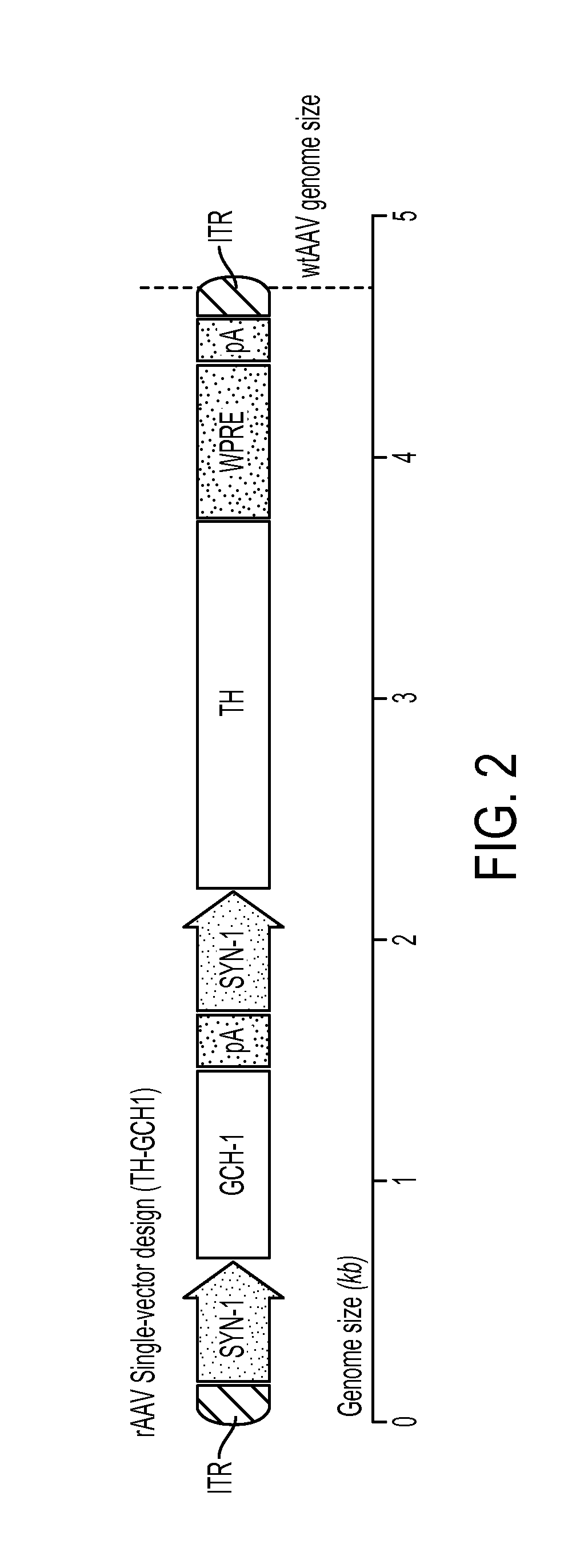
Novel viral vector construct for neuron specific optimized continuous dopa synthesis in vivo
InactiveUS20150065560A1Reduce adverse effectsHigh expressionNervous disorderHydrolasesDiseaseCatecholamine
Owner:GENEPOD THERAPEUTICS
Cell system for alleviating syndromes of Parkinson's disease in a mammal
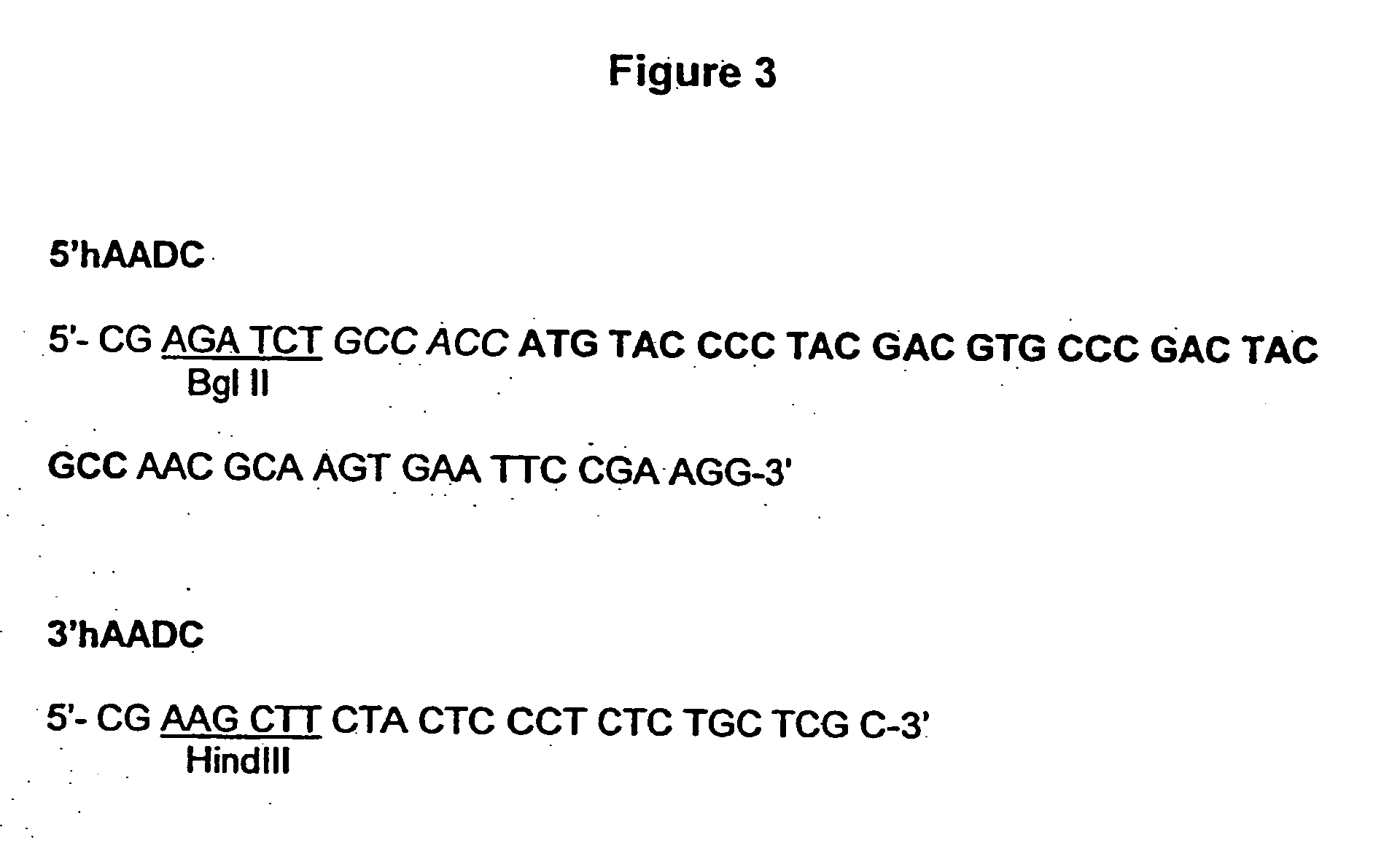
A cell system for treating neurodegenerative disorders in a mammal is provided. The cell system includes a population of neurons differentiated from umbilical mesenchymal stem cells for expressing at least one of tyrosine hydroxylase (TH), dopamine-β-hydroxylase (DBH), glutamate decarboxylase (GAD), aromatic L-amino acid decarboxylase (AADC) and dopaminergic transporter (DAT) in a cell culture. A method for treating neurodegenerative disorders in a mammal is also provided. The method comprises the steps of differentiating umbilical mesenchymal stem cells into a population of neurons that express at least one of TH, DBH, GAD, AADC and DAT in a cell culture, and transplanting the population of neurons into the brain of the mammal.
Owner:FU YU SHOW +1
Skin equivalents derived from umbilical cord mesenchymal stem/progenitor cells and umbilical cord epithelial stem/progenitor cells
The present invention relates to a skin equivalent and a method for producing the same, wherein the skin equivalent comprises a scaffold and stem / progenitor cells isolated from the amniotic membrane of umbilical cord. These stem / progenitor cells may be mesenchymal (UCMC) and / or epithelial (UCEC) stem cells, which may then be further differentiated to fibroblast and keratinocytes. Further described is a method for isolating stem / progenitor cells from the amniotic membrane of umbilical cord, wherein the method comprises separating the amniotic membrane from the other components of the umbilical cord in vitro, culturing the amniotic membrane tissue under conditions allowing cell proliferation, and isolating the stem / progenitor cells from the tissue cultures. The invention also refers to therapeutic uses of these skin equivalents. Another aspect of the invention relates to the generation of a mucin-producing cell using stem / progenitor cells obtained from the amniotic membrane of umbilical cord and therapeutic uses of such mucin-producing cells. In yet another aspect, the invention relates to a method for generating an insulin-producing cell using stem / progenitor cells isolated from the amniotic membrane of umbilical cord and therapeutic uses thereof. The invention further refers to a method of treating a bone or cartilage disorder using UCMC. Furthermore, the invention refers to a method of generating a dopamin and tyrosin hydroxylase as well as a HLA-G and hepatocytes using UCMC and / or UCEC. The present invention also refers to a method of inducing proliferation of aged keratinocytes using UCMC.
Owner:CELLRESEARCH CORP PTE LTD
Pharmaceuticals containing multipotential precursor cells from tissues containing sensory receptors
Current sources of neural stem and progenitor cells for neural transplantation are essentially inaccessible in living animals. This invention relates to neural precursor cells (stem cells, progenitor cells or a combination of both types of cells) isolated from the olfactory epithelium of mammals that can be passaged and expanded, and that will differentiate into cell types of the central nervous system (CNS), including astrocytes, oligodendrocytes, and tyrosine-hydroxylase-positive neurons. These precursor cells provide an accessible source for autologous transplantation in CNS, PNS, spinal cord and other damaged tissues.
Owner:MCGILL UNIV
Derivation of terminally differentiated dopaminergic neurons from human embryonic stem cells
InactiveUS7674620B2Simple methodIncrease percentageNervous disorderCulture processDiseaseNeurulation
The present disclosure is directed to improved methods for efficiently producing neuroprogenitor cells and differentiated neural cells such as dopaminergic neurons and serotonergic neurons from pluripotent stem cells, for example human embryonic stem cells. Using the disclosed methods, cell populations containing a high proportion of cells positive for tyrosine hydroxylase, a specific marker for dopaminergic neurons, have been isolated. The neuroprogenitor cells and terminally differentiated cells of the present disclosure can be generated in large quantities, and therefore may serve as an excellent source for cell replacement therapy in neurological disorders such as Parkinson's disease.
Owner:RELIANCE LIFE SCI PVT
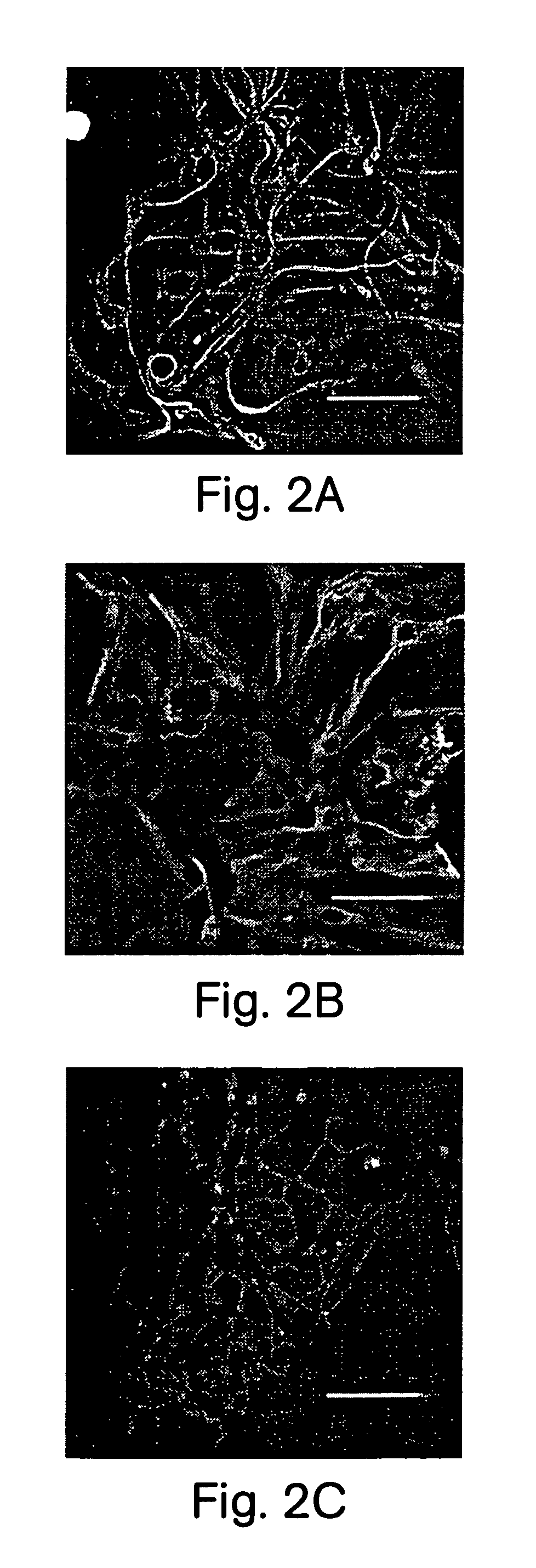
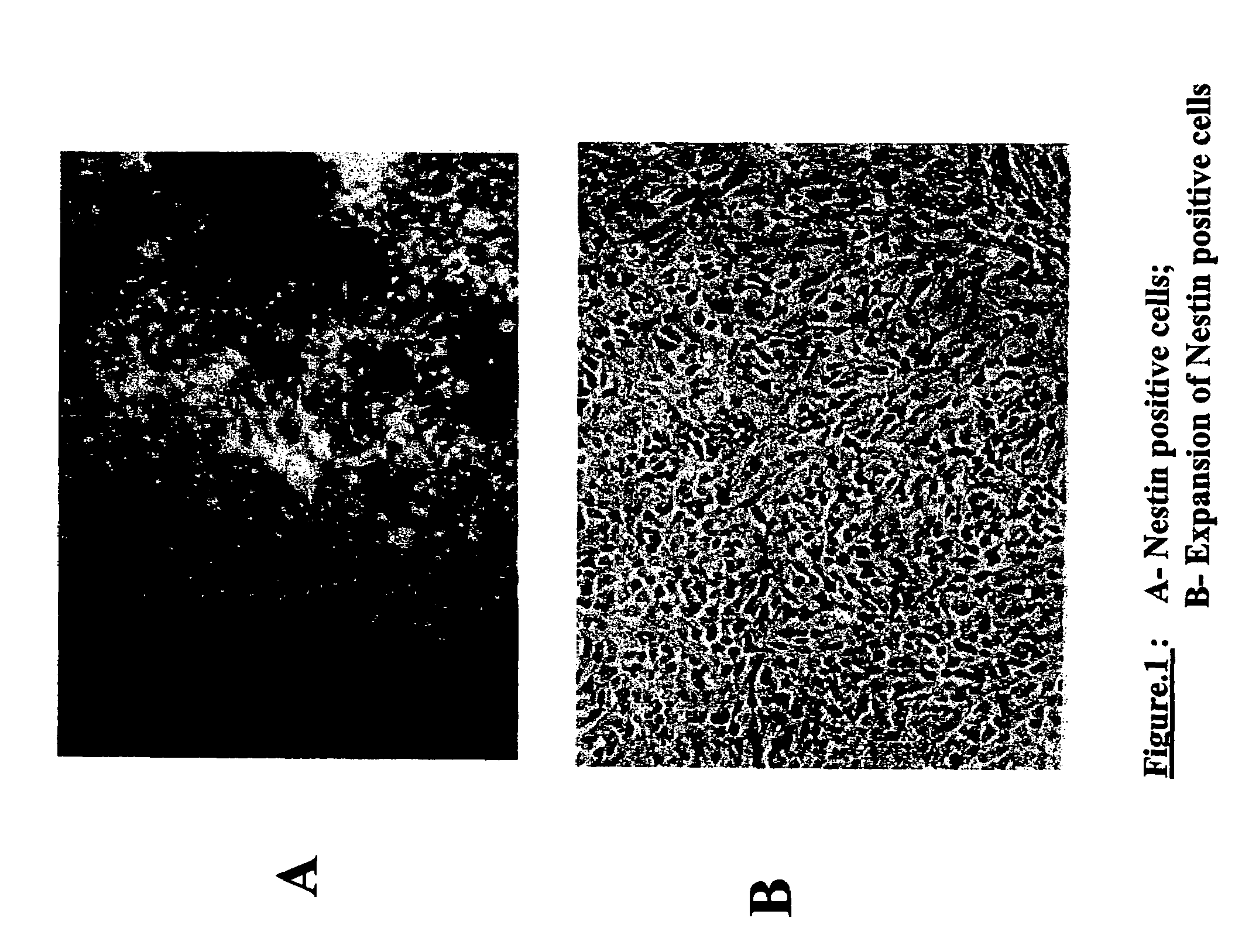
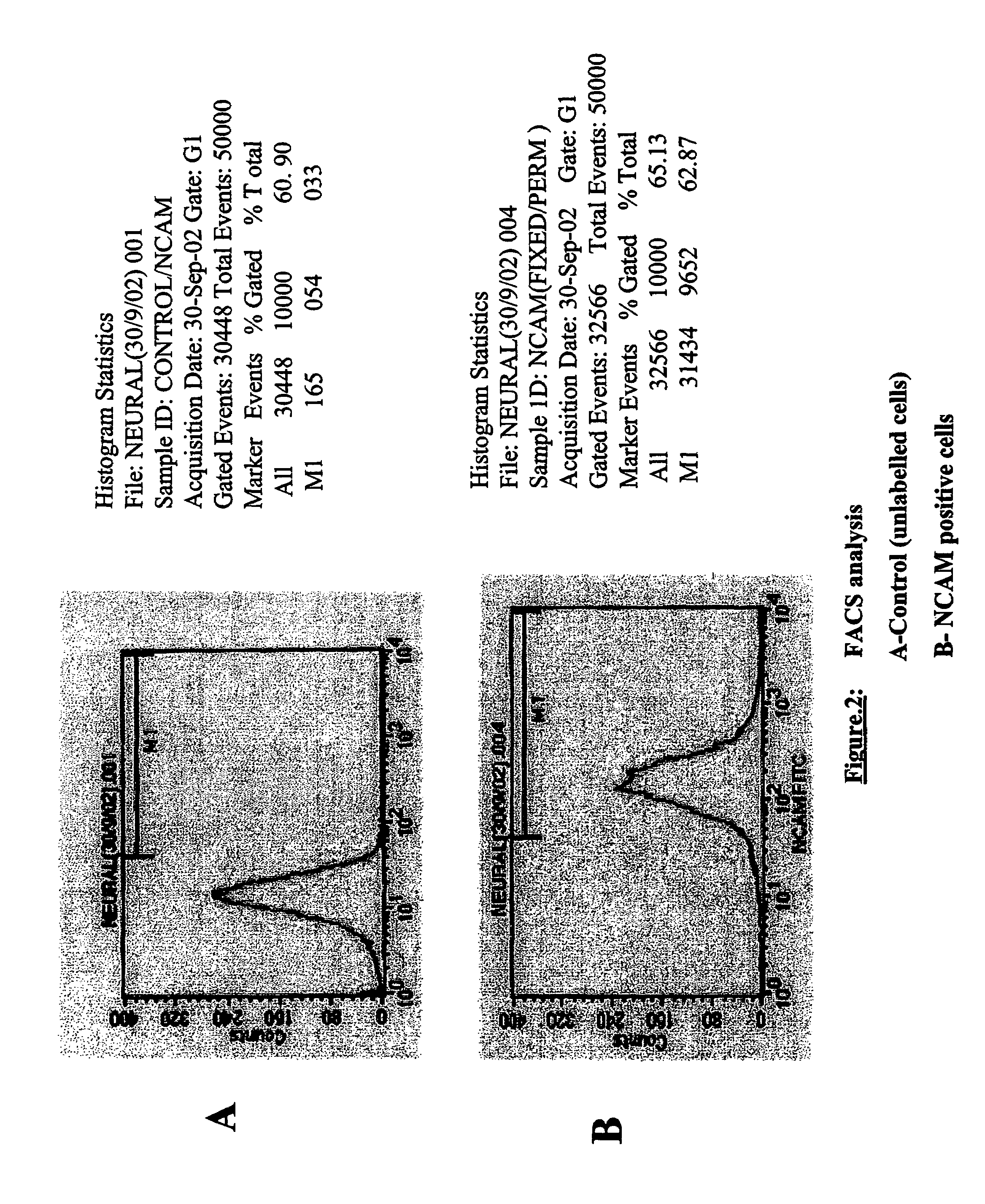
Production of tyrosine hydroxylase positive neurons
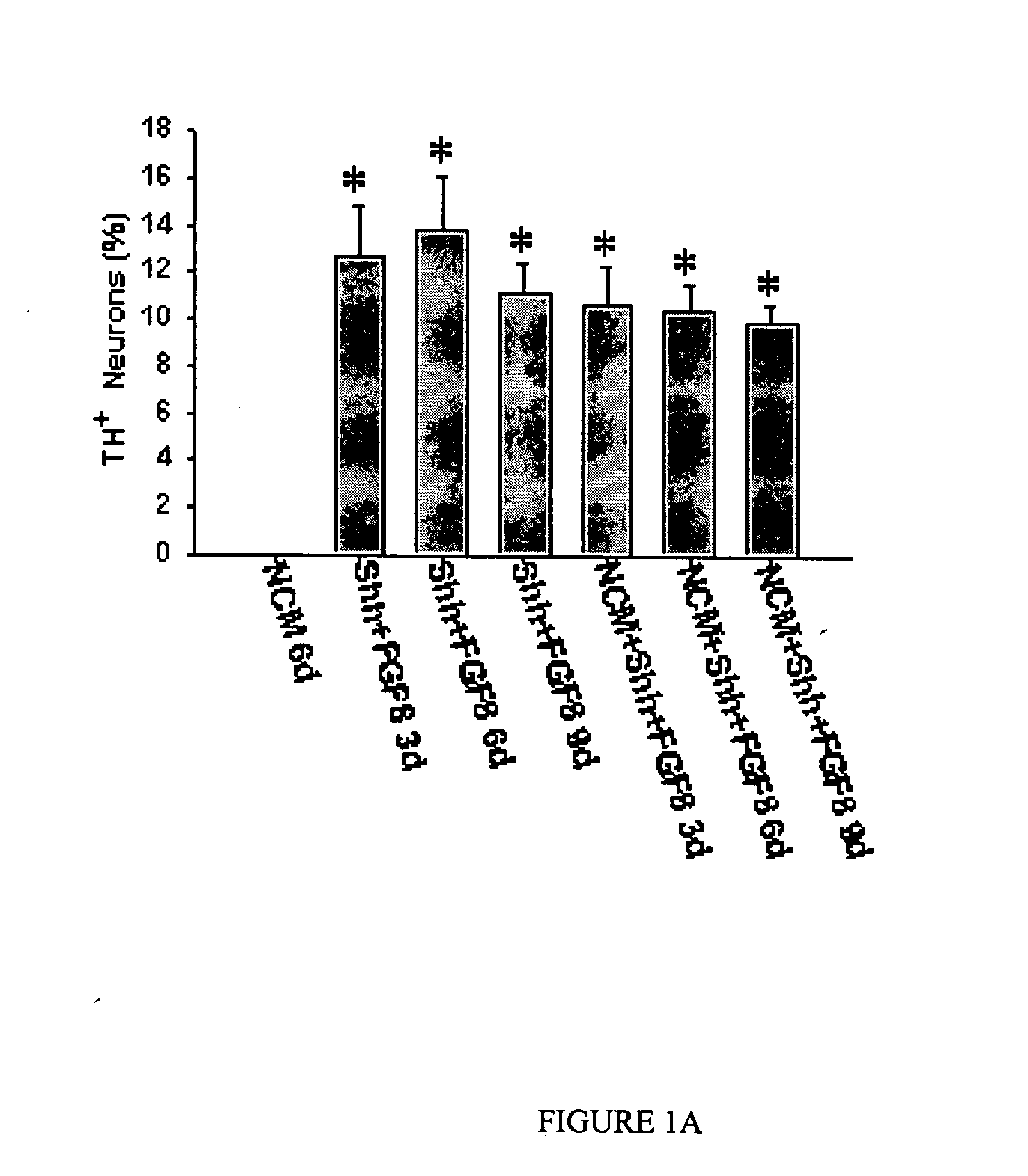
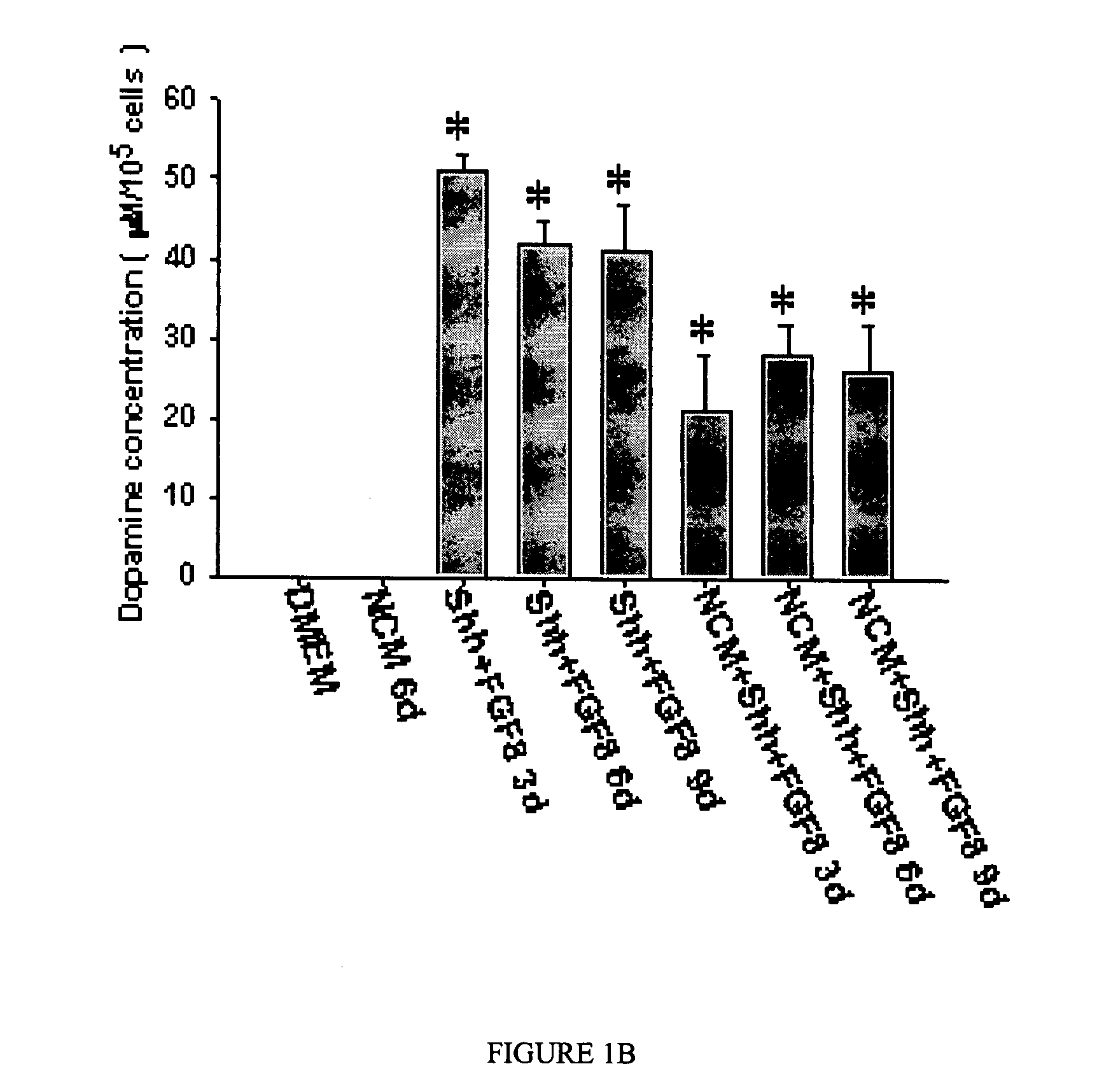
The present invention relates to a method of producing neurons that express the enzyme tyrosine hydroxylase (TH) by subjecting neural stem cells to FGF-1, a protein kinase A activator, a protein kinase C activator, and dopamine / L-DOPA. Surprisingly, when forskolin is used as a protein kinase A activator, it requires only low levels of FGF-1 and forskolin to efficiently produce TH positive neurons from fetal or adult neural stem cells. Also provided are compositions used to produce TH positive neurons and the resulting neural cell culture, as well as a method of treating disease or conditions which are associated with dopamine neuron loss or dysfunction.
Owner:STEM CELL THERAPEUTICS
Dopaminergic neurons and proliferation-competent precursor cells for treating Parkinson's disease
InactiveUS20050042749A1Promote generationHigh proportionGenetically modified cellsMicrobiological testing/measurementProgenitorNervous system
This disclosure provides improved methods for obtaining populations of neural progenitor cells and differentiated neurons from pluripotent stem cells. The technology can be used to produce progenitors that proliferate through at least 40 doublings, while maintaining the ability to differentiate into a variety of different neural phenotypes. Cell populations have been obtained that contain a high proportion of cells staining for tyrosine hydroxylase, which is a feature of dopaminergic neurons. The neural progenitors and terminally differentiated neurons of this invention can be generated in large quantities for use in drug screening and the treatment of clinically important neurological disorders, such as Parkinson's disease.
Owner:ASTERIAS BIOTHERAPEUTICS INC
Mutant NURR1 gene in Parkinson's disease
InactiveUS7037657B2Avoid harmful effectsInhibiting transcriptionSugar derivativesMicrobiological testing/measurementTyrosine hydroxylaseIn vivo
The identification of mutations in NURR1 provides molecular tools for the development of diagnostic, prophylactic and therapeutic agents for Parkinson's Disease. In specific embodiments, two point mutations are identified in exon 1 of the NURR1 gene in 10 / 107 (9.3%) cases of familial Parkinson's disease (PD). The mutations reduce NURR1 gene expression (mRNA and protein levels) by 87–95% and decrease tyrosine hydroxylase (a rate-limited dopamine synthesis enzyme) gene expression in vitro. It is also demonstrated that in vivo NURR1 mRNA levels in the lymphocytes from the PD patients with the exon 1 mutation are reduced by 68–84%, and in over 50% sporadic PD patients the NURR1 mRNA levels in lymphocytes are significantly reduced. A homozygous polymorphism is identified in intron 6 of NURR1 that correlates with the presence of Parkinson's disease. A splicing variant in NURR1 exon 5 is identified.
Owner:BAYLOR COLLEGE OF MEDICINE
Tyrosine hydroxylase variants and methods of use thereof
ActiveUS20170306301A1Lower Level RequirementsImprove the level ofMicrobiological testing/measurementOxidoreductasesIncreased tyrosineGene product
The present disclosure provides a variant tyrosine hydroxylase that provides for increased production of L-DOPA in a host cell that expresses the tyrosine hydroxylase. The present disclosure provides nucleic acids encoding the variant tyrosine hydroxylase, and host cells genetically modified with the nucleic acids. The present disclosure provides methods of making L-DOPA in a host cell. The present disclosure provides methods of making a benzylisoquinoline alkaloid (BIA), or a BIA precursor. The present disclosure provides methods of detecting L-DOPA level in a cell. The present disclosure provides methods of identifying tyrosine hydroxylase variants that provide for increased L-DOPA production; and methods of identifying gene products that provide for increased tyrosine production.
Owner:VALORBEC S E C +1
Pharmaceutical composition for preventing and treating Parkinson's disease and preparation method thereof
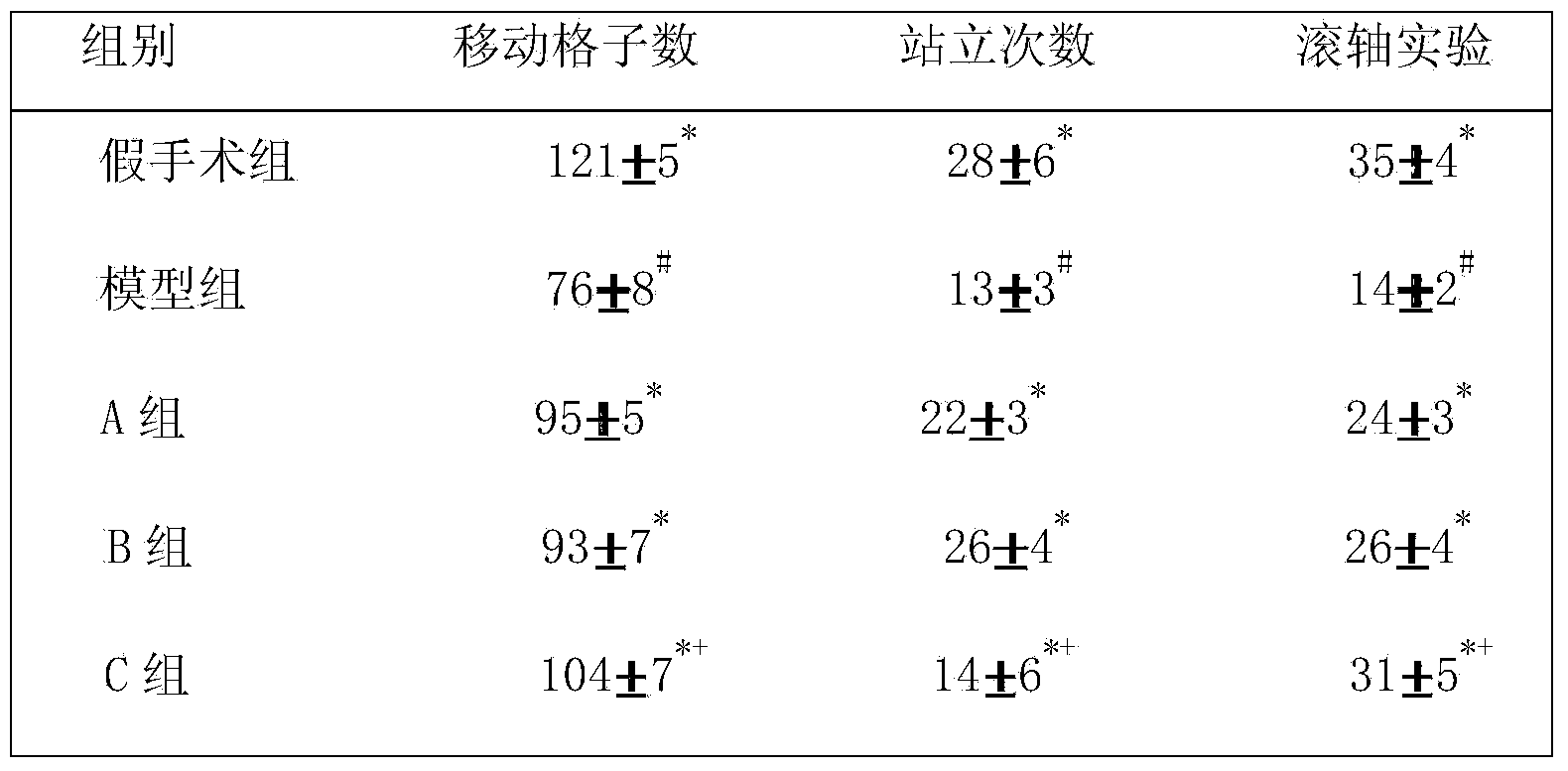
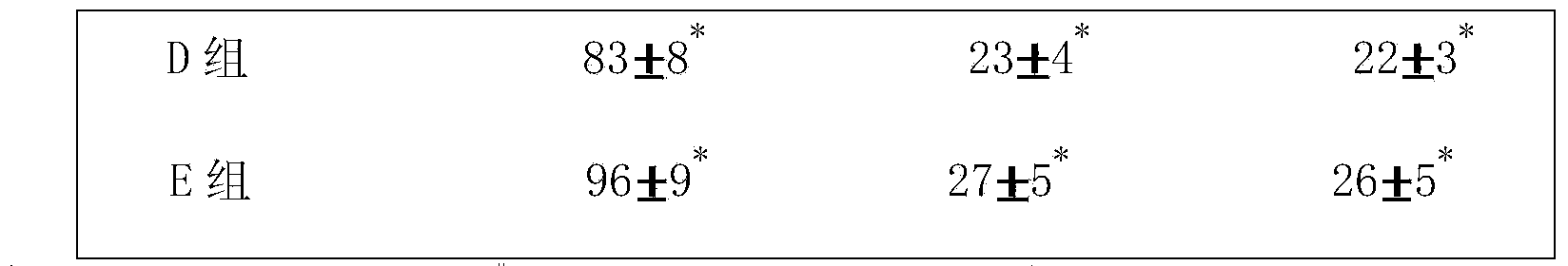
ActiveCN103720959ALess medicinalImprove quality controlNervous disorderUnknown materialsTyrosine hydroxylaseBULK ACTIVE INGREDIENT
The invention relates to a pharmaceutical composition for preventing and treating Parkinson's disease. The pharmaceutical composition consists of active ingredients and medically acceptable auxiliary materials, and is characterized in that the active ingredients consist of the following components in percentage by weight: rhizoma acori graminei volatile oil extracted from rhizoma acori graminei accounting for 25-45 percent and tortoise plastron water extract extracted from tortoise plastron accounting for55-75 percent. By adopting the pharmaceutical composition, neurological integrals of Parkinson's model rats can be obviously improved, the content of monoamine neurotransmitters in rat brains is increased, reduction of tyrosine hydroxylase in brain is inhibited, and the effect of preventing and treating the Parkinson's disease is obvious.
Owner:THE FIRST AFFILIATED HOSPITAL OF GUANGZHOU UNIV OF CHINESE MEDICINE
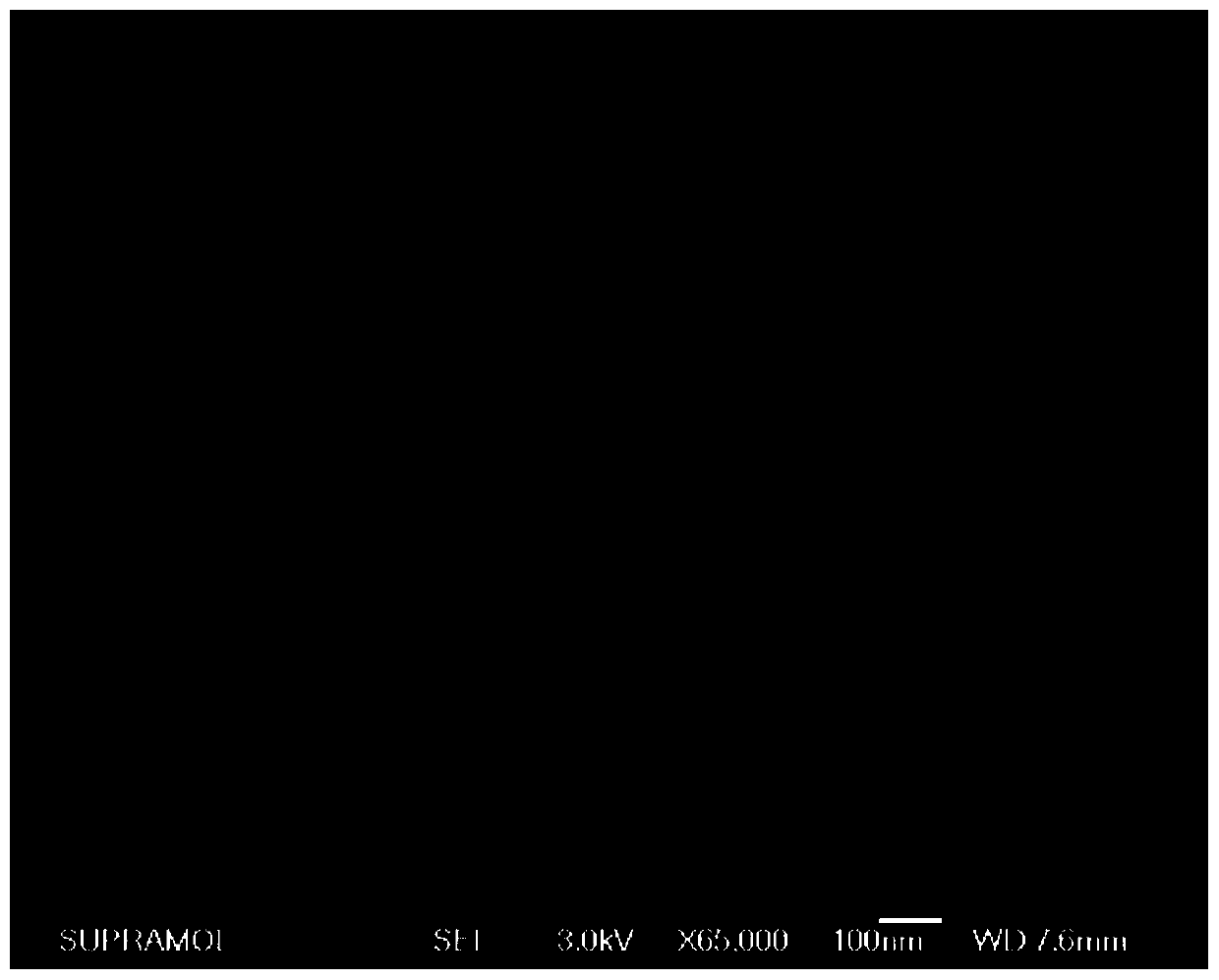
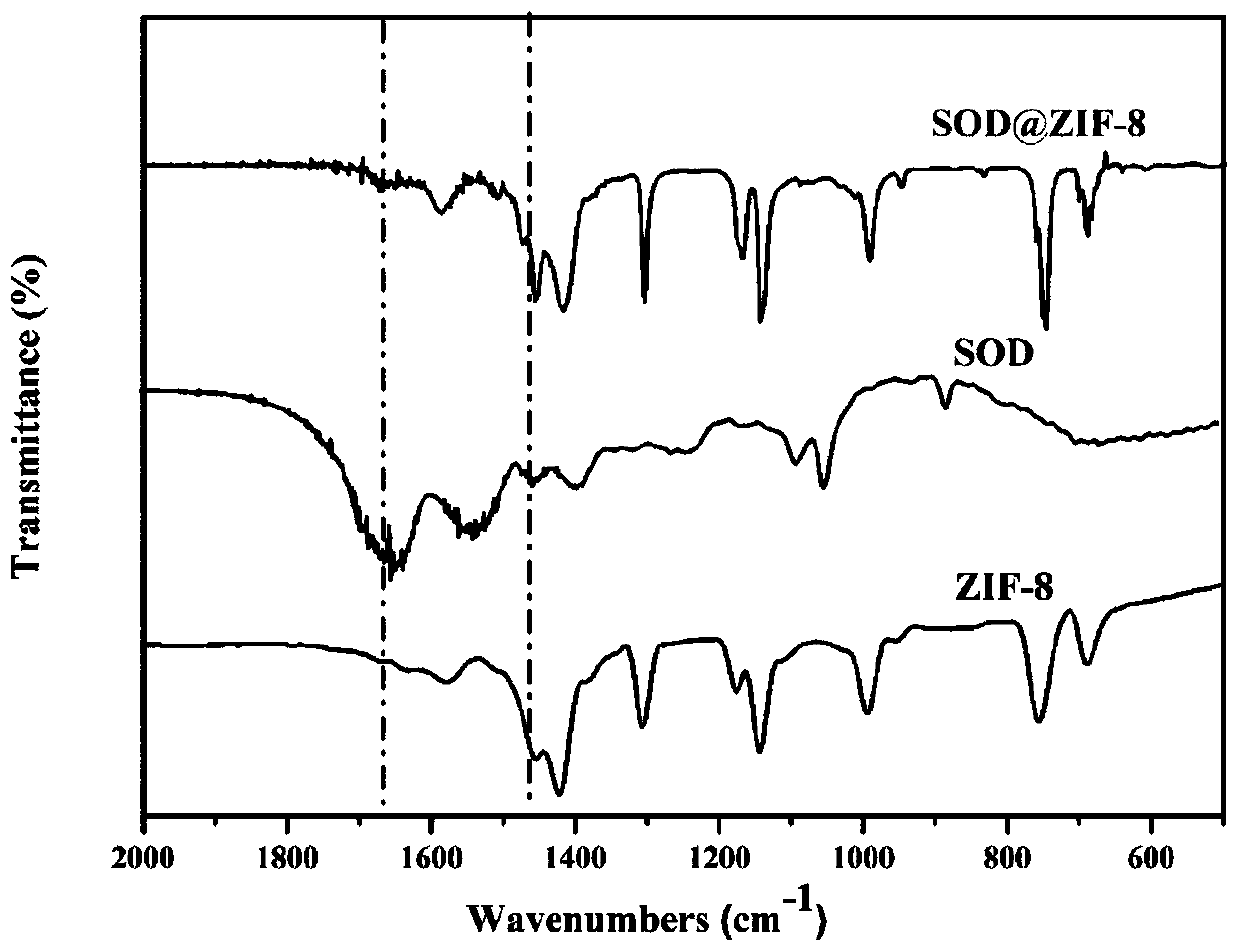
Metal-organic framework-superoxide dismutase assembly, preparation method and application thereof in preparation of drugs for treating Parkinson
InactiveCN109833468AProtection stabilityEasy to synthesizeNervous disorderPeptide/protein ingredientsSuper oxide dismutaseTreatment effect
The invention provides a metal-organic framework-superoxide dismutase assembly, a preparation method and an application thereof in preparation of drugs for treating Parkinson, and belongs to the technical field of biology. The framework makes use of biomimetic mineralization assembly strategy to immobilize SOD enzyme molecules into a metal-organic framework ZIF-8, coordination bonds are formed between SOD amino and zinc ions of ZIF-8, so that metal organic framework-SOD enzyme assembly (SOD@ZIF-8) are constructed based on enzymes. The temperature stability of SOD enzyme molecules and pH tolerance are improved through the frame work, by treating the SOD@ZIF-8 assembly as a therapeutic drug, at the cellular level, active oxygen produced by stimulation of MPP+ can be removed effectively, so as to relieve apoptosis caused by active oxygen; at the animal level, after intravenous chemotherapy by the assembly, the barrier in sports ability of Parkinson model mouse constructed by MPTP can be relieved, the expression level of tyrosine hydroxylase in substantia nigra is improved, the therapeutic effect is good.
Owner:JILIN UNIV
Gene expression system and regulation thereof
InactiveUS20170114346A1Eliminate fluctuationsPromote resultsOrganic active ingredientsNervous disorderDiseaseNucleotide
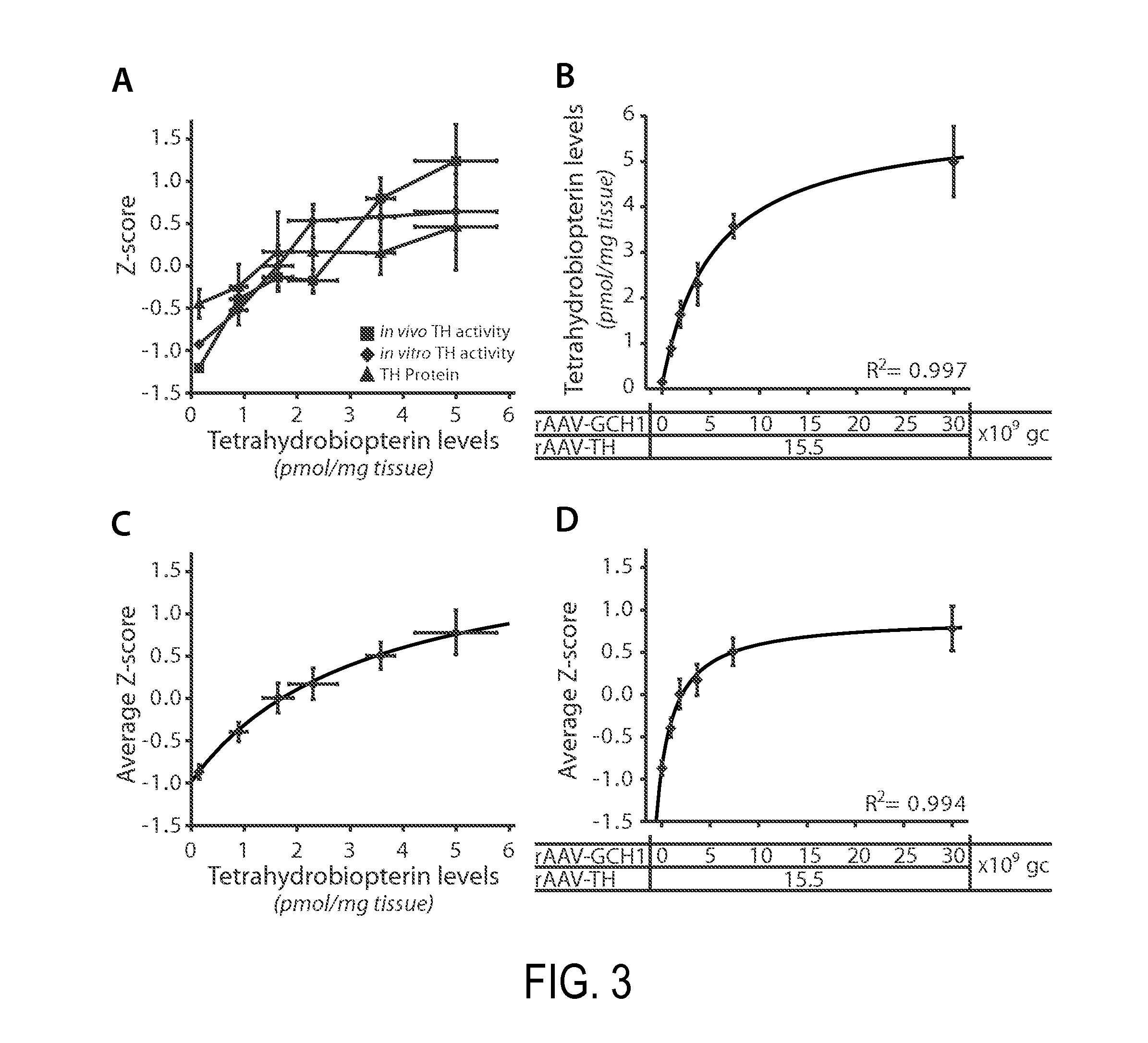
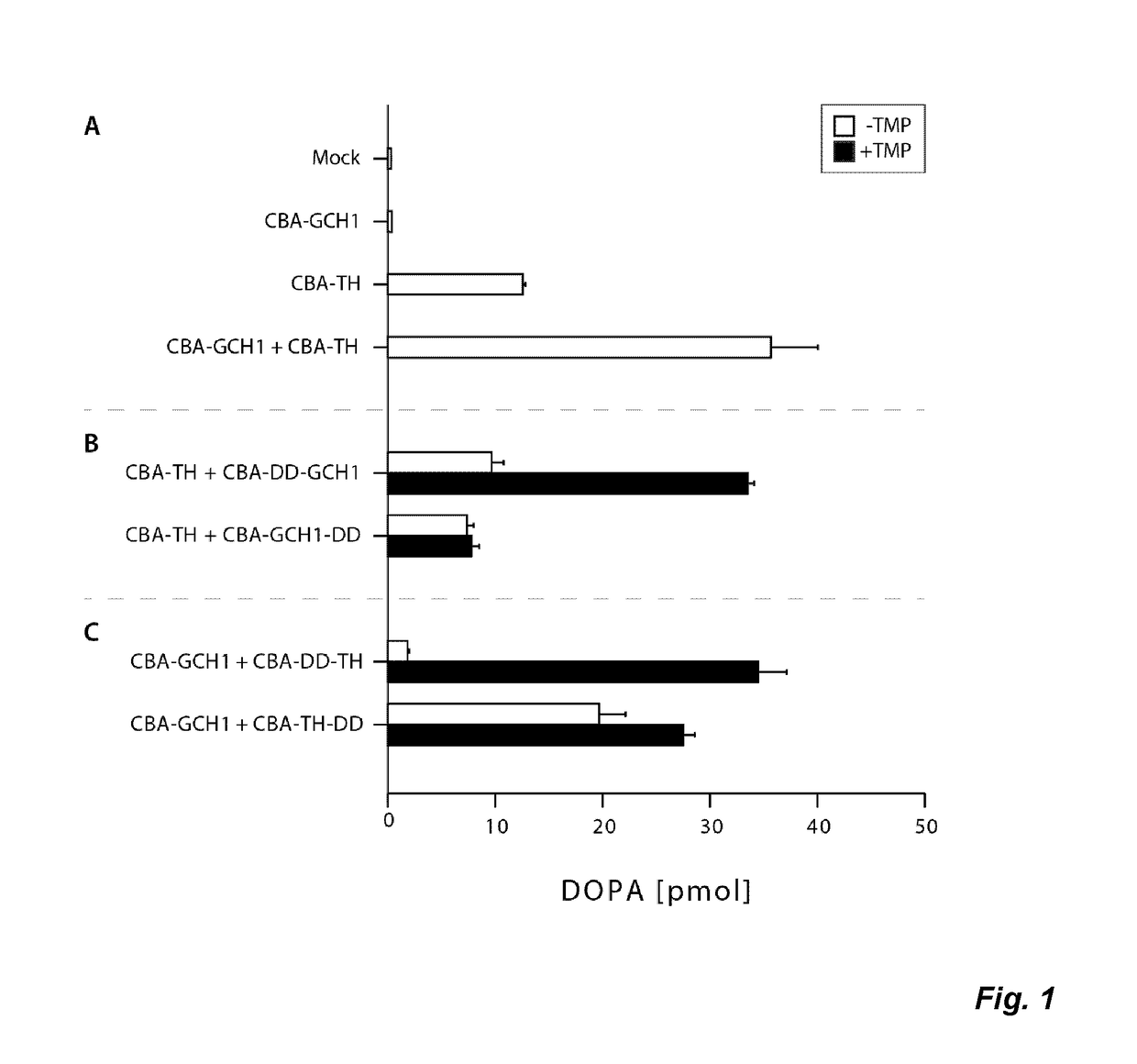
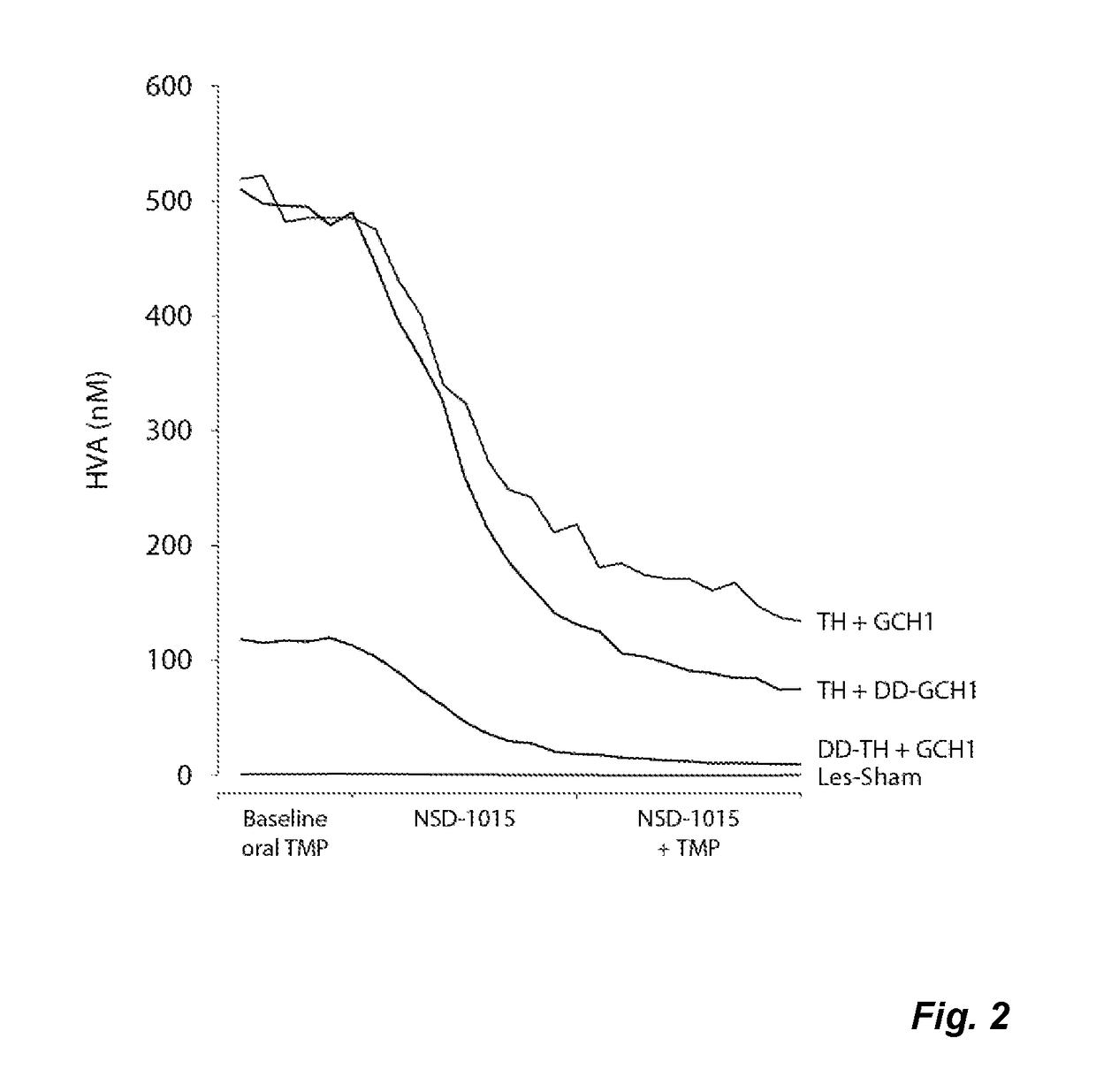
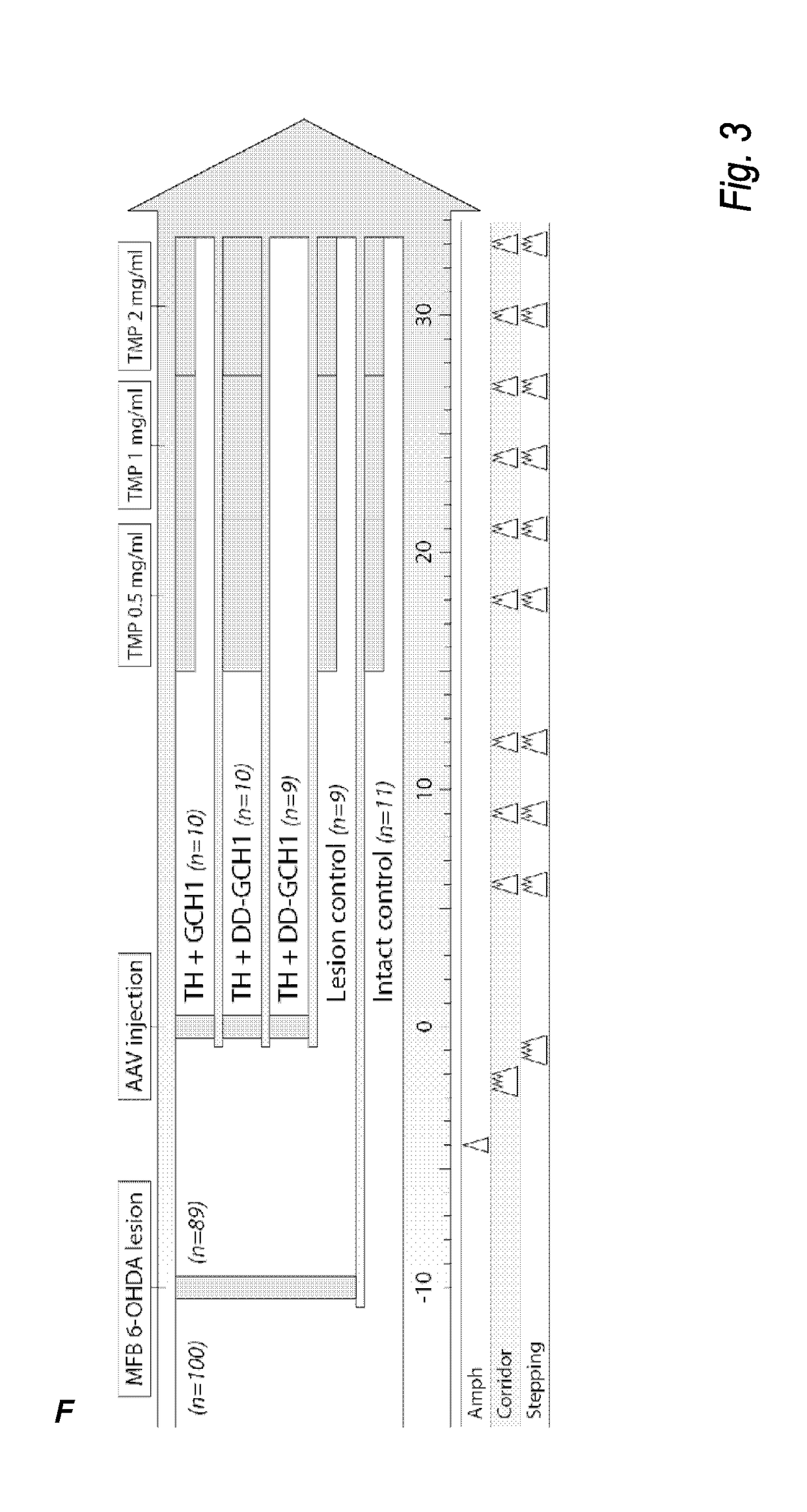
The present invention relates to a novel gene expression system comprising: a) a first nucleotide sequence encoding a fusion polypeptide of: a1) a destabilizing domain (DD) based on DHFR, and a2) a GTPcyclohydrolase 1 (GCH1) polypeptide, or a biologically active fragment or variant thereof; and b) a second nucleotide sequence encoding a tyrosine hydroxylase (TH) polypeptide, or a biologically active fragment or variant thereof. The invention also relates to use of this gene expression system together with a ligand binding to a destabilizing domain (DD) based on dihydrofolate reductase (DHFR) for treatment of diseases associated with a reduced dopamine level, such as Parkinson's disease.
Owner:BRAINGENE AB
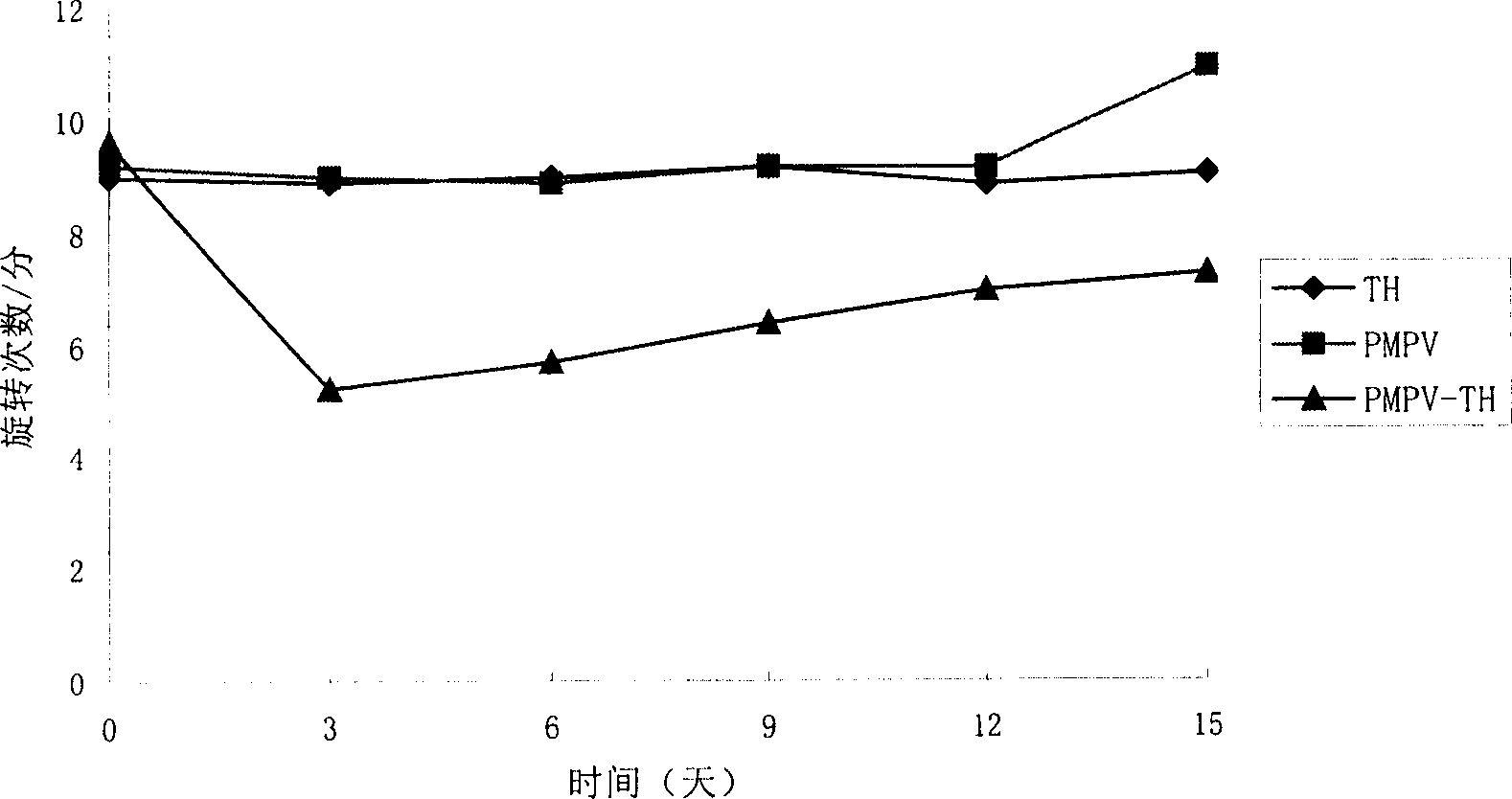
Sporoderm-broken germination-activated ganoderma lucidum spores for protection of dopaminergic neurons and treatment of Parkinson's disease
InactiveUS7357933B2Improves tyrosine hydrolase (TH) activitySpeed up the conversion processBiocideLichen medical ingredientsDiseaseMammal
This invention provides a method for treating Parkinson's disease (PD), particularly early stage of PD by orally administering to a mammal sporoderm-broken germination activated Ganoderma lucidum spore powders (GASP) to reduce and / or release the symptom of rotatory behavior in PD, to reduce the progression of neuron apoptosis and to improve the tyrosine hydroxylase (TH) activity so as to increase the conversion of hydroxydopamine (DOPA) to dopamine in the dopaminergic neurons. This invention also provides a method for reducing neuron apoptosis and a method for improving TH activity in dopaminergic neurons by orally administering to a mammal GASP.
Owner:ENHAN TECH HLDG INT
Regulation of tyrosine hydroxylase
InactiveUS6617311B1UpregulationNervous disorderDipeptide ingredientsCentral nervous systemTyrosine hydroxylase
This invention relates to methods of regulating the effect of tyrosine hydroxylase (TH). In particular it relates to increasing the effective amount of TH in the central nervous systems (CNS) for the purpose of increasing TH-mediated dopamine production in the treatment of conditions such as Parkinson's disease.
Owner:ENDOCRINZ +1
Methods and compounds for treatment or prevention of substance-related disorders
InactiveUS20090258869A1Ameliorating and eliminating effectAmeliorate and eliminate effectBiocideNervous disorderTyrosine hydroxylaseHsp Inhibitor
The present disclosure provides methods of treating or preventing a substance-related disorder using Hsp90 inhibitors, Hsp90 modulators, tyrosine hydroxylase modulators, and modulators that reduce the interaction between Hsp90 and tyrosine hydroxylase.
Owner:RGT UNIV OF CALIFORNIA
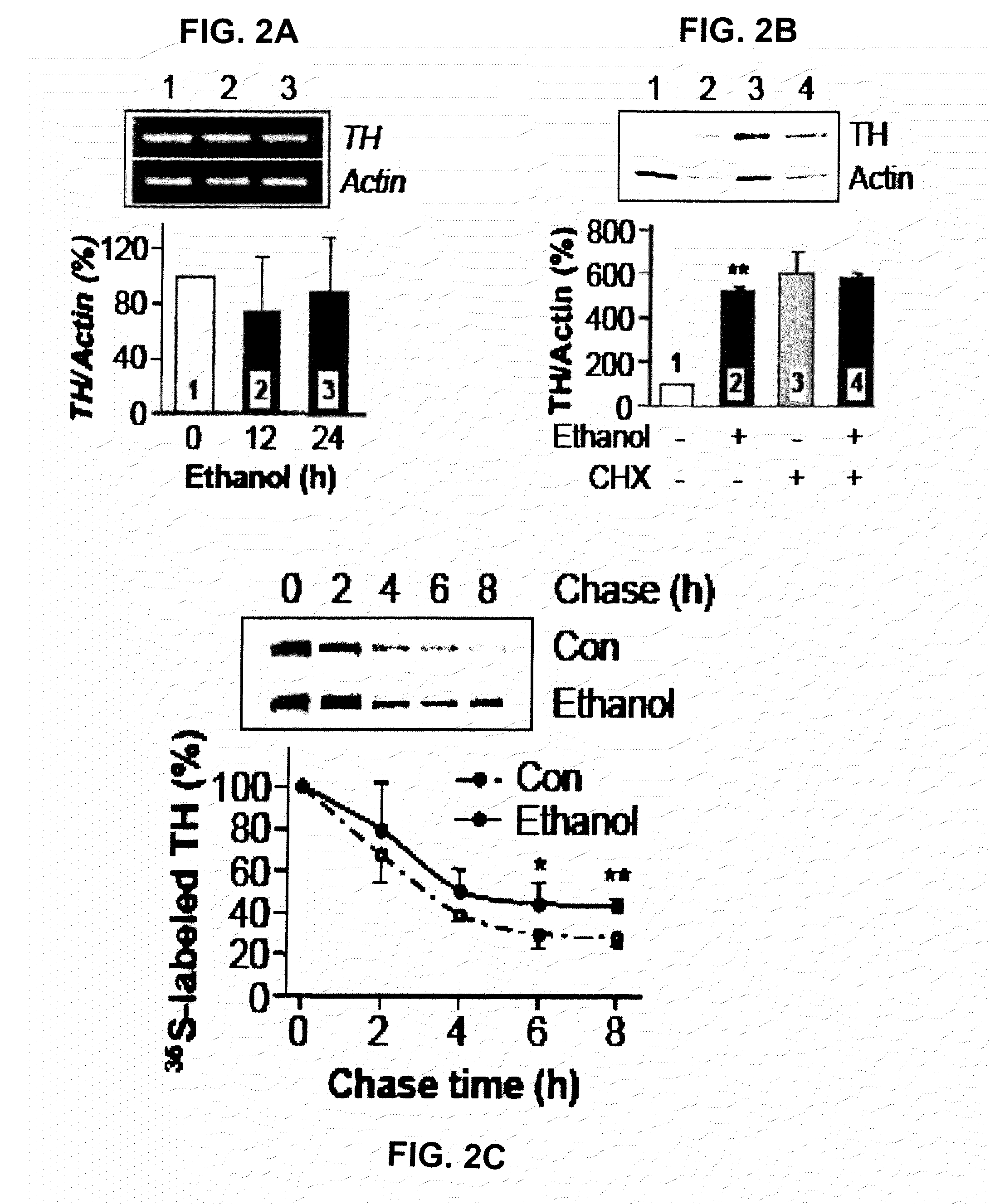
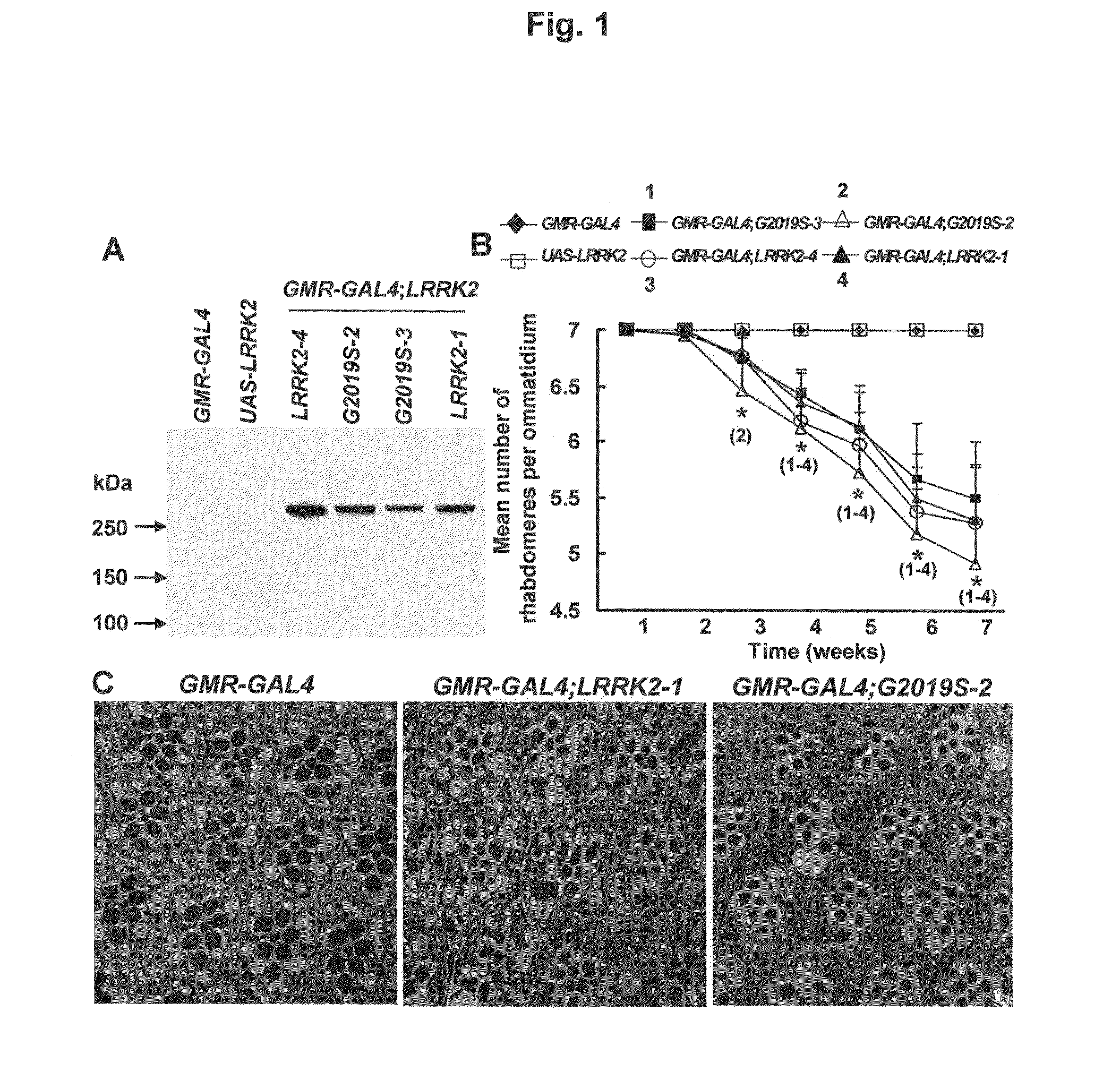
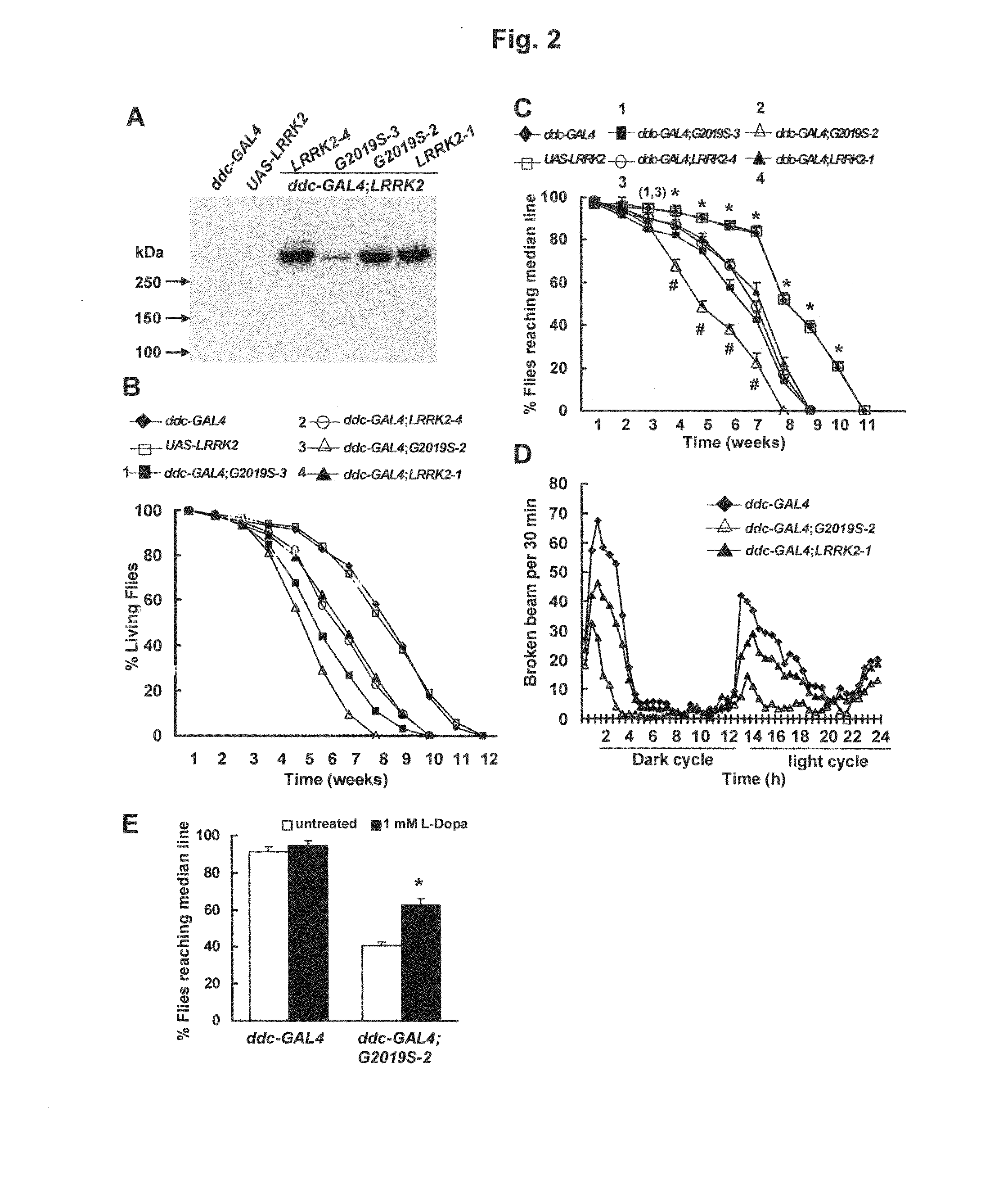
Leucine-rich repeat kinase (LRRK2) drosophila model for parkinson's disease: wildtype1 (WT1) and G2019S mutant flies
InactiveUS20100175140A1Effective preventionEffective treatmentCompounds screening/testingGenetic engineeringNeuronal degenerationWild type
Mutations in the leucine-rich repeat kinase (LRRK2) gene cause late-onset autosomal dominant Parkinson's disease (PD) with pleiomorphic pathology. Previously, we and others found that expression of mutant LRRK2 causes neuronal degeneration in cell culture. Here we used the GAL4 / UAS system to generate transgenic Drosophila expressing either wild-type (WT1) human LRRK2 or LRRK2-G2019S, the most common mutation associated with PD. Expression of either WT1 human LRRK2 or LRRK2-G2019S in the photoreceptor cells caused retinal degeneration. Expression of WT1 LRRK2 or LRRK2-G2019S in neurons produced adult-onset selective loss of dopaminergic neurons, locomotor dysfunction, and early mortality. Expression of mutant G2019S-LRRK2 caused a more severe parkinsonism-like phenotype than expression of equivalent levels of WT1 LRRK2. Treatment with L-DOPA improved mutant LRRK2-induced locomotor impairment but did not prevent the loss of tyrosine hydroxylase (TH)-positive neurons. To our knowledge, this is the first in vivo “gain-of-function” model which recapitulates several key features of LRRK2-linked human parkinsonism. These flies may provide a useful model for studying LRRK2-linked pathogenesis and for future therapeutic screens for PD intervention.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Derivation of neural stem cells from embryonic stem cells and methods of use thereof
Provided is a method for the derivation of neural stem cells (NSCs) from embryonic stem cells (ESCs) and the use of the NSCs for treatment of various neural disorders. The NSCs that are derived from the ESCs are tissue-specific multipotent NSCs with a stable growth rate, unlimited self-renewal capacity, and a predictable differentiation profile. Being both non-tumorigenic and engraftable, the NSCs of the present invention have utility in repopulation stroke-damaged tissue. The NSCs of the present invention may be differentiated to produce tyrosine-hydroxylase expressing neurons, which may be used as a source of dopaminergic neurons for subjects suffering from a condition characterized by dopaminergic dysfunction, such as Parkinson's disease.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Recombinant plasmids for producing hydroxyl salidroside and genetically engineered bacteria and application thereof
InactiveCN112501194AIncrease productionAchieve productionFungiMicroorganism based processesSalidrosideEnzyme Gene
The invention relates to the field of bioengineering, and particularly discloses recombinant plasmids for producing hydroxyl salidroside and genetically engineered bacteria and application of the recombinant plasmids and the genetically engineered bacteria. According to the invention, tyrosine hydroxylase BvTH, AAS enzyme gene and glycosidase UGTs gene are respectively cloned to a plasmid vector pTrc99a to obtain recombinant plasmids PTAX4511 and RrTG1068, and then the recombinant plasmids are transferred into engineering bacteria NVSL4869; through overexpression of tyrosine hydroxylase BvTH,AAS enzyme gene and glycosidase UGTs gene, saccharomyces cerevisiae can obtain the capacity of producing target enzyme, so that the purpose of producing hydroxyl salidroside is achieved. The strain and plasmid disclosed by the invention can be used for producing a downstream derivative hydroxyl salidroside of an important drug molecule salidroside by taking tyrosine as a substrate under the condition of not influencing the survival of saccharomyces cerevisiae, so that the application of salidroside in pharmacology and medicine is broadened.
Owner:CHONGQING UNIV
Method of building animal model for parkinsonism
InactiveCN101204585AExacerbated deathRecombinant DNA-technologyIn-vivo testing preparationsGrowth hormoneTyrosine hydroxylase
The invention relates to the biomedical field, in particular to a parkinsonism mouse model, a method for establishing the parkinsonism mouse model and application of the parkinsonism mouse model. The invention transfers an extrinsic BAG5-Flag expression cassette to a genome of the animal model, and the expression cassette comprises: (a) a promoter of the rat tyrosine hydroxylase, (b) a part of Beta-globin gene and BAG5-Flag, and (c) a human somatotropin mini gene. The invention also provides the method for establishing the parkinsonism mouse model, and the parkinsonism mouse model obtained by the method of the invention can be used for studying an ubiquitin-protein degradation system and a pathology process mechanism, and screen medicine applied to delay and treatment of parkinsonism.
Owner:FUDAN UNIV
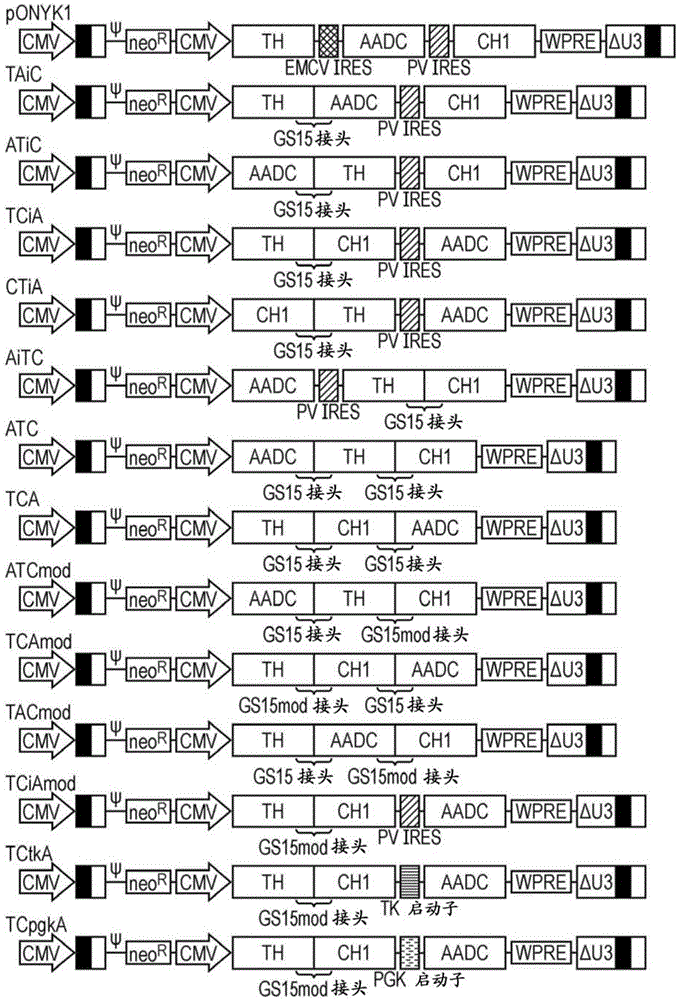
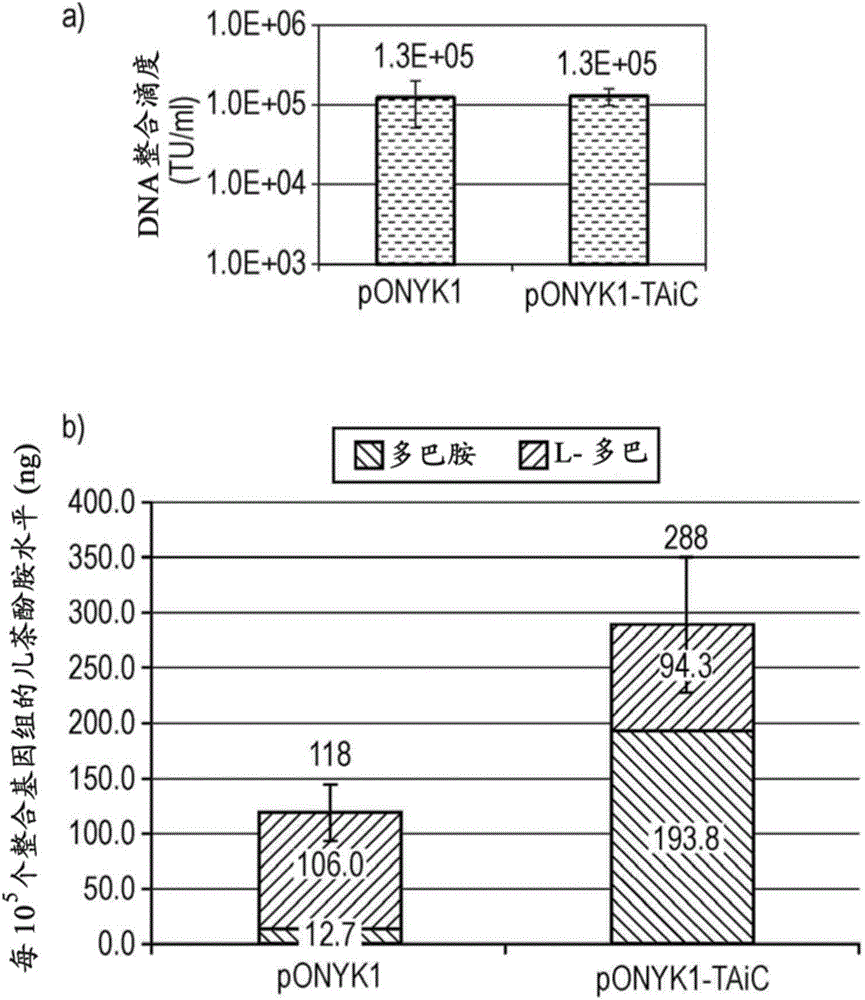
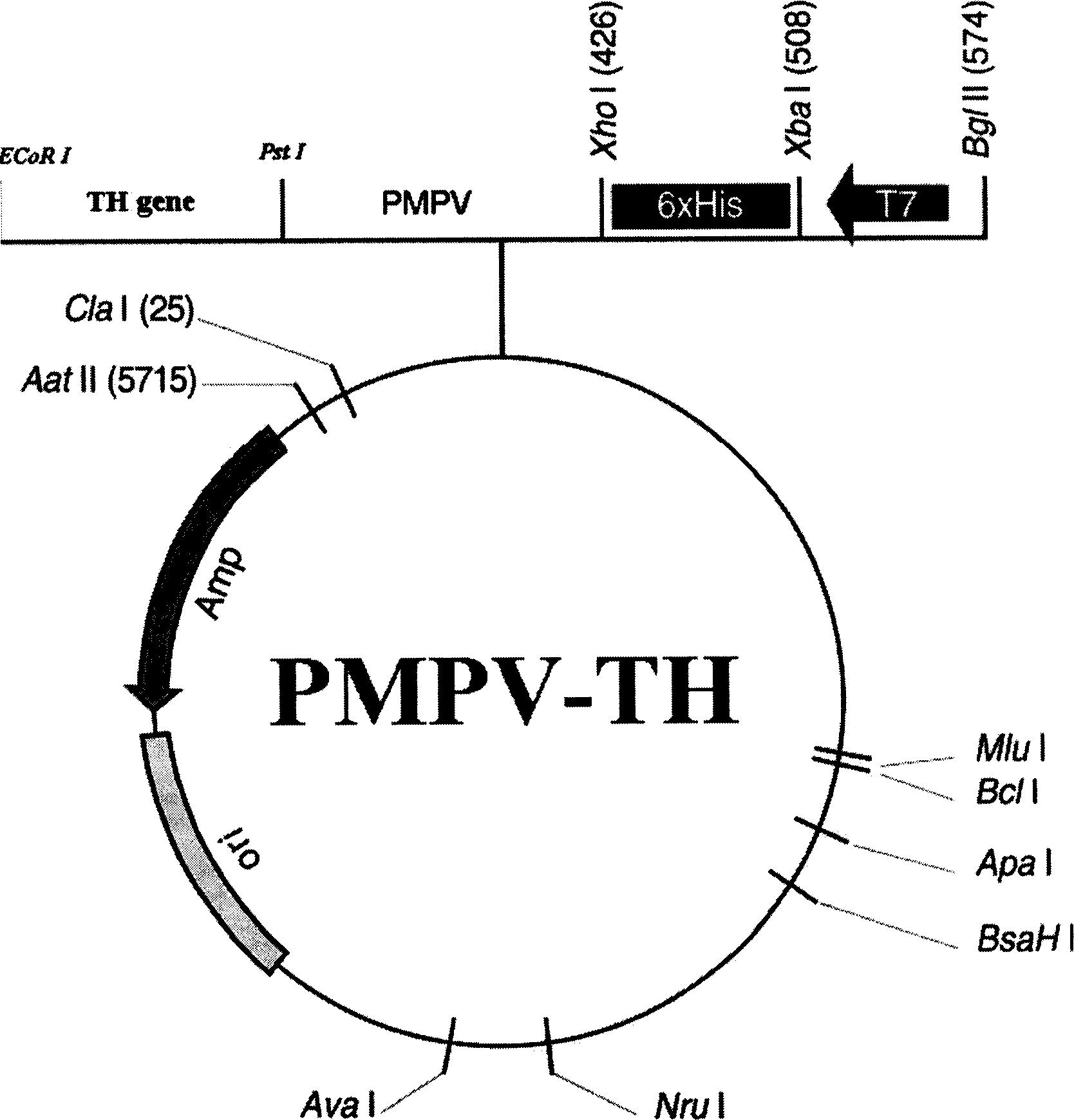
Construct
The present invention provides a construct comprising (i) a nucleotide sequence which encodes tyrosine hydroxylase (TH), (ii) a nucleotide sequence which encodes GTP- cyclohydrolase I (CH1) and (iii) a nucleotide sequence which encodes Aromatic Amino Acid Dopa Decarboxylase (AADC) wherein the nucleotide sequence encoding TH is linked to the nucleotide sequence encoding CHI such that they encode a fusion protein TH-CH1. The invention also provides a viral vector comprising such a nucleotide sequence and its use in the treatment and / or prevention of Parkinson's disease.
Owner:OXFORD BIOMEDICA (UK) LTD
Serial medicine carriers, tyrosine hydrozylase fusion protein as new carrier and its prepn
The serial medicine carriers are named polypeptide mediated penetrating vector and belongs to membrane penetrating peptide family. They have the functions of penetrating cell membrane and cytoblast membrane, and may carry medicine molecule to penetrate physiological barriers, such as cell membrane, blood brain barrier, placenta barrier, etc. The new carrier-tyrosinie hydrozylase fusion protein is one new kind of protein with the features of both new carrier and tyrosinie hydrozylase, and it may reach human brain substantia nigra pathologic change part via blood brain barrier to treat parkinsonism.
Owner:牛勃
Production of tyrosine hydroxylase positive neurons
Owner:STEM CELL THERAPEUTICS
Application of seabuckthorn berries oil in preparing medicament for treating nervus deformability disease of the old
InactiveCN101129424AIncrease the number ofIncrease beta receptorsNervous disorderPlant ingredientsAmnesiaG-csf therapy
Owner:SHAANXI TIANKUI BIOMEDICAL TECH
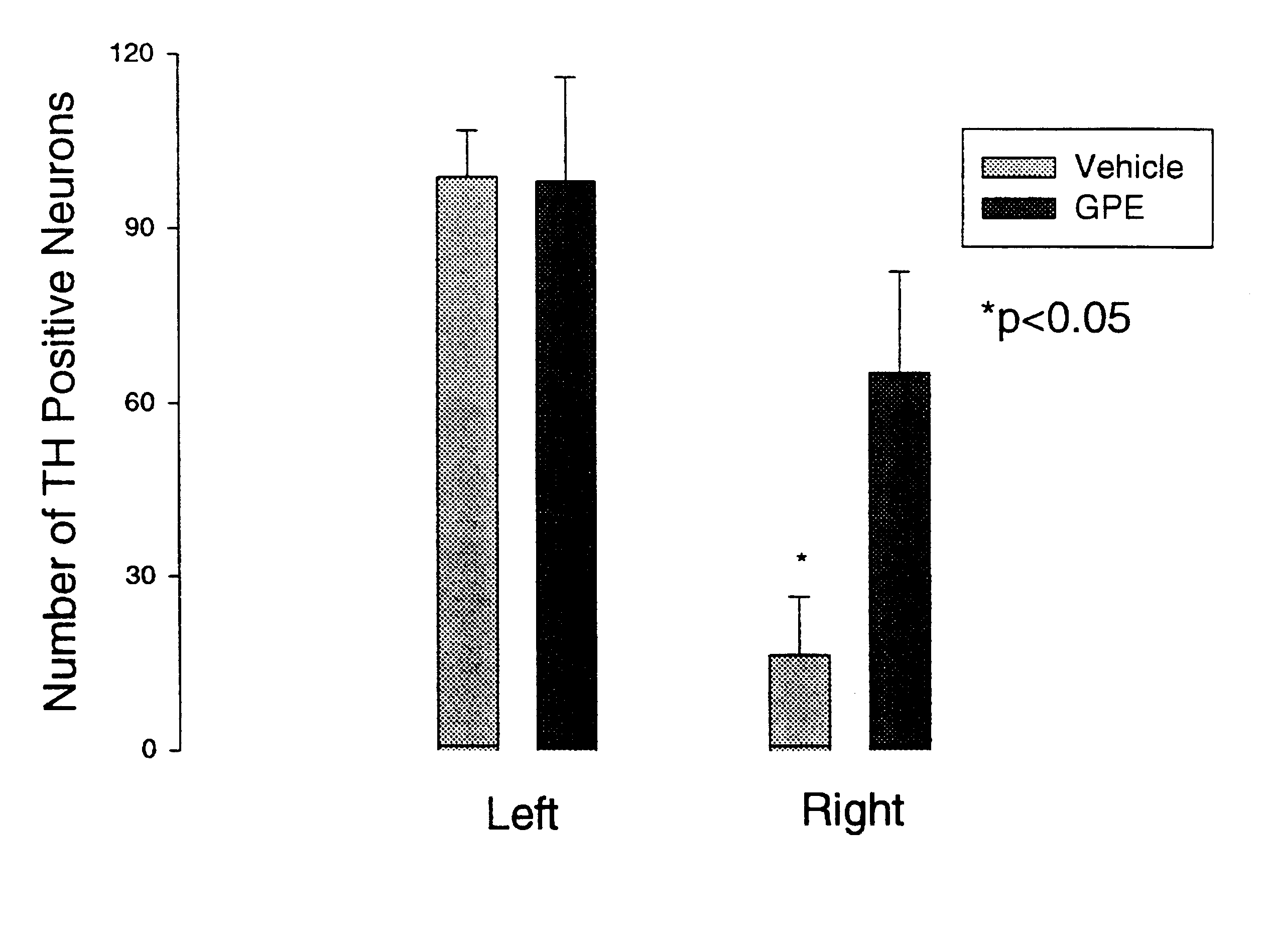
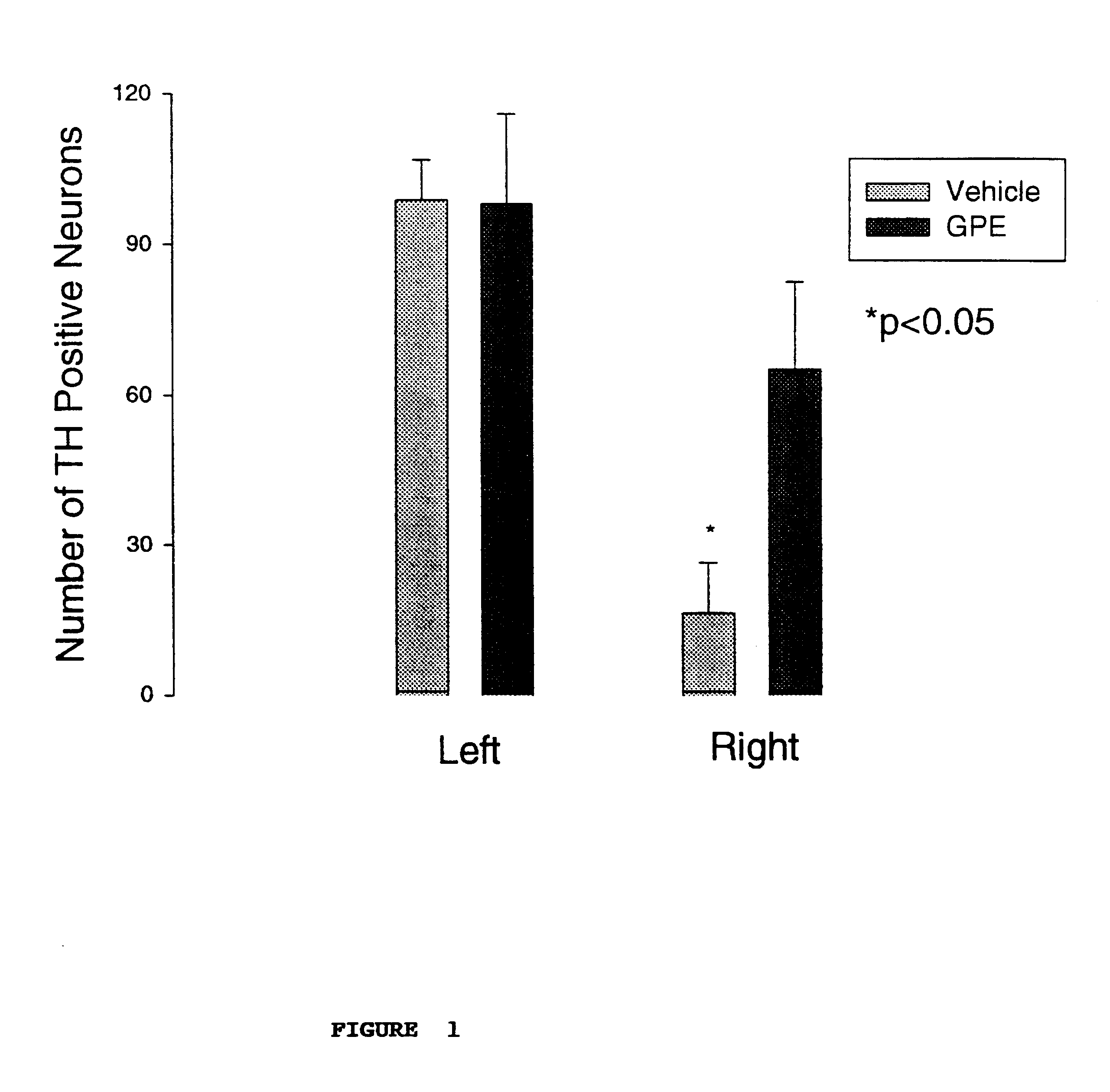
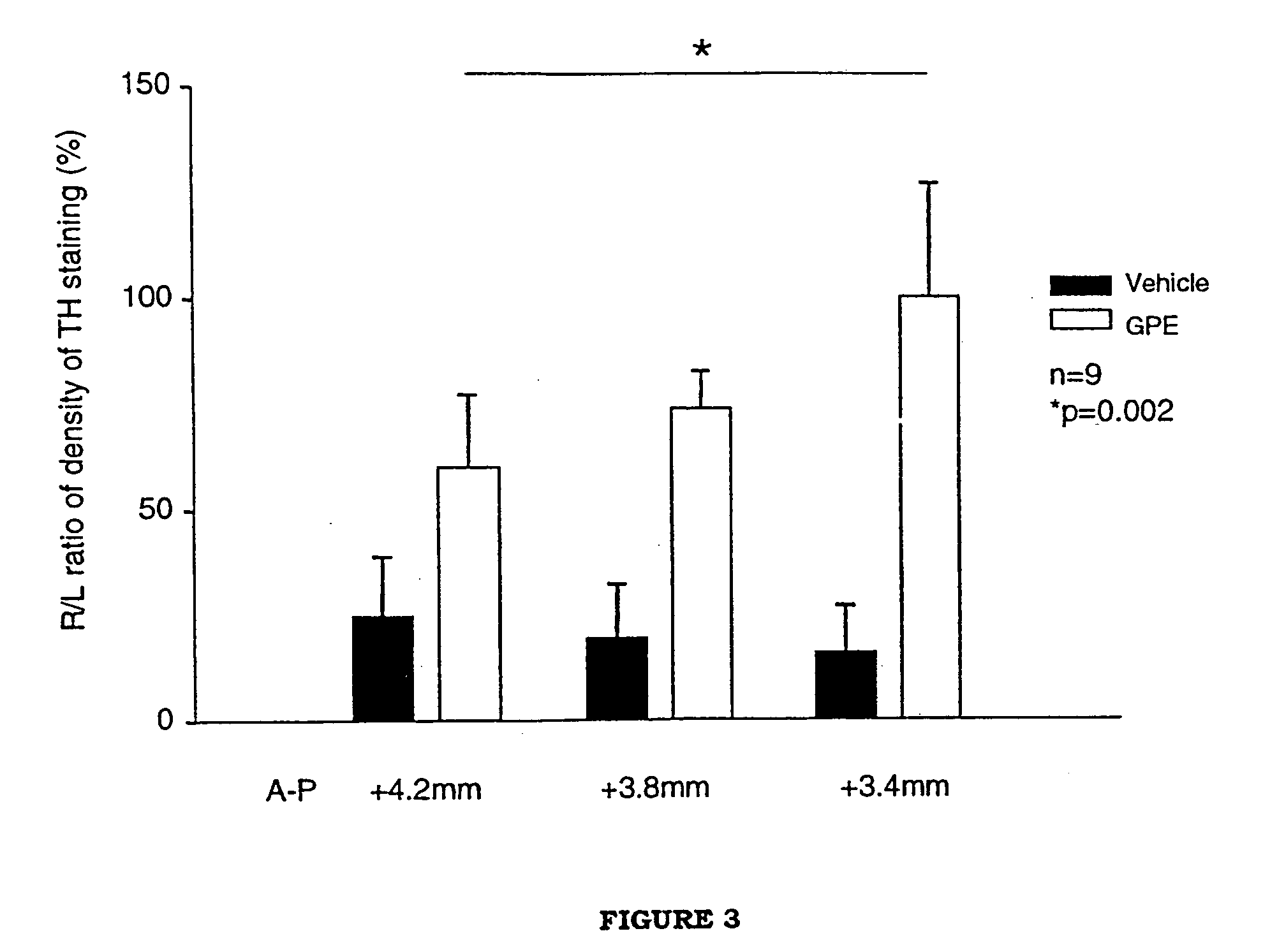
Regulation of tyrosine hydroxylase by gpe
Embodiments of this invention include methods for increasing the amount of the enzyme tyrosine hydroxylase (TH) in the central nervous system (CNS) of mammals in need of an increase in TH. Methods include the use of the tripeptide, gly-pro-glu (GPE) to increase TH in the CNS. GPE can increase the amount of TH and / or decrease the loss of TH in conditions characterized by a loss of dopamine, such as Parkinson's disease and CNS injury. GPE may act to increase the expression of TH or by inhibiting a decrease in TH expression within the CNS or by inhibiting the loss of TH-containing neurons within the CNS. By increasing the amounts of TH in the CNS, GPE can increase the amount of the neurotransmitter, dopamine, in areas of the CNS responsible for adverse symptoms of neural injury or disease.
Owner:NEUREN PHARMA LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com