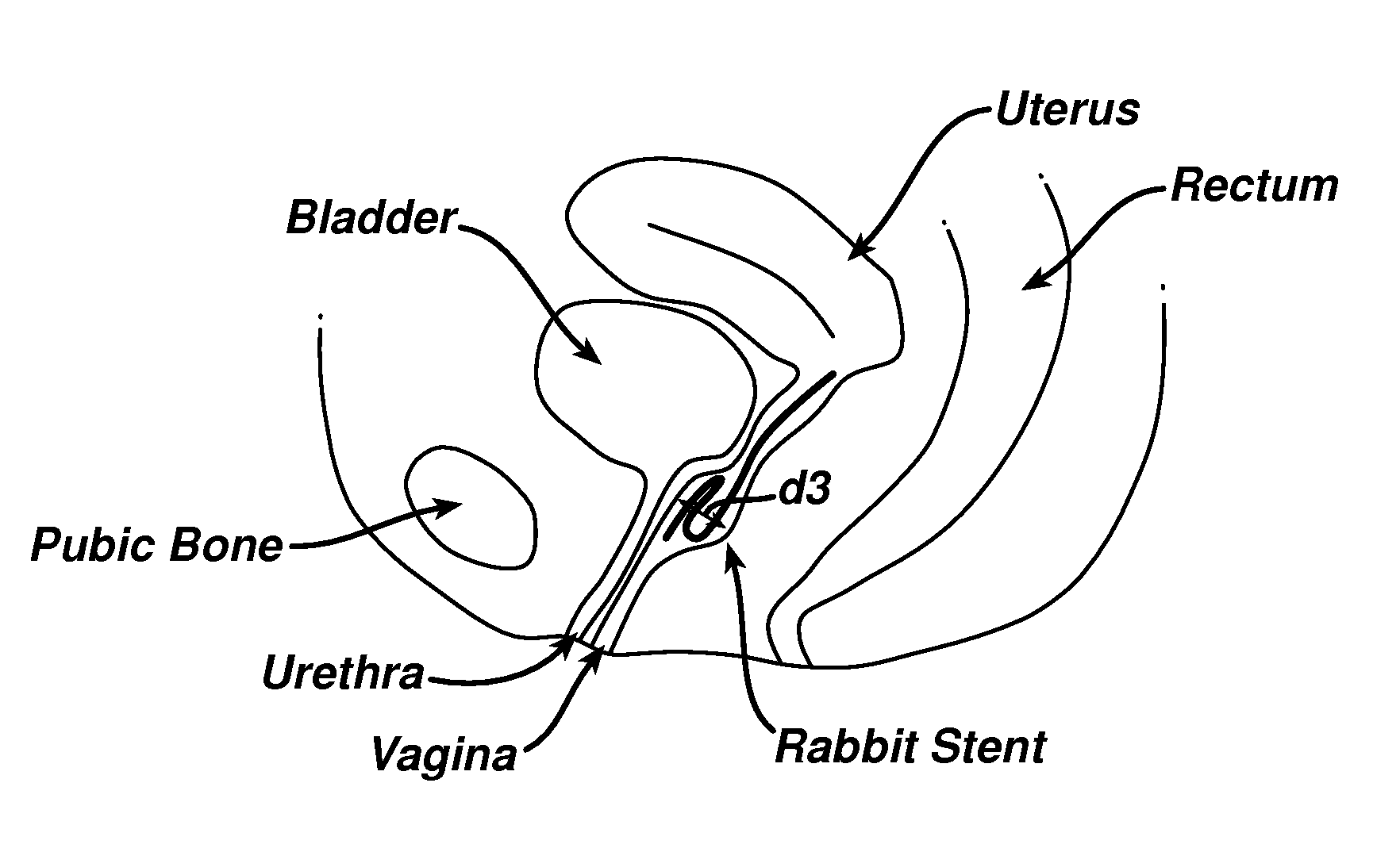
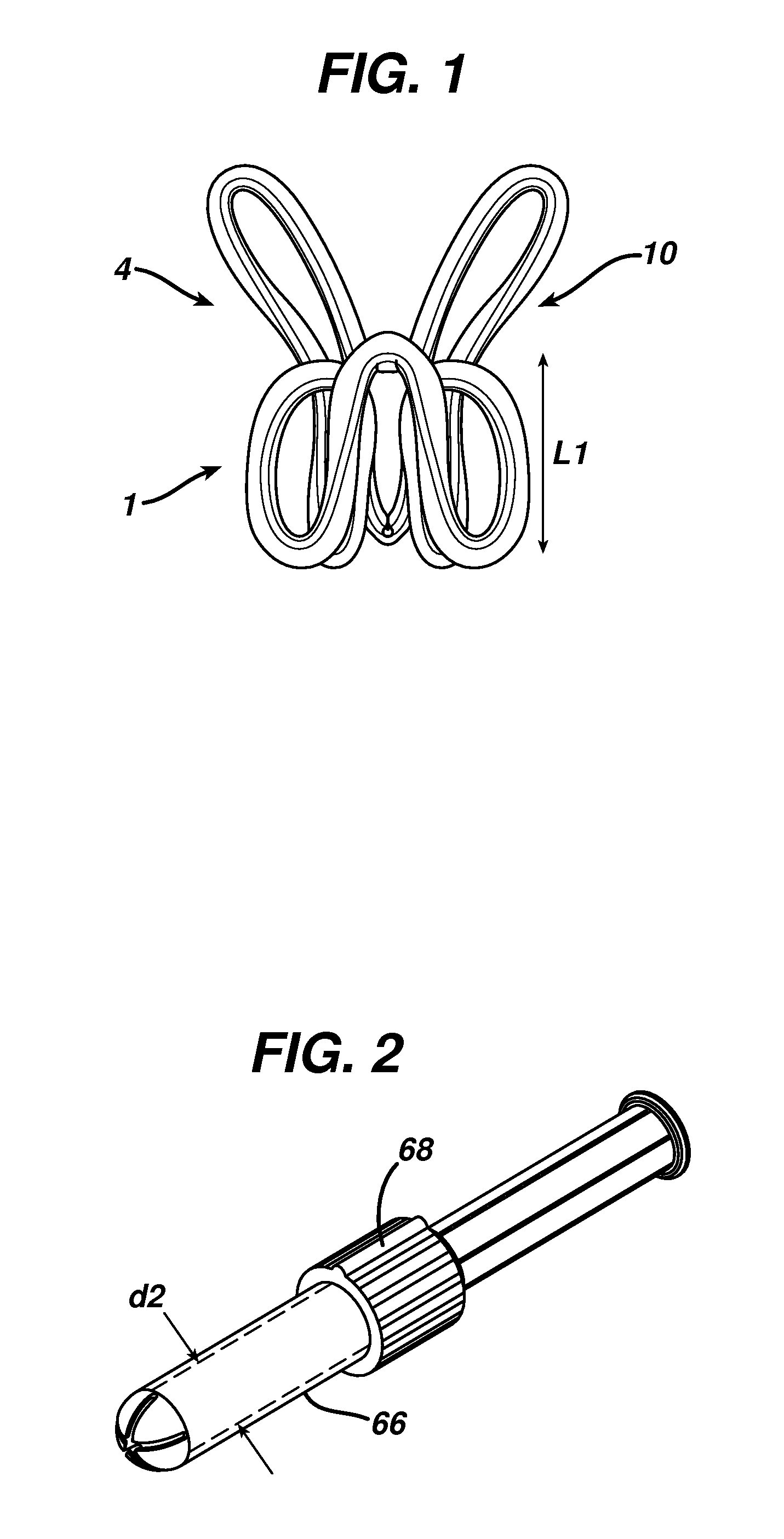
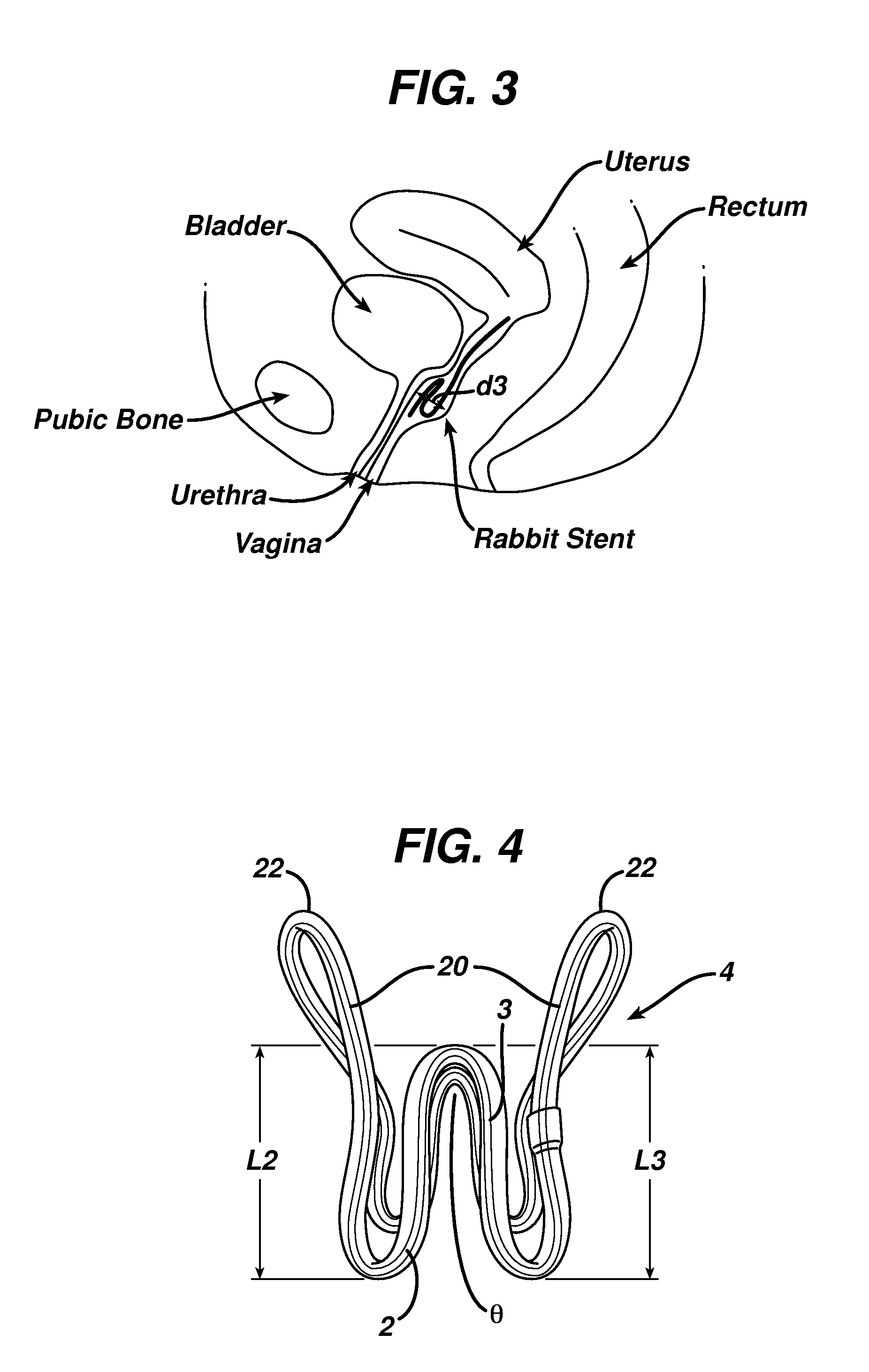
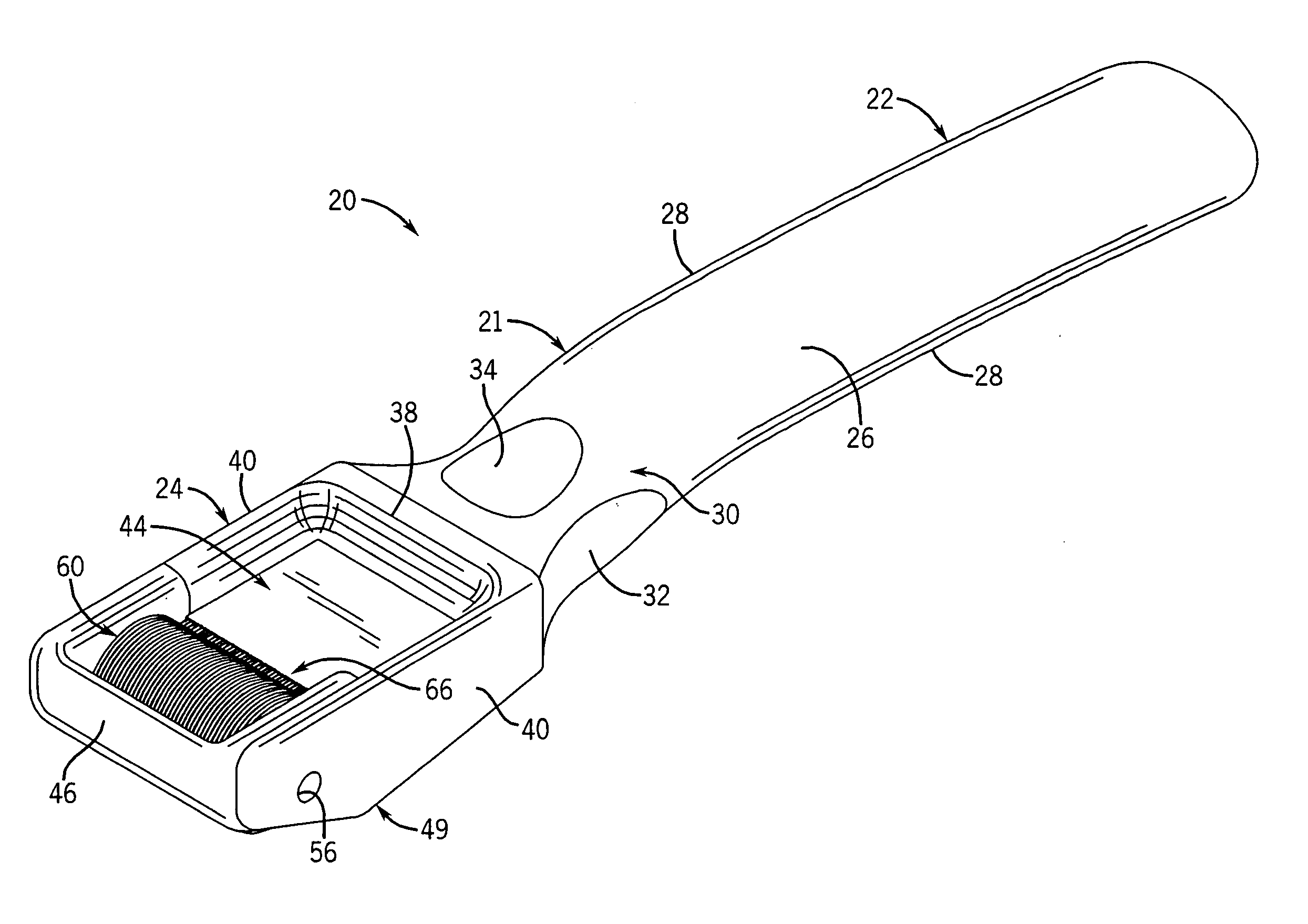
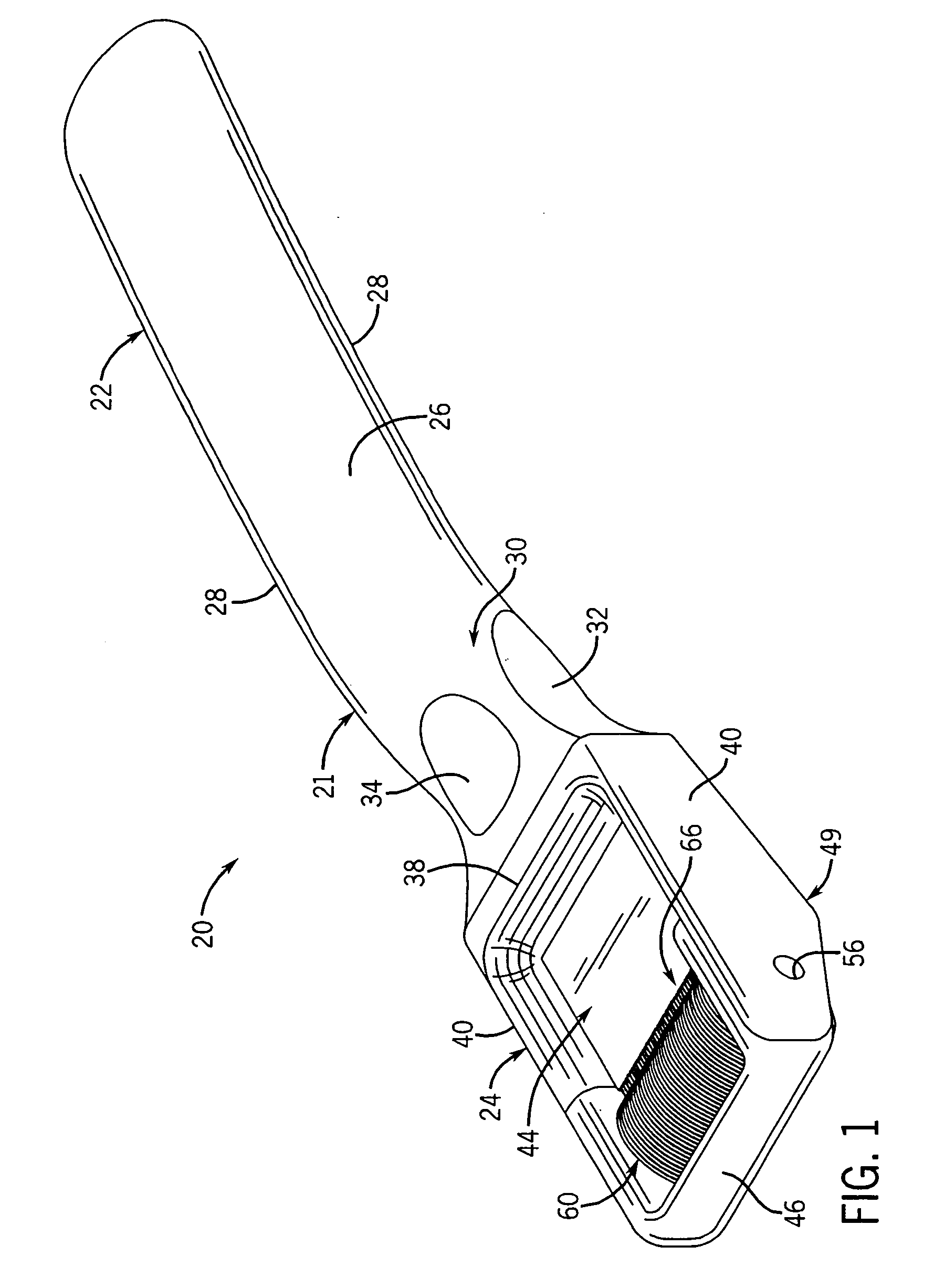
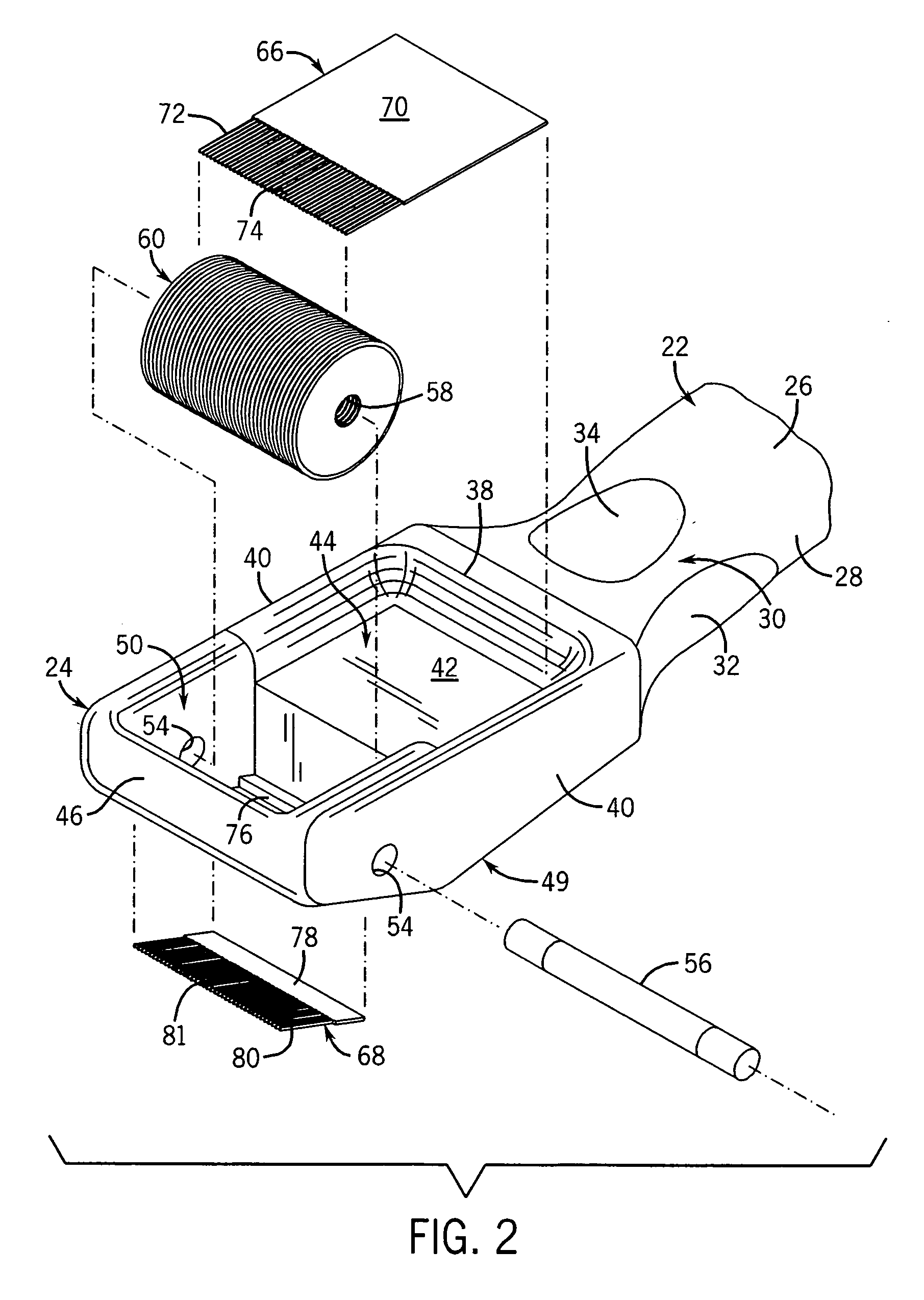
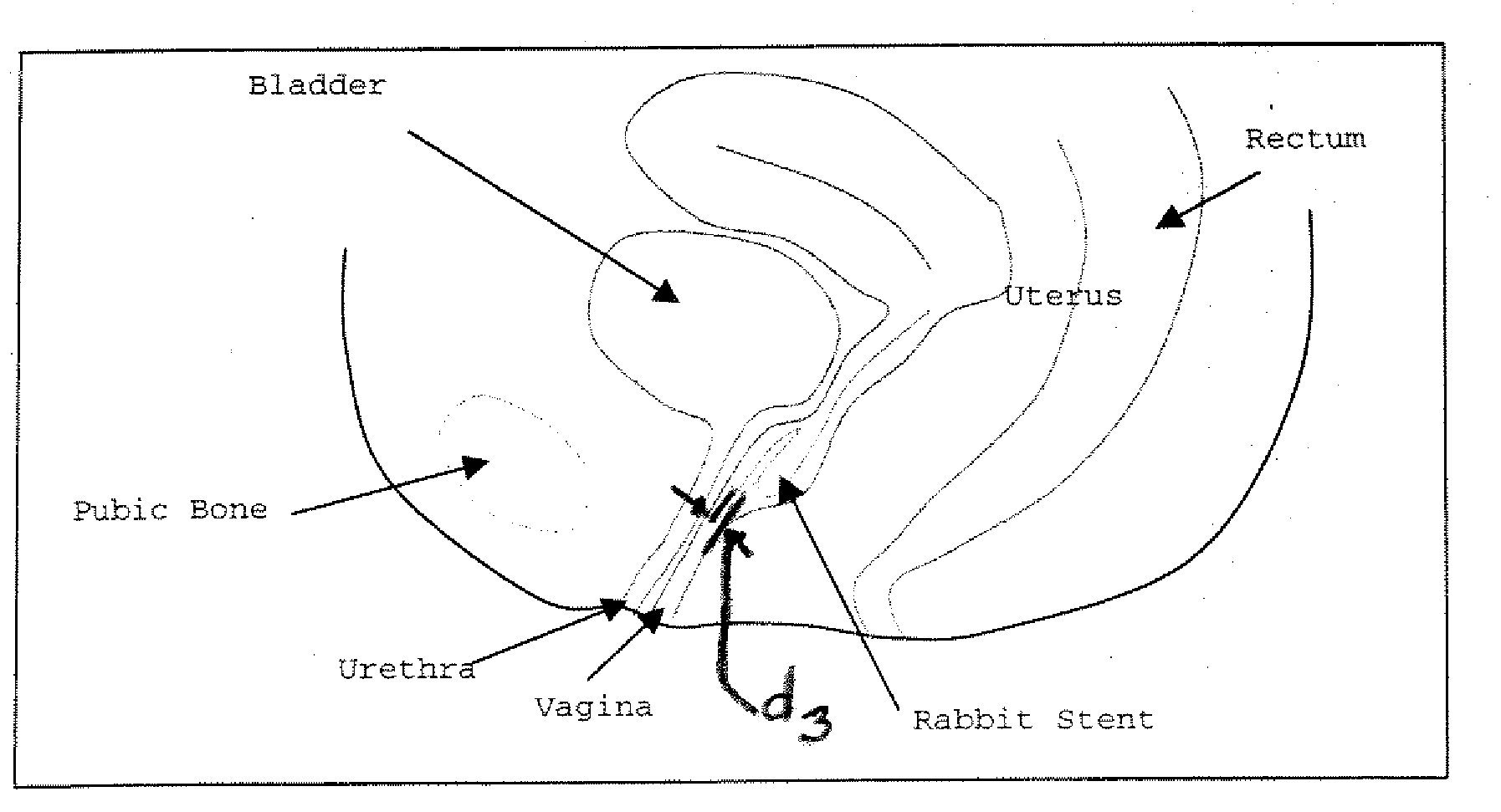
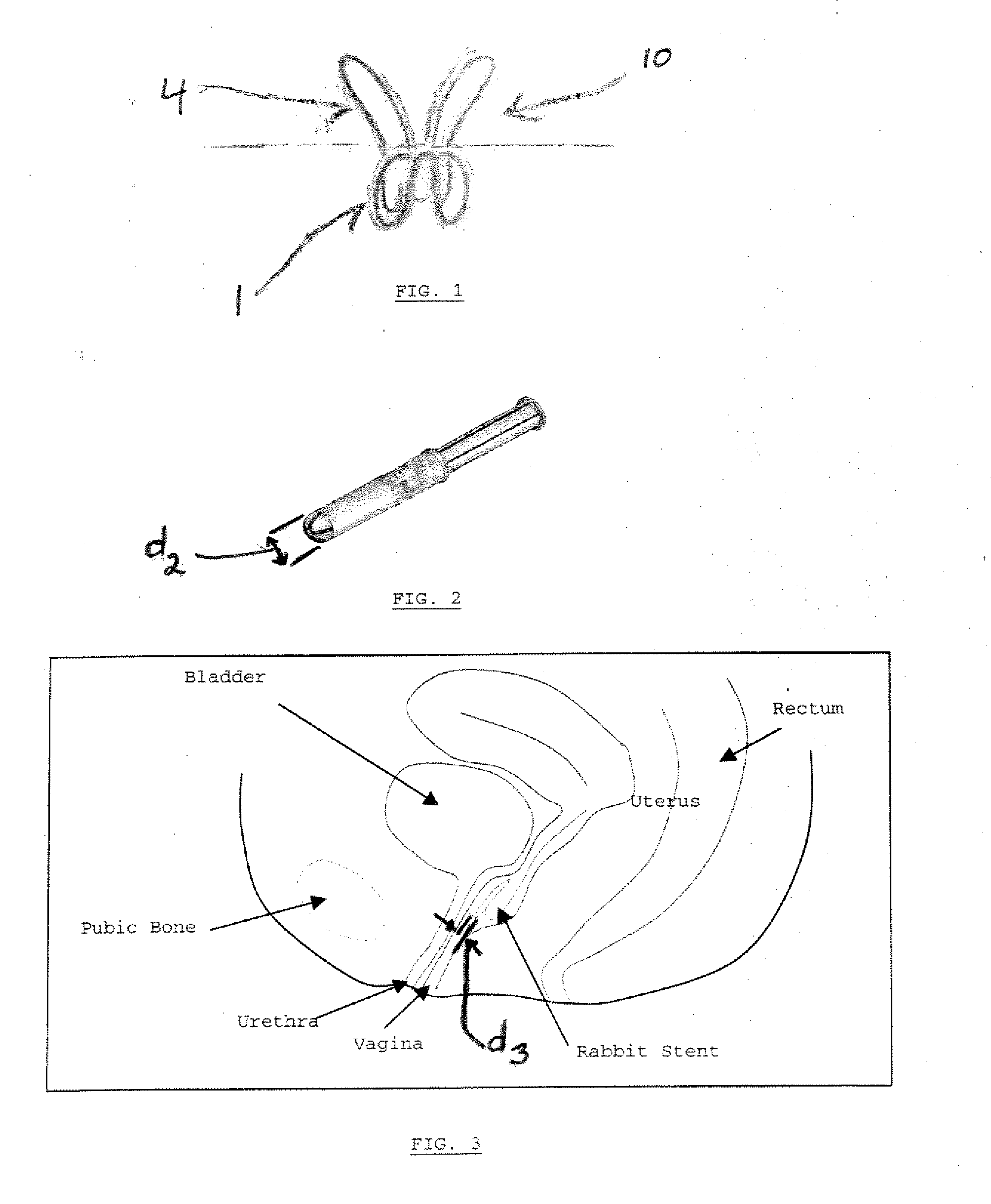
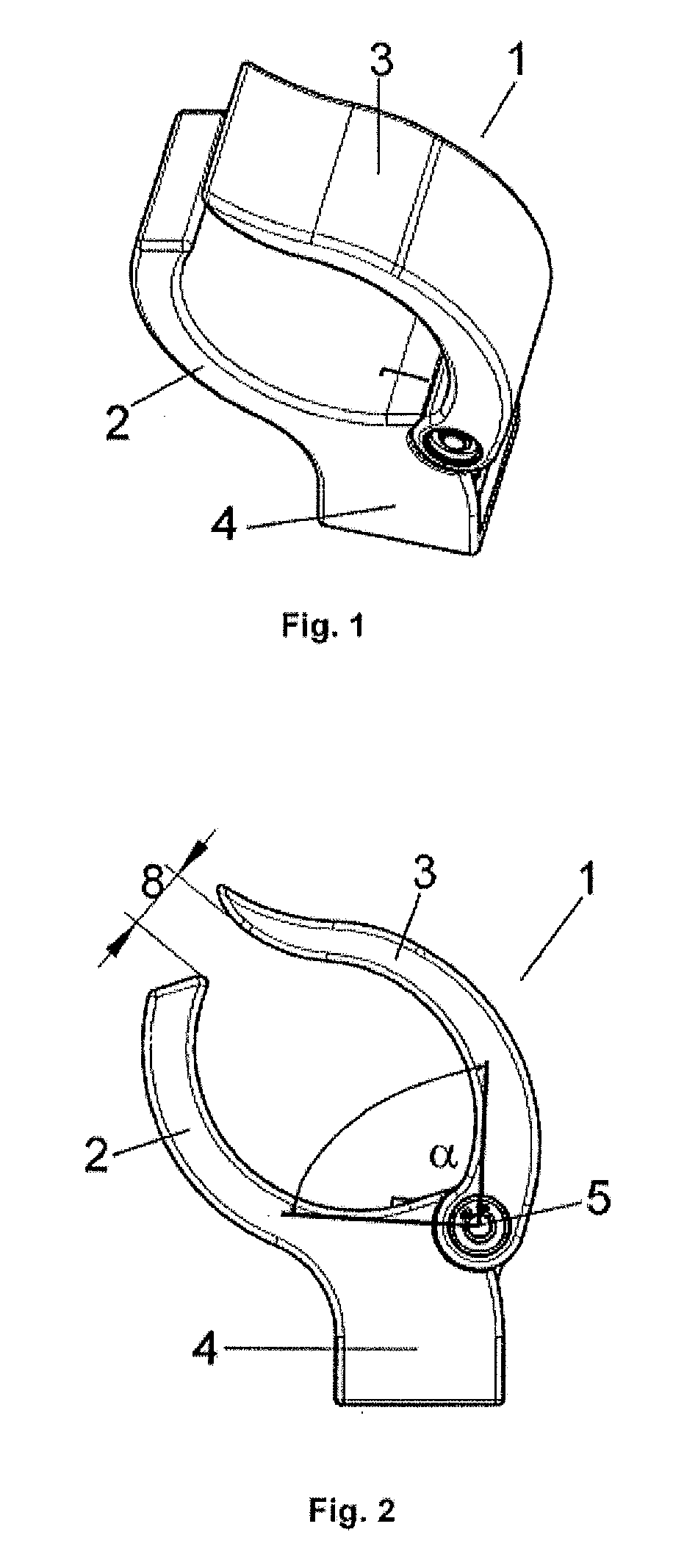
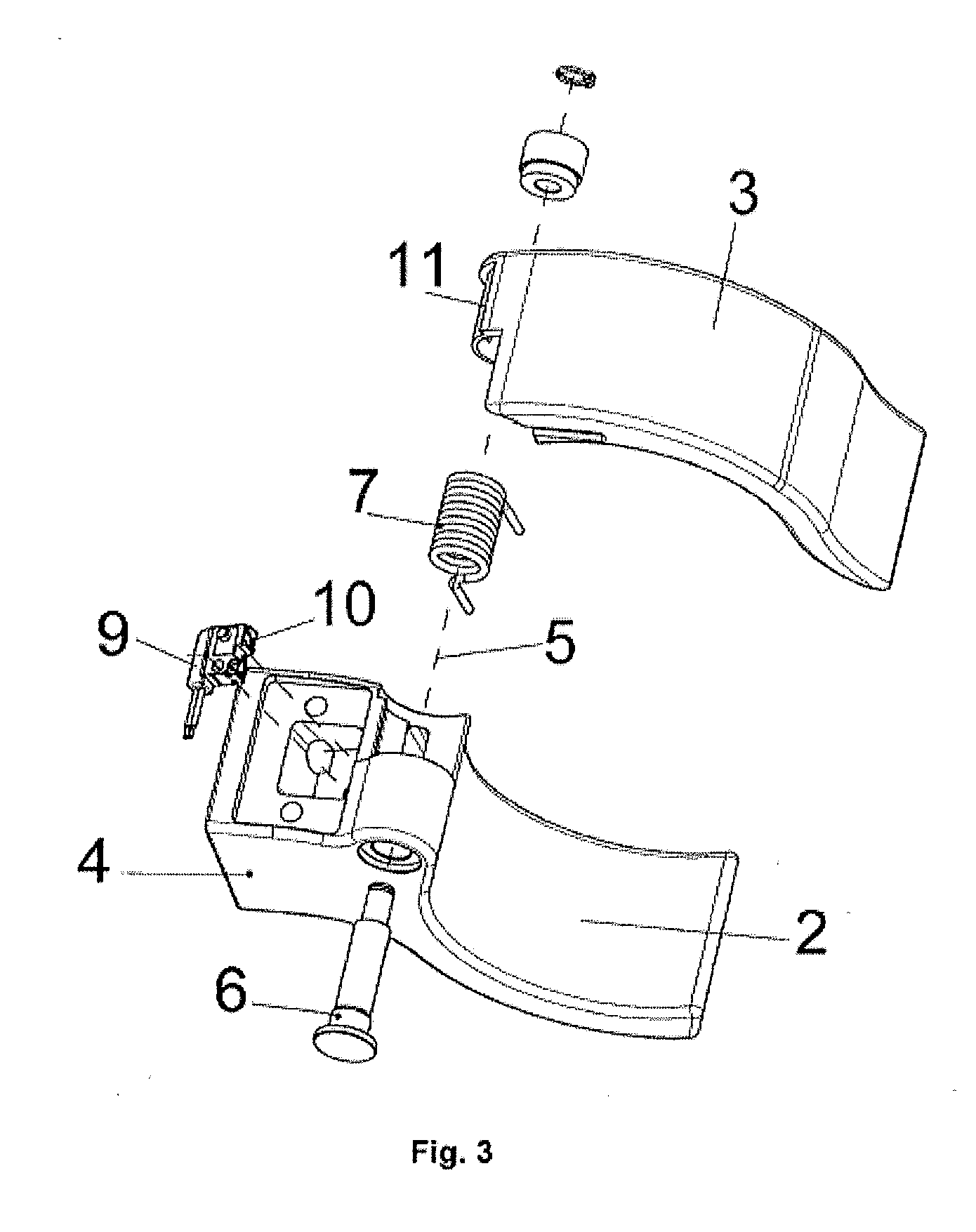
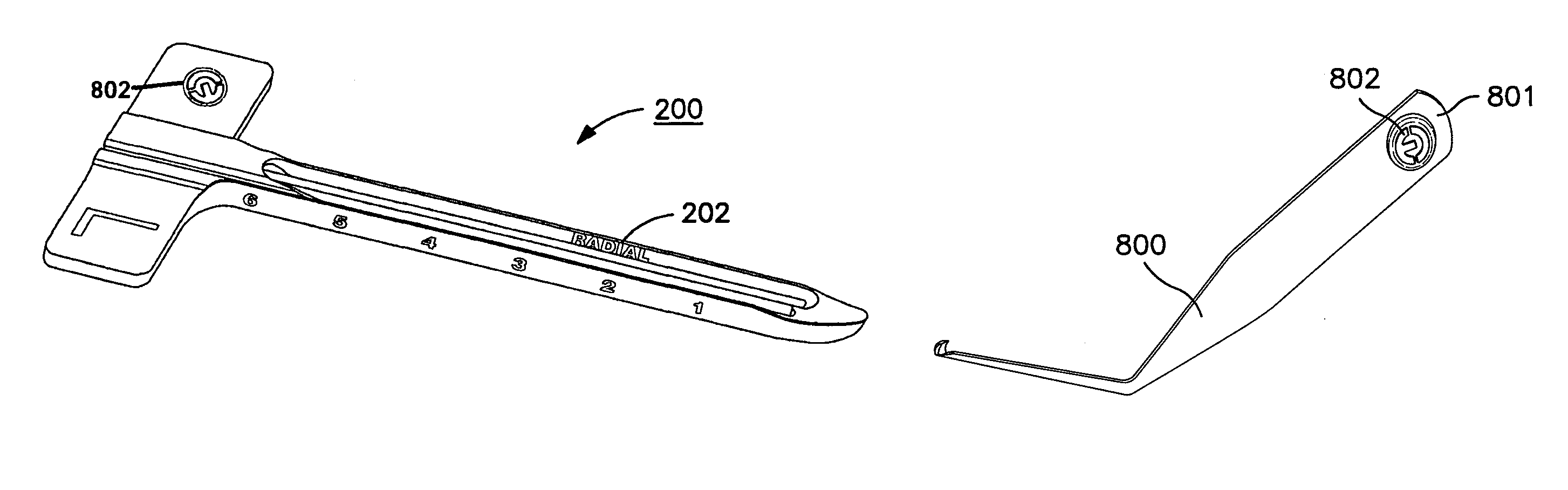
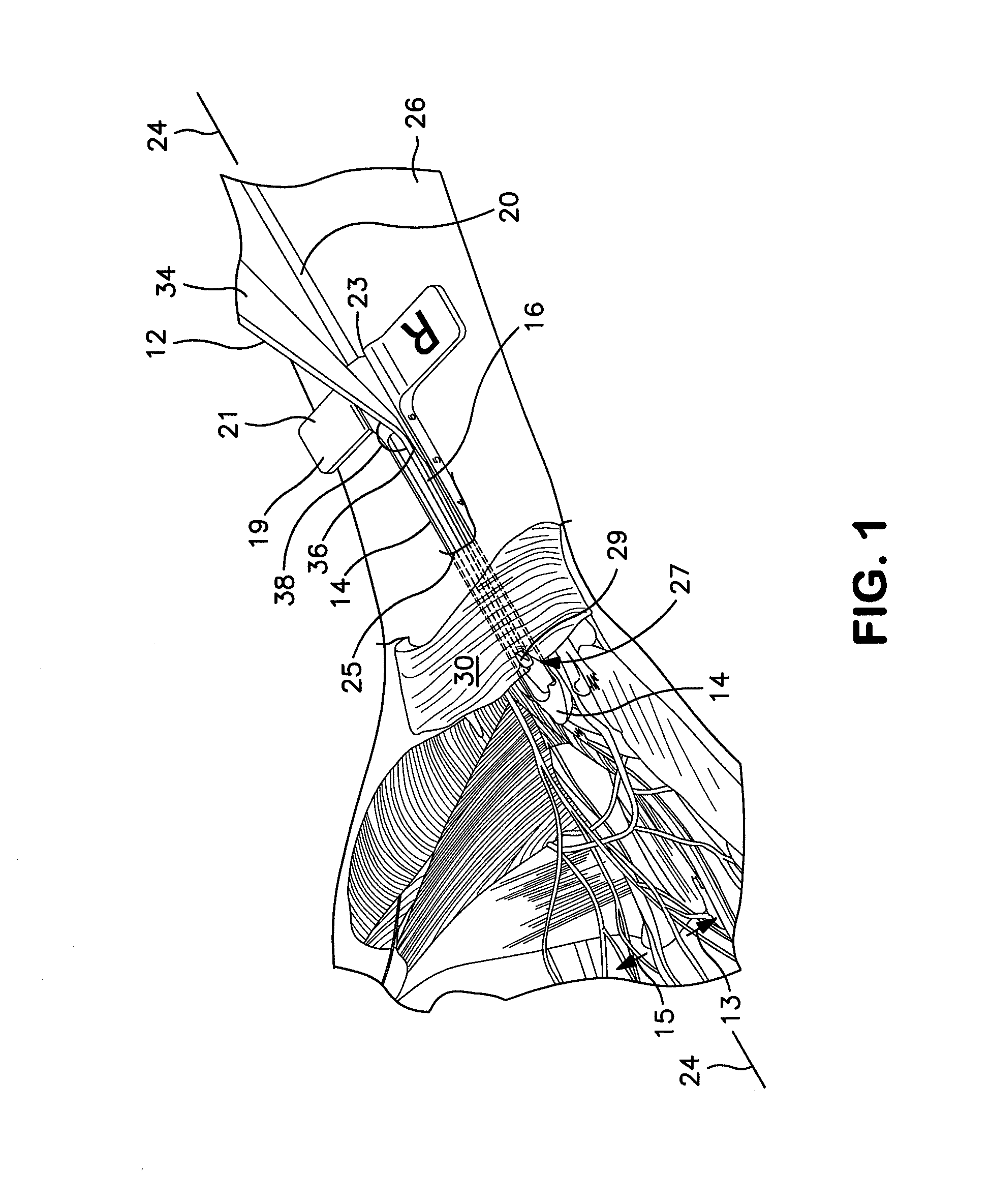
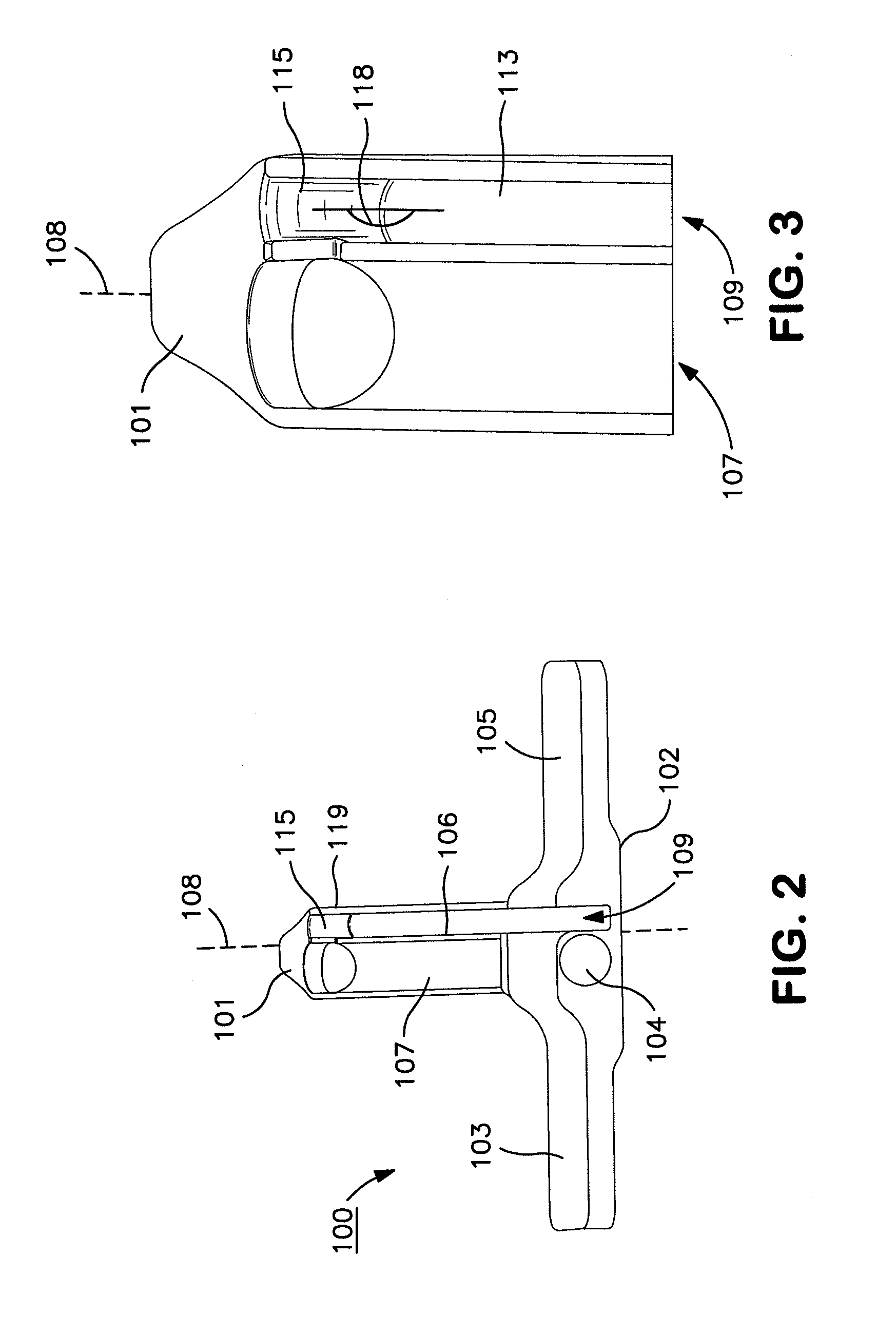
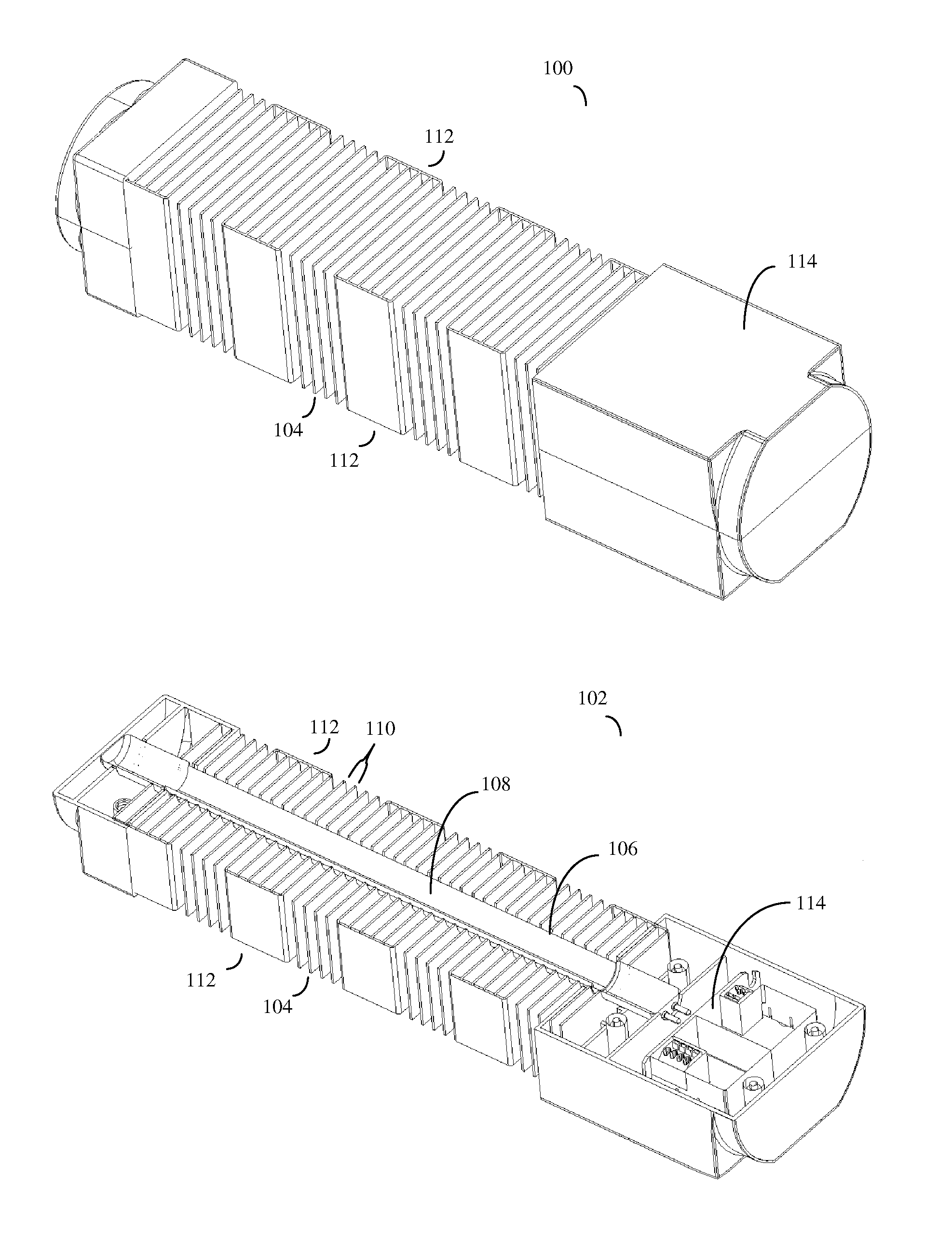
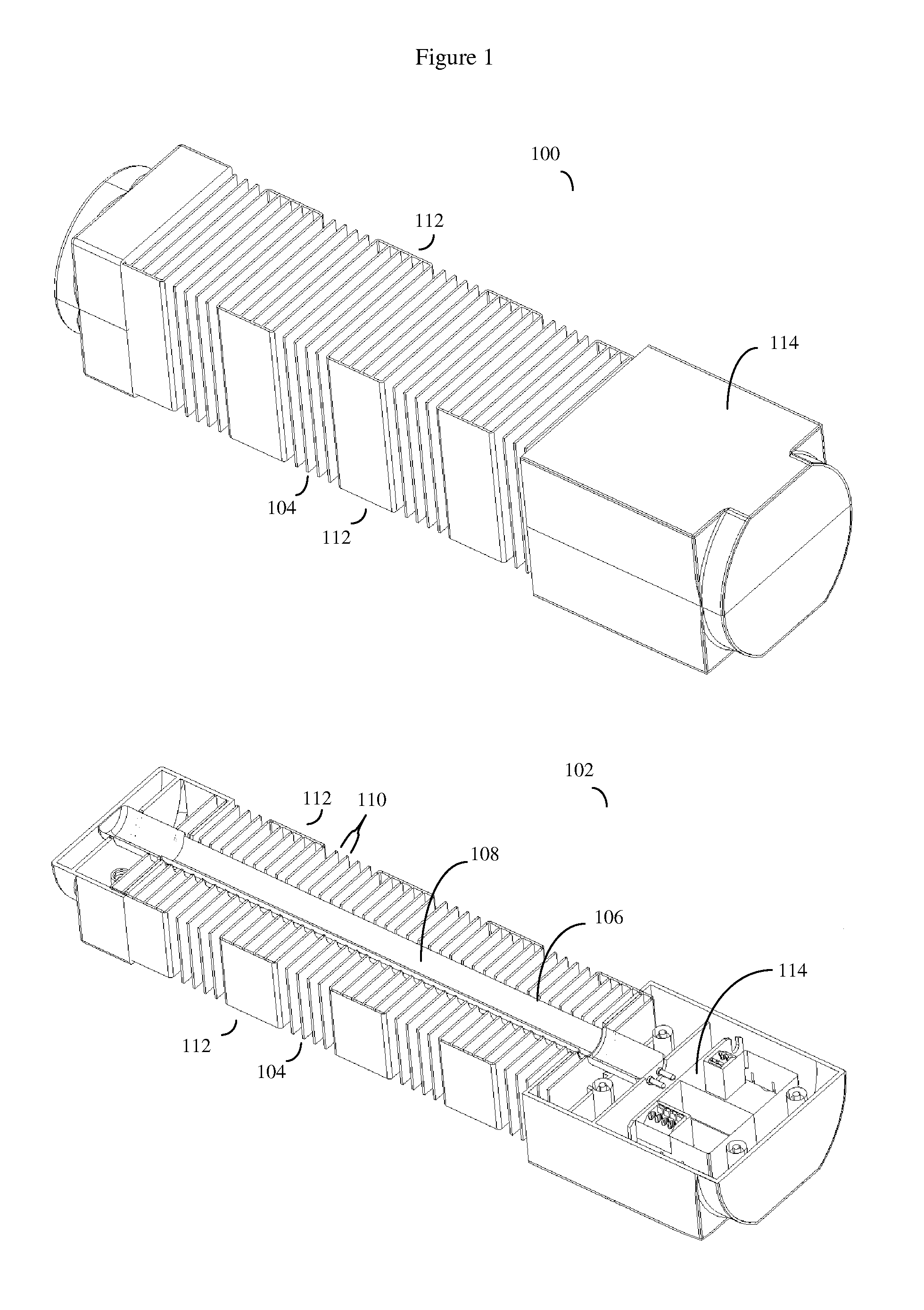
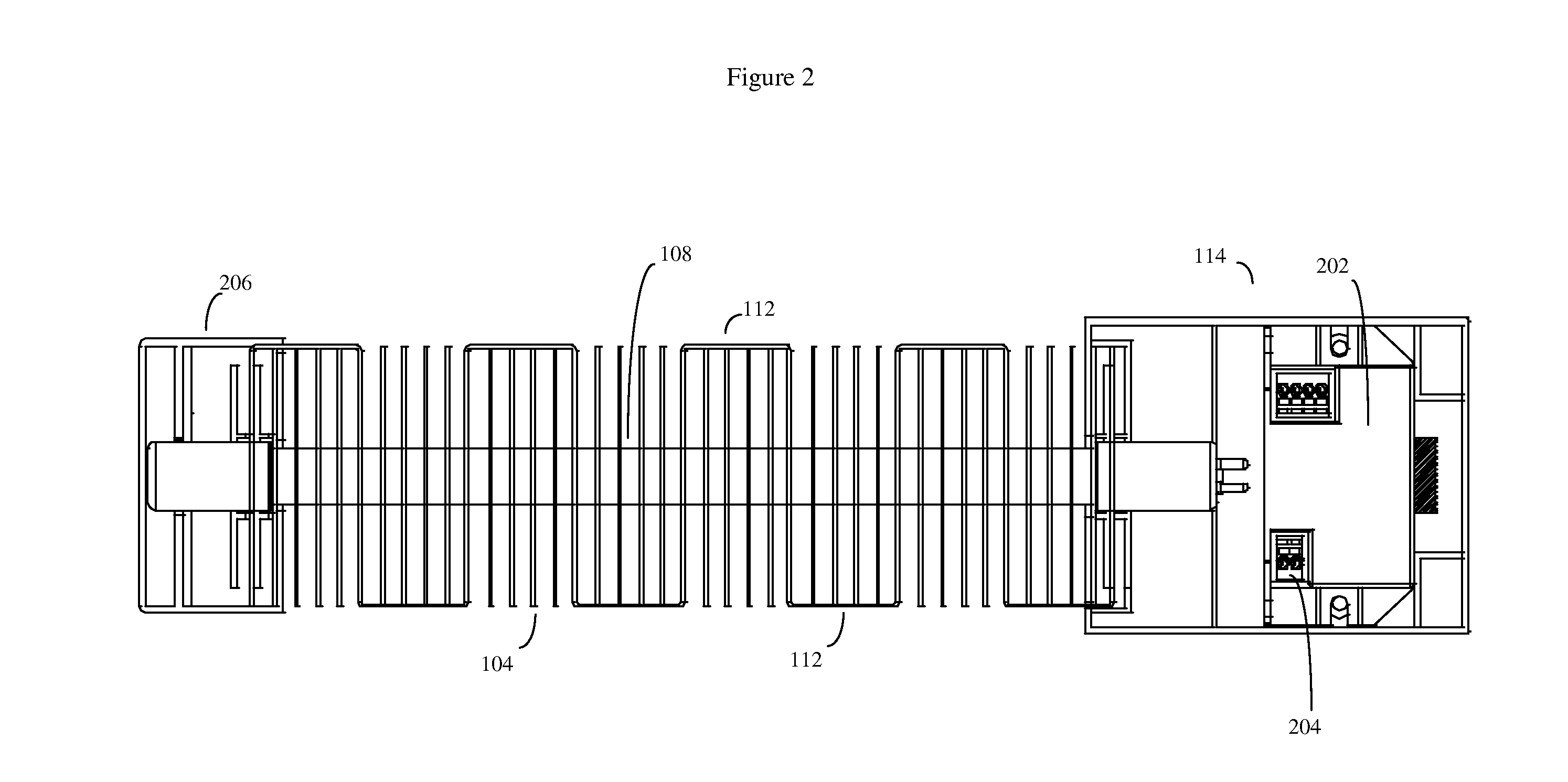
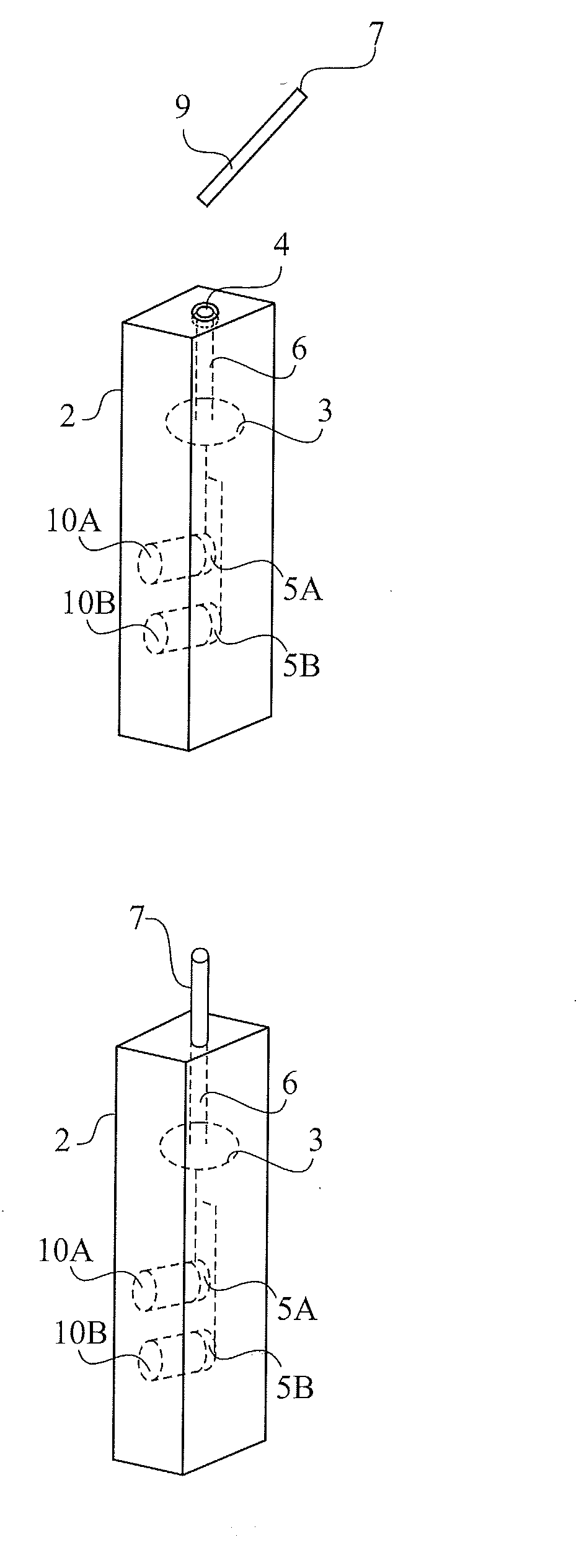
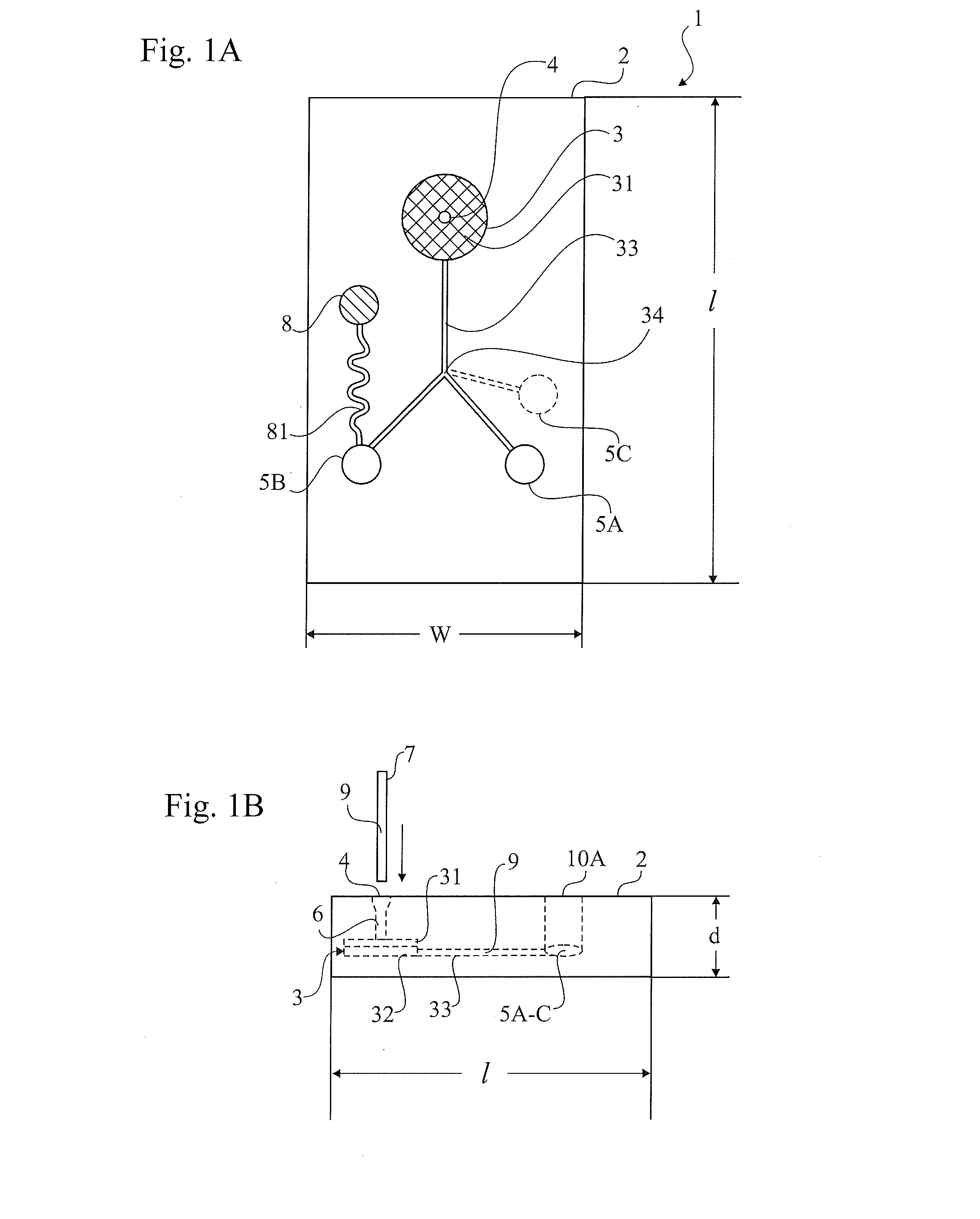
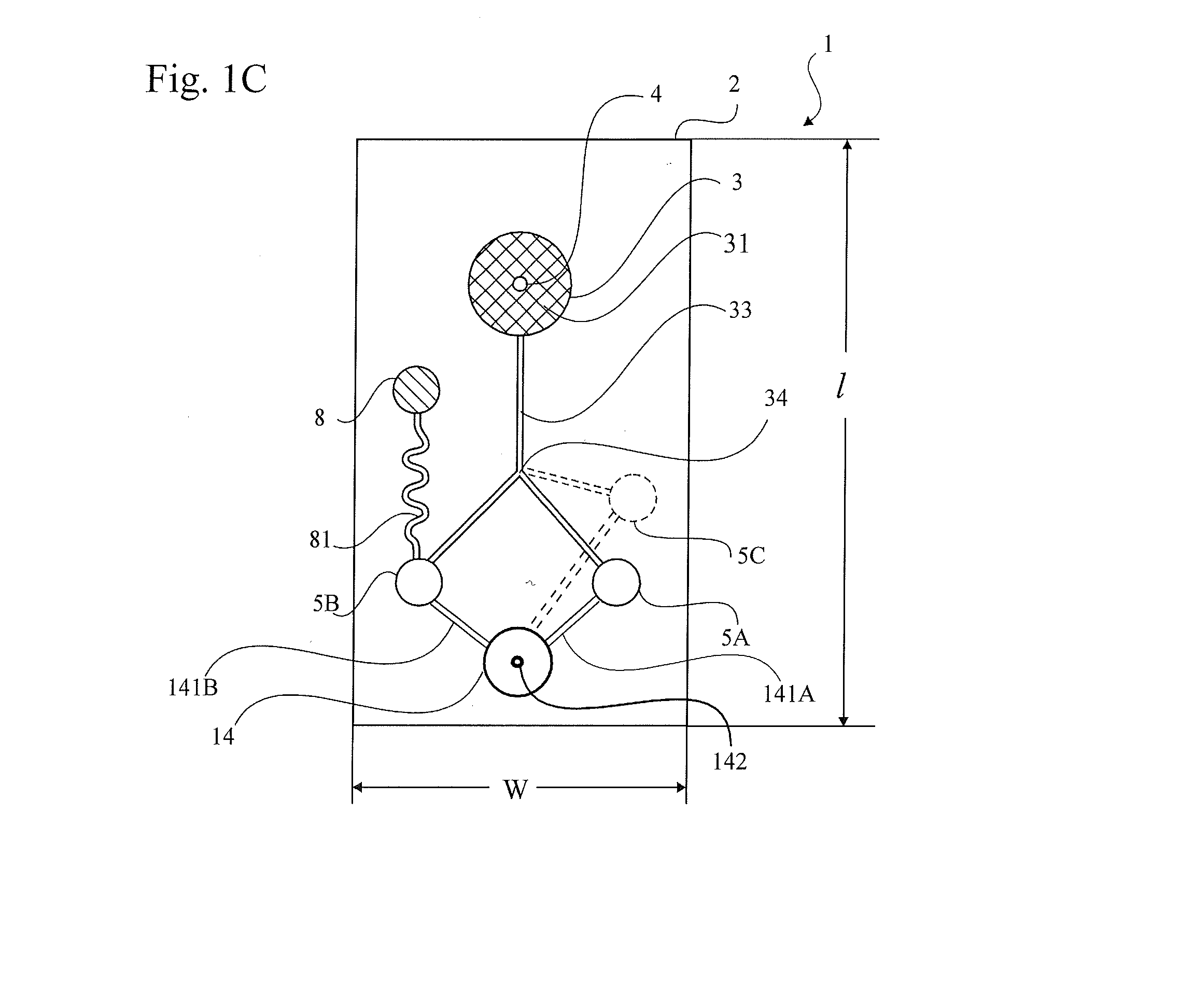
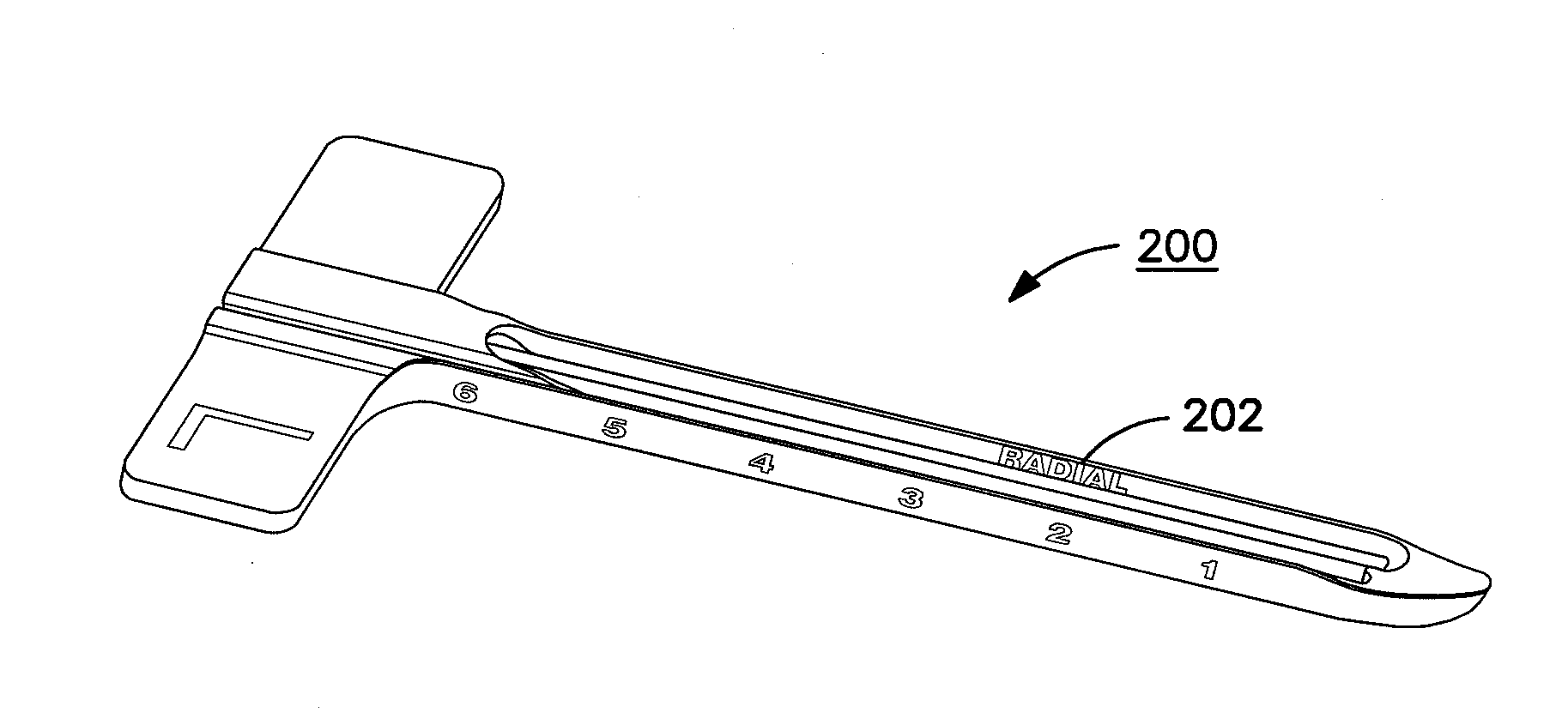
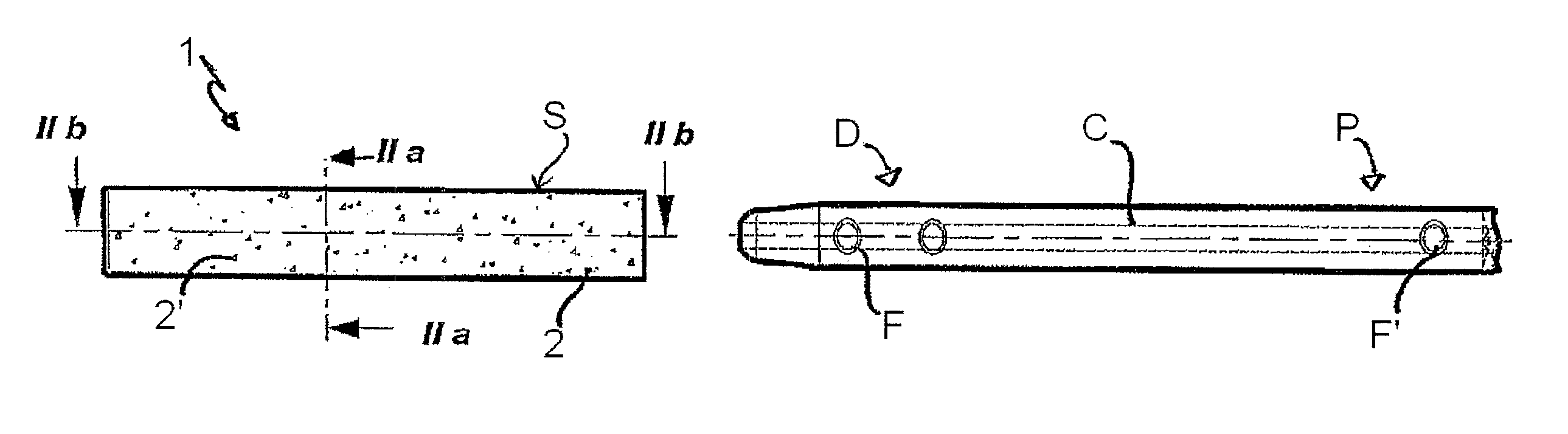
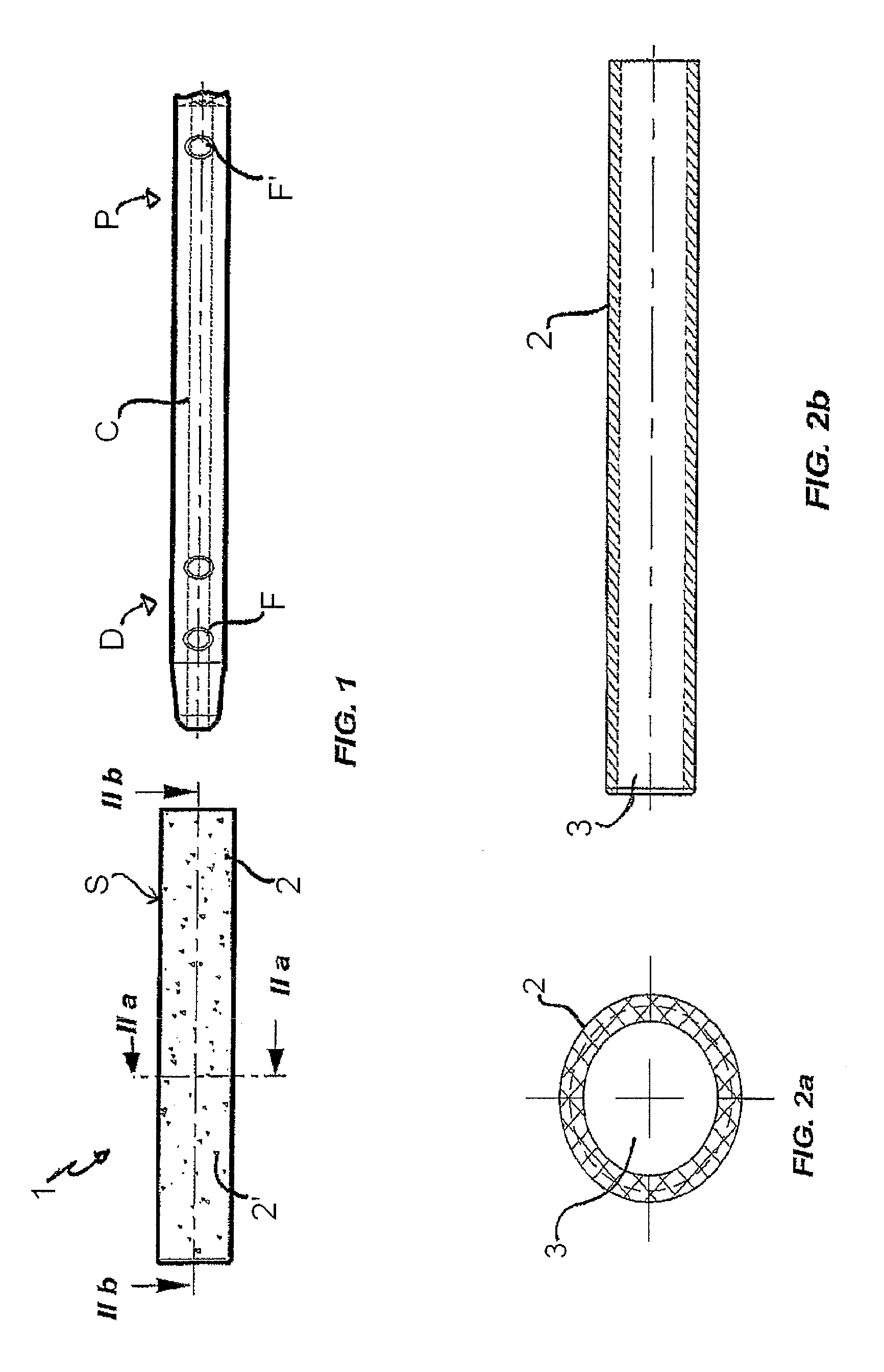
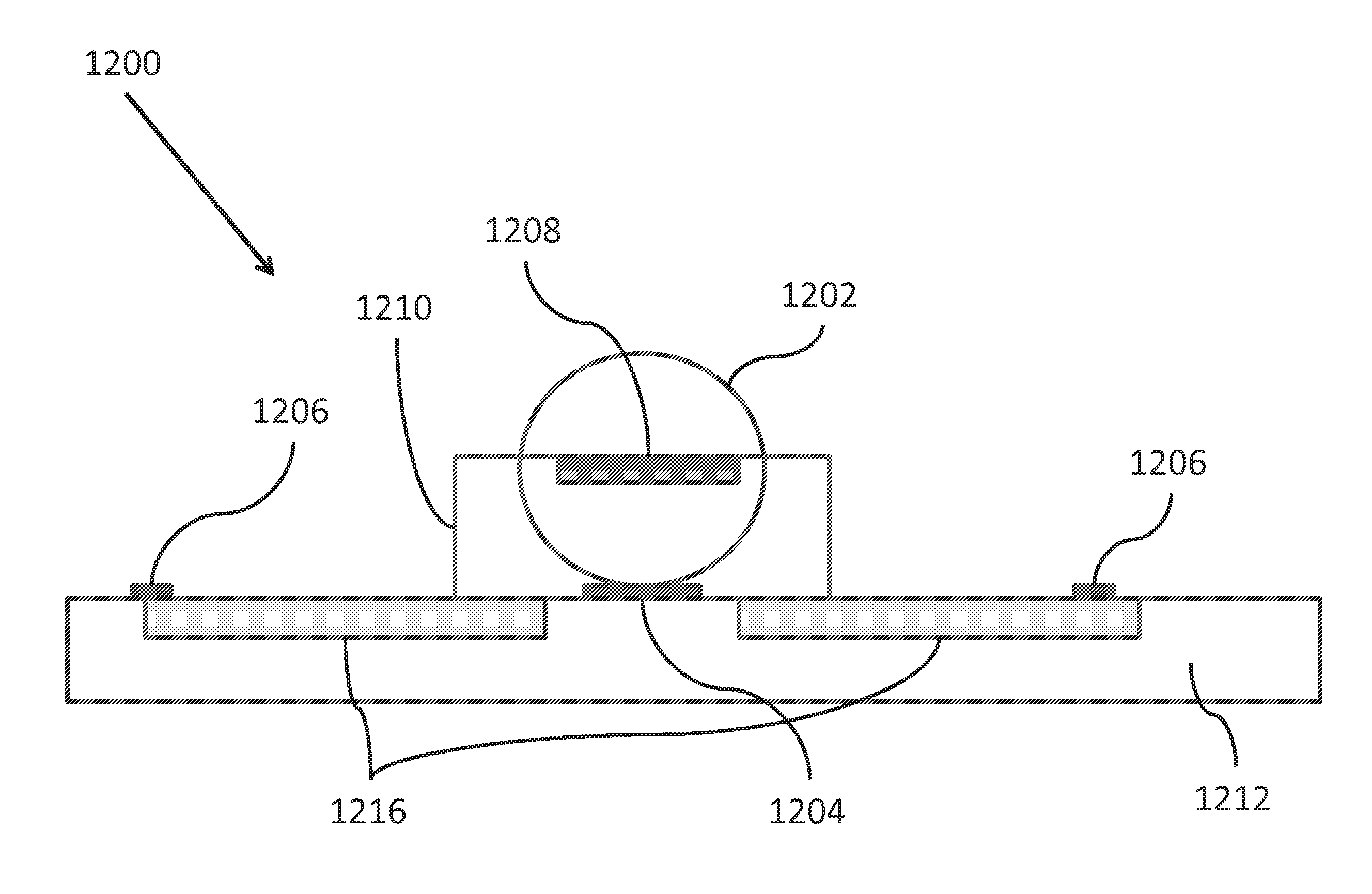
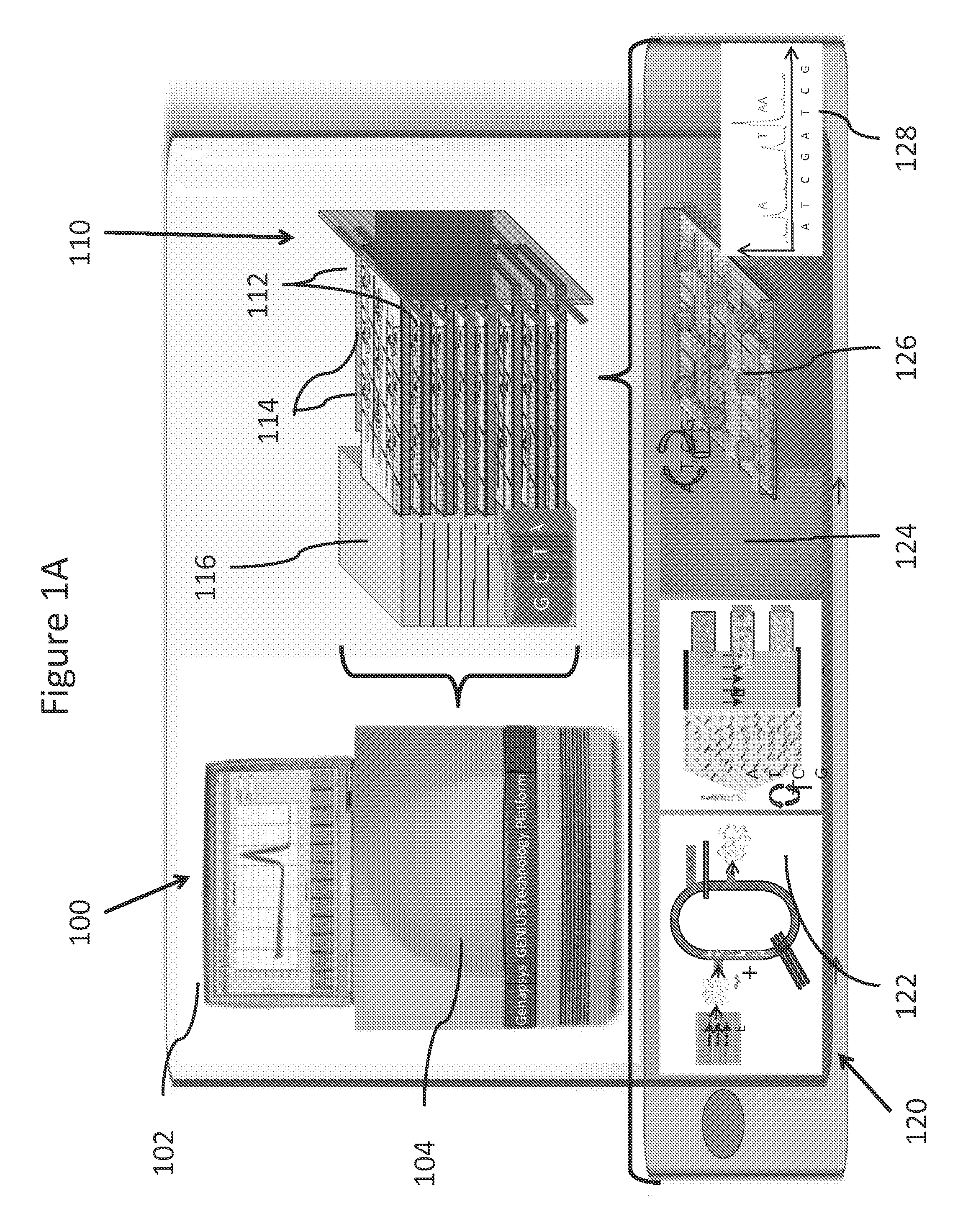
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
118 results about "Single-Use Device" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Single-use medical device reprocessing is the disinfection, cleaning, remanufacturing, testing, packaging and labeling, and sterilization among other steps, of a used, (or, in some cases, a device opened from its original packaging but unused), medical device to be put in service again.
Temperature measuring device and temperature measuring method
InactiveUS20050141591A1Low production costFree replacementThermometer detailsElectric signal transmission systemsSingle-Use DeviceMeasurement device
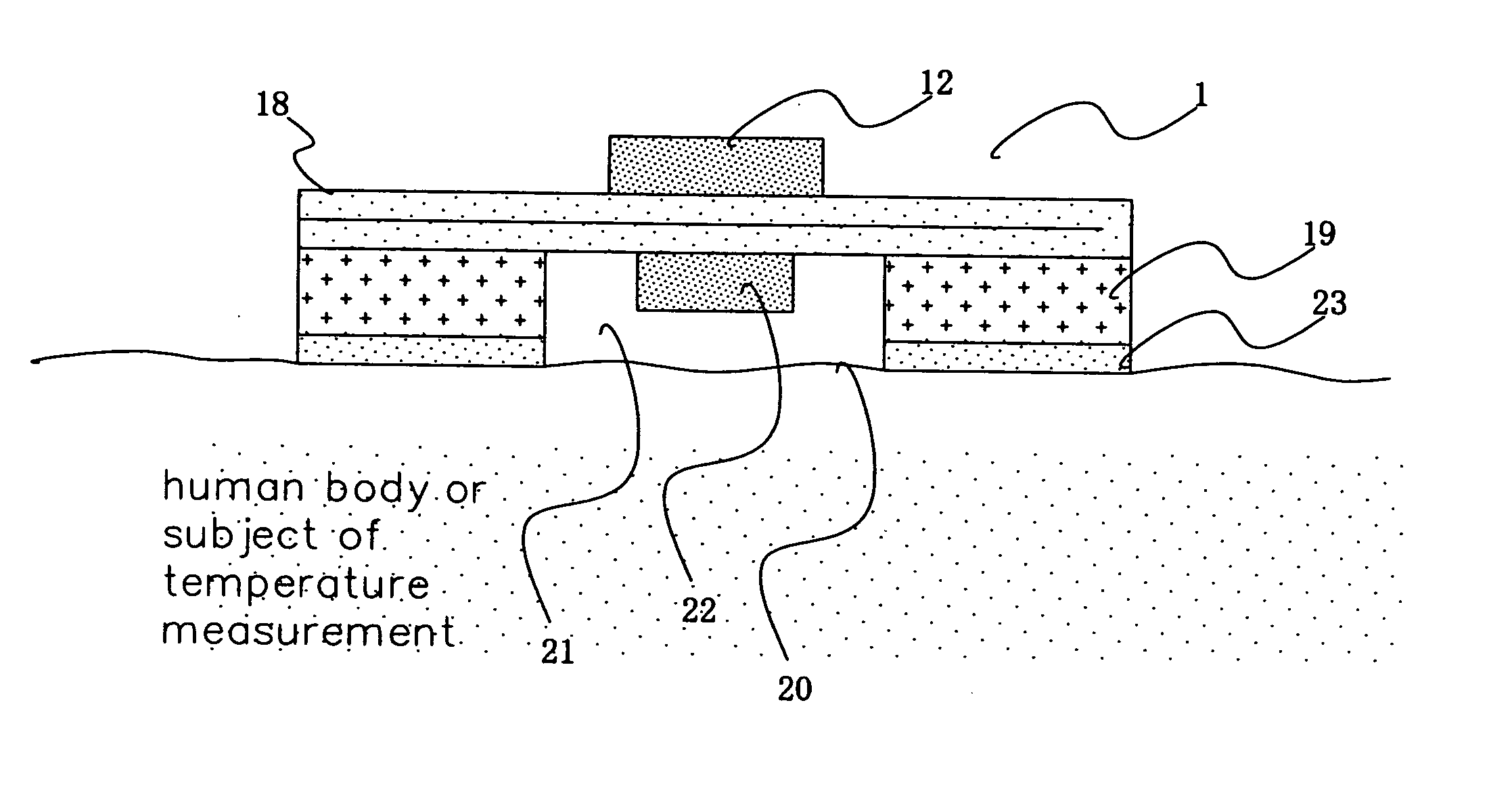
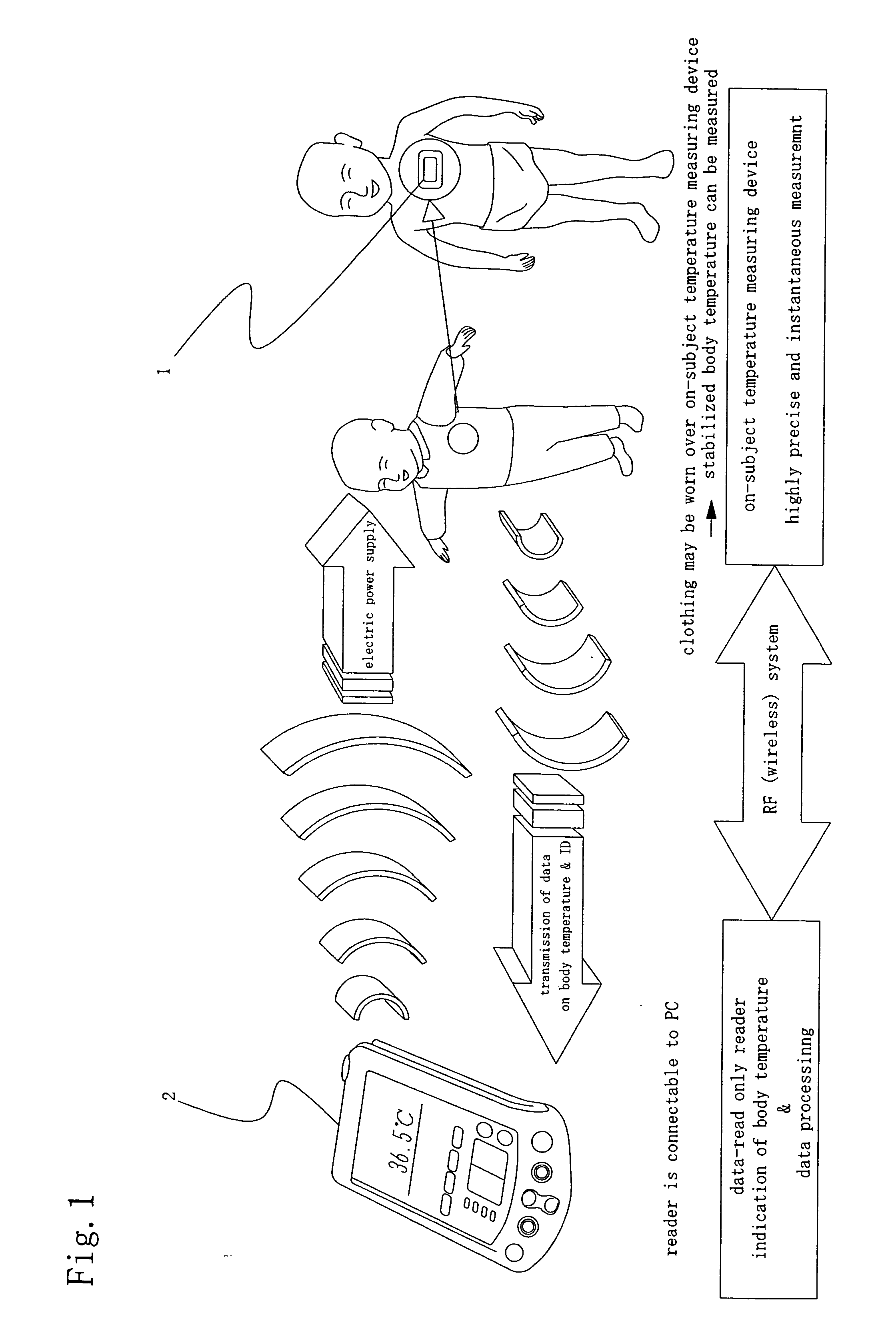
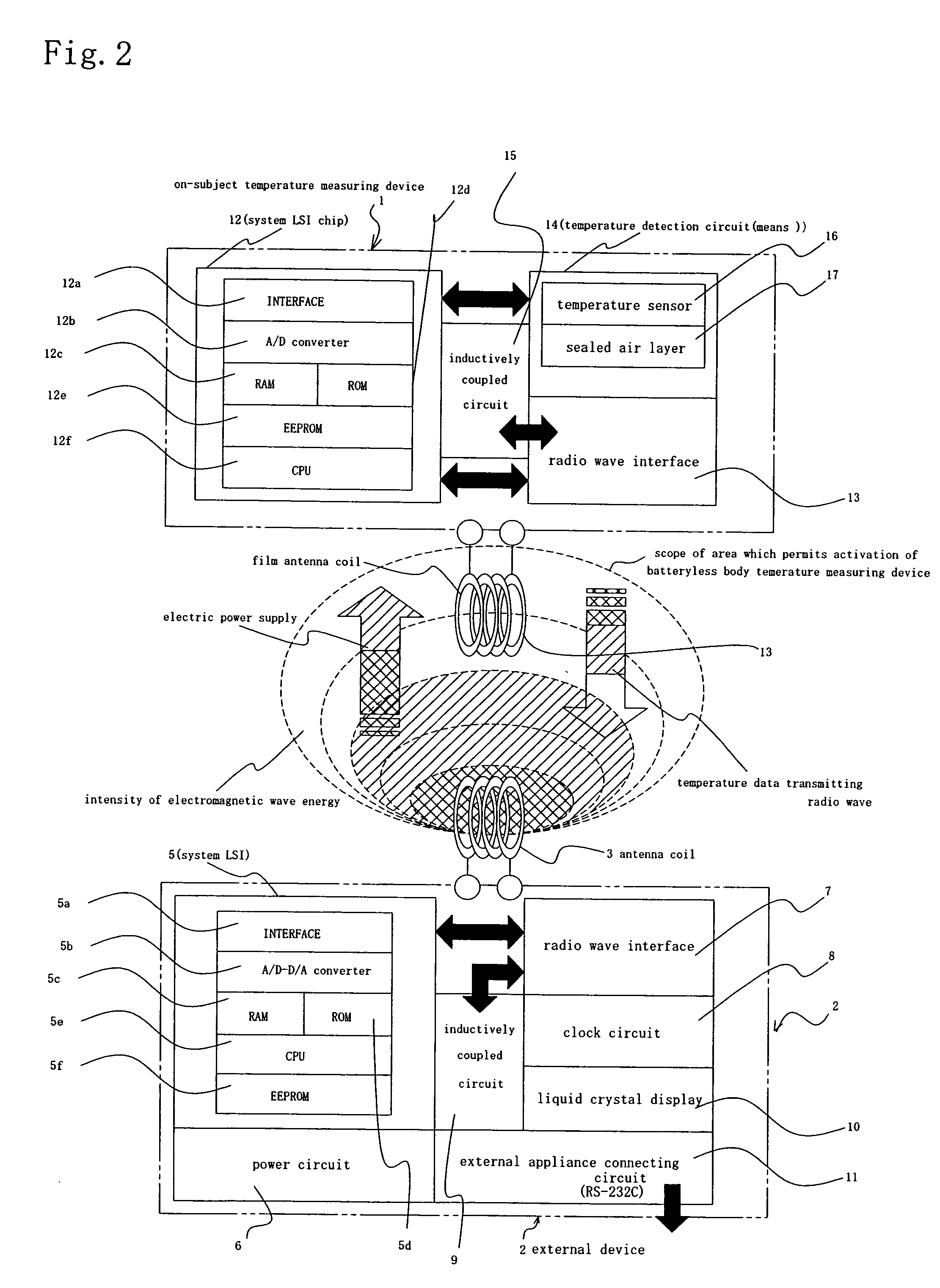
A temperature measuring device and a temperature measuring method are provided which are capable of obtaining precise measured temperature values with respect even to old persons, sucklings or infants, which device may be formed into a disposable type according to need, which method may be carried out using such a disposable type device according to need, and which enable precise temperature measurement in real time. An on-subject temperature measuring device 1, which is attached to a subject when a temperature of the subject is measured, receives a radio wave from a reader 2 as an external device and is thereby electrically powered. Using the electric power, temperature measurement is performed in the on-subject temperature measuring device 1. The results of the measurement are transmitted through radio waves to the reader 2 in the form of a temperature of the subject and ID data. The reader 2 is so constructed as to be connectable to a personal computer (not shown), and data processing by the personal computer is performed according to need.
Owner:SAKANO KAZUHITO +2
Integrated microfluidic assay devices and methods
InactiveUS20090325276A1Low costDeep insightBioreactor/fermenter combinationsBiological substance pretreatmentsAntigenPoint of care
Combinations of microfluidic diagnostic testing modules for simultaneous evaluations of serological and molecular biological targets are provided, and include panel testing for both antibodies (or antigens) and nucleic acid targets in one single-use device. These improvements are directed to evaluating the overall progress and activity of a pathogenic process in real time, at the point of care, not merely the presence or absence of a particular diagnostic marker, which can often be incomplete or misleading.
Owner:PERKINELMER HEALTH SCIENCES INC
Methods and devices for automated biopsy and collection of soft tissue
InactiveUS7226424B2Improved and more operationEasy and inexpensive to fabricateUltrasonic/sonic/infrasonic diagnosticsSurgical needlesSingle-Use DeviceMultiple use
Instruments for performing percutaneous biopsy procedures are disclosed, which have advantageous features for improving functionality and performance over prior art devices. These instruments comprise two types, single-use devices, and multiple-use devices having active tissue capture capability. Improved features include the ability to retrieve and evaluate multiple tissue samples during a single insertion procedure, without physical handling of the samples, as well as constructional features, such as a molded tissue cassette housing, variant vacuum port embodiments suited for different tissue environments, and a method for backflushing the instrument to remove biological debris, among others.
Owner:DEVICOR MEDICAL PROD
Hydraulically actuated pump for long duration medicament administration
ActiveUS20050119618A1Precise pressure regulationHigh viscosityPositive displacement pump componentsMicroneedlesHydraulic cylinderSingle-Use Device
Presently disclosed is a hydraulic pump device and its use thereof, especially in a fluid delivery system. In one embodiment, the fluid delivery system is an inexpensive, single-use device for slow dosing medicament applications. The fluid delivery system may employ a spring-compressed bellows crank or other combination of simple mechanisms operating according to the well-known peristaltic principle to force a volume of ultrapure bio-inert hydraulic fluid through an aperture, thereby expanding one chamber of a two chamber hydraulic cylinder. The second, fluid storage chamber, containing the medicament, is emptied through a conventional orifice in response to the expansion of the pump chamber. The medicament may thence flow through any suitable infusion set into a patient removeably attached thereto.
Owner:MANNKIND CORP
Single use device for blood microsampling
ActiveUS7357808B2Prevent the spread of diseaseSensorsBlood sampling devicesSingle-Use DeviceEngineering
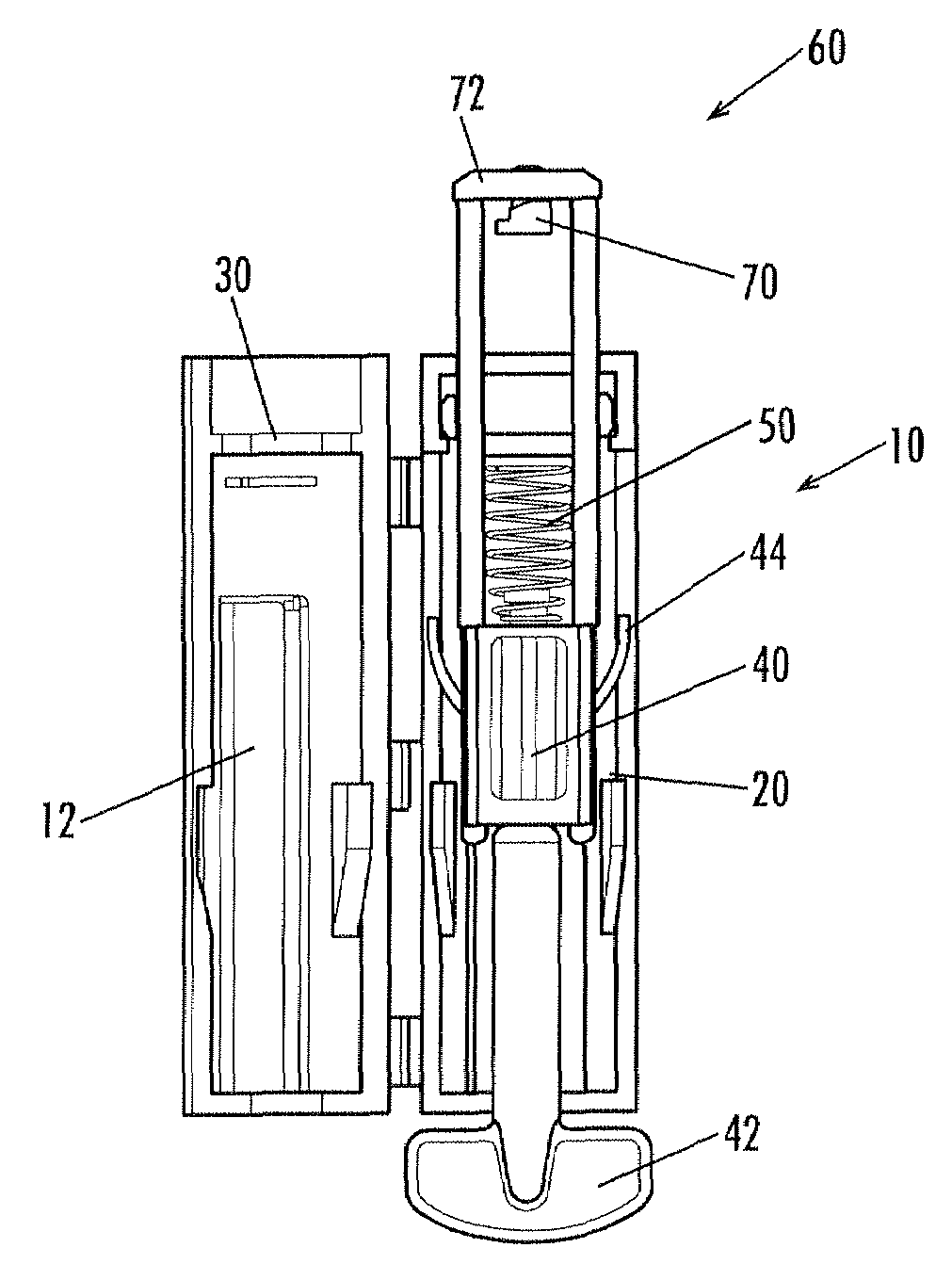
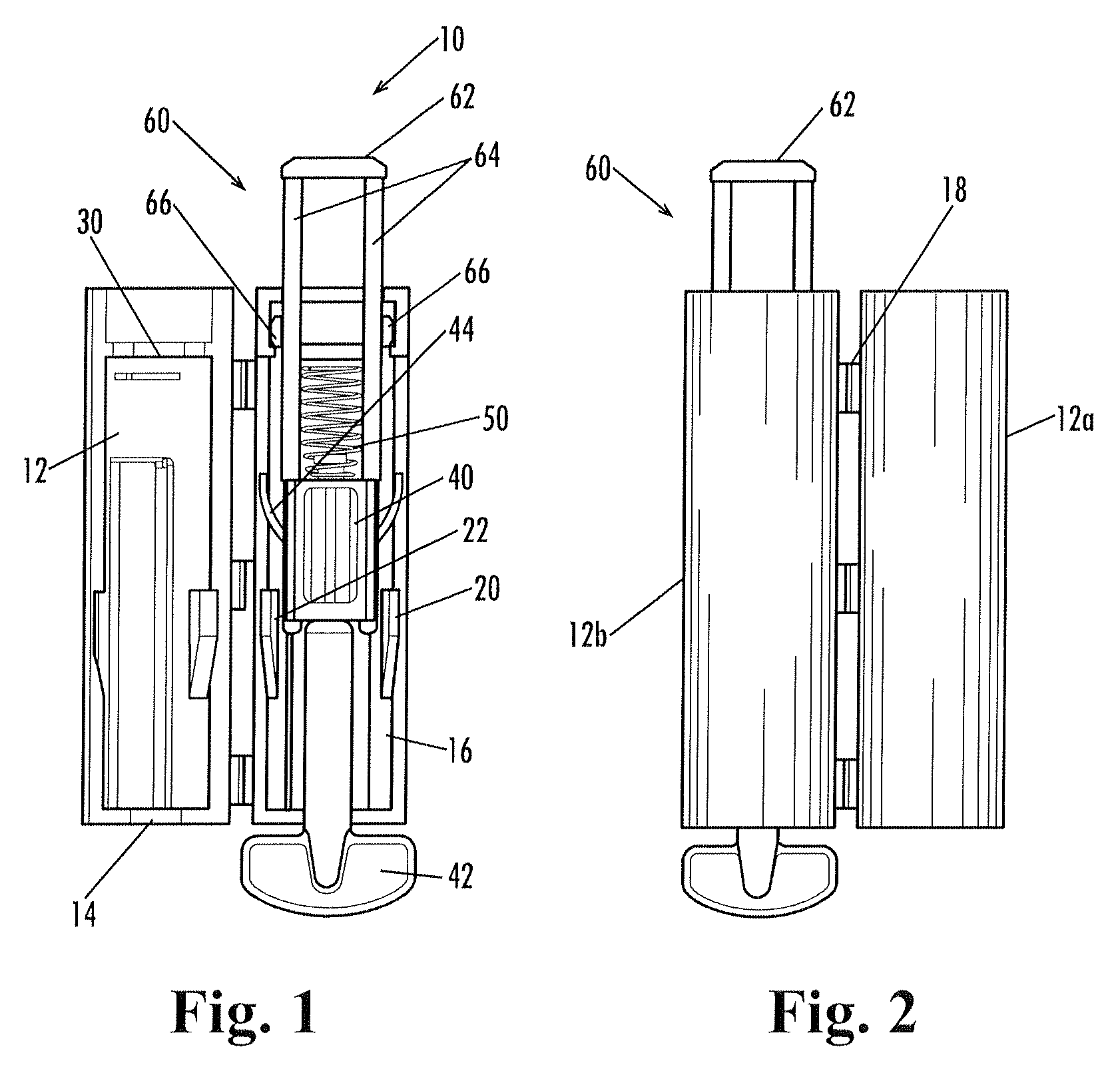
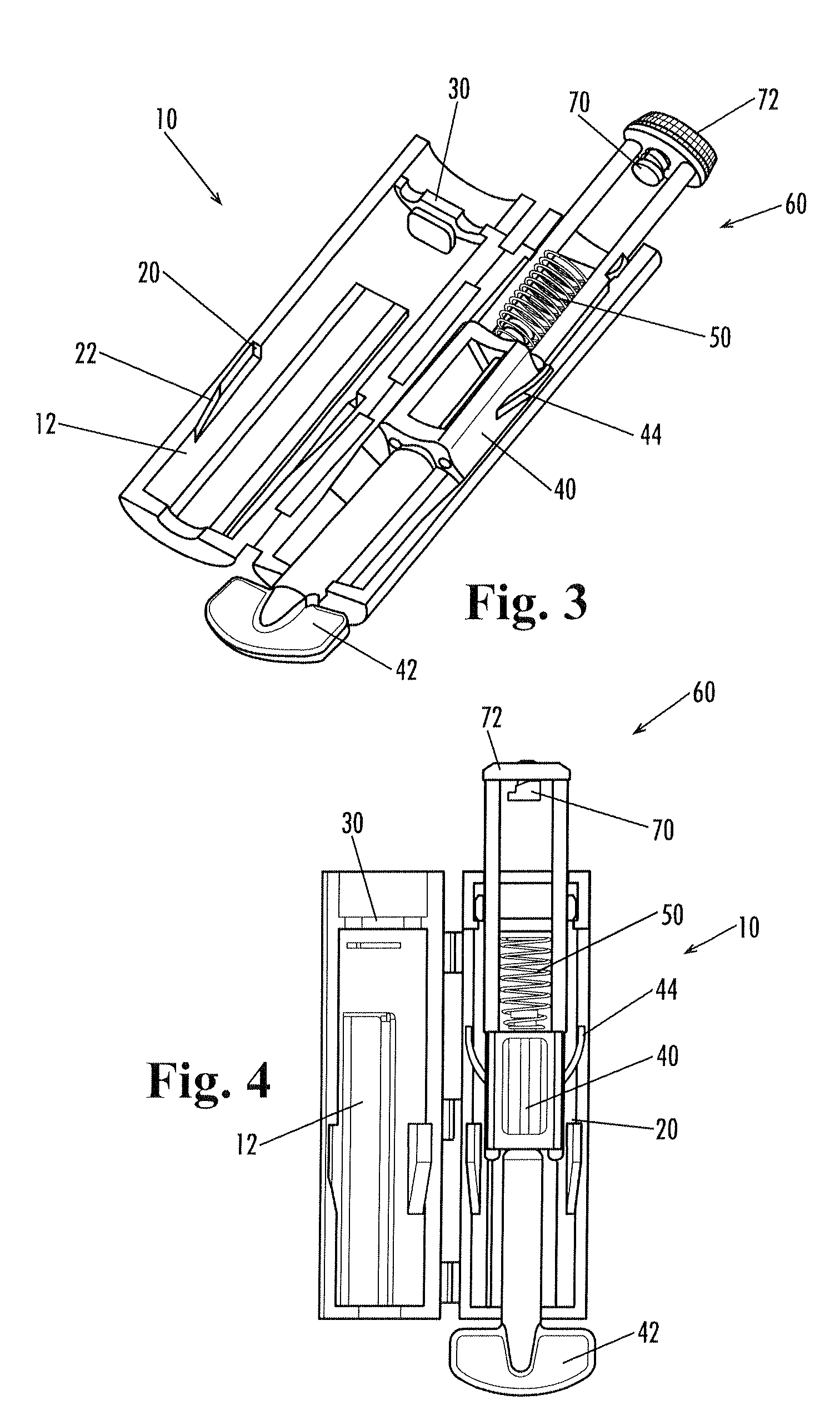
A lancing device including a spring-driven lancet translationally mounted within a housing. The lancet has at least one resilient finger extending outwardly therefrom and engageable within a cooperating recess formed in the housing to prevent re-use of the lancing device. A depth-adjustment knob is optionally included for contacting the housing to limit the travel of the lancet and thereby control the depth of penetration.
Owner:FACET TECH LLC
Device for surface stimulation of acupuncture points
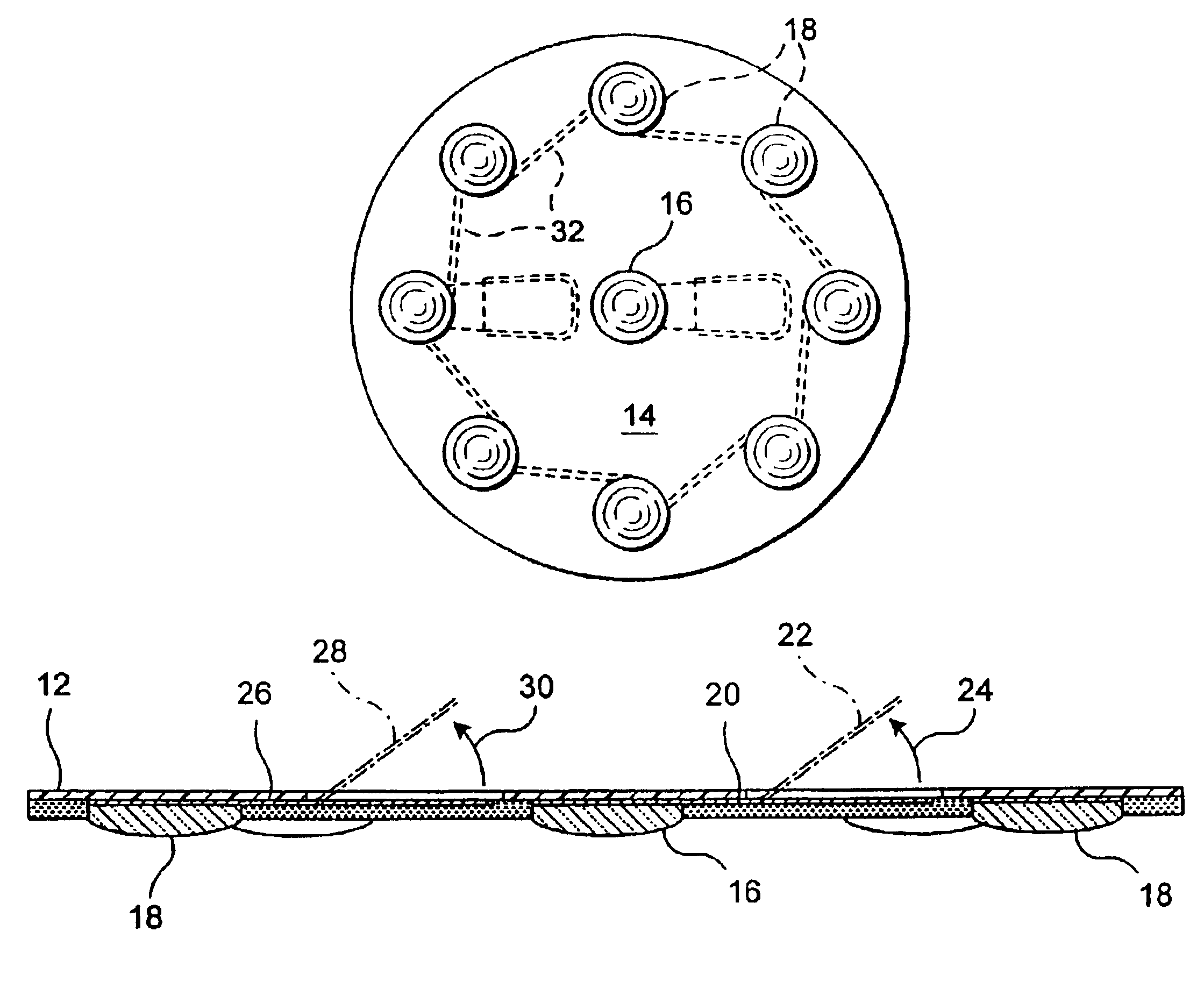
InactiveUS6961622B2Reduced Power RequirementsCurrent lossExternal electrodesDevices using electric currentsPolyesterSingle-Use Device
A device, which is preferably disc shaped, has two primary layers. A first layer has one side, which is the lower side in use, for adhering to a patient's skin surface, a second, upper layer on the underside of which electrical circuitry is printed or affixed so that the electrical circuitry is sandwiched between the layers. The device can be made in several sizes to accommodate patient size and location of the acupuncture point to be stimulated. Two distinct forms of the device are presented: a single use and a reusable device for. In the single use device, the first, lower layer is preferably a foam with non-conductive adhesive on both sides: the bottom side for adhering to the skin and the top side for adhering to the upper polyester disc. The holes through this lower foam layer include, preferably, eight holes spaced concentrically about one central hole in the middle of the lower foam layer. All these holes are, preferably, overfilled with an electrically conductive gel that projects from the lower foam layer. The conductive circuitry preferably printed on the underside of the upper layer provides a series connection to the gel in each of the eight concentric holes and a separate connection to the gel in the center hole when the upper layer is adhered to the lower layer. The printed circuitry may be a silver / silver chloride polymer film, also provides for a tab(s) which can be permanently or temporarily affixed to an integral or remote simulator through direct contact or wire leads. In the reusable device a pressure-sensitive adhesive material forms the lower layer which allows for multiple applications to a patient's skin. The adhesive lower layer is transparent to show the circular central electrode the annular electrode. The electrodes are preferably silver / silver chloride polymer film. In either configuration, metal core insulated leads can be use for electrical connection with the opposite ends of the leads connected to jacks for connection to an impulse stimulator or can end in electrically conductive tabs.
Owner:THE RUSSELL GRP
Hydraulically actuated pump for long duration medicament administration
ActiveUS7530968B2Accurate doseIncrease volumePositive displacement pump componentsMicroneedlesHydraulic cylinderSingle-Use Device
Presently disclosed is a hydraulic pump device and use thereof, especially in a fluid delivery system. In one embodiment, the fluid delivery system is an inexpensive, single-use device for slow dosing medicament applications. The fluid delivery system may employ a spring-compressed bellows crank or other combination of simple mechanisms operating according to the well-known peristaltic principle to force a volume of ultrapure bio-inert hydraulic fluid through an aperture, thereby expanding one chamber of a two chamber hydraulic cylinder. The second, fluid storage chamber, containing the medicament, is emptied through a conventional orifice in response to the expansion of the pump chamber. The medicament may thence flow through any suitable infusion set into a patient removeably attached thereto.
Owner:MANNKIND CORP
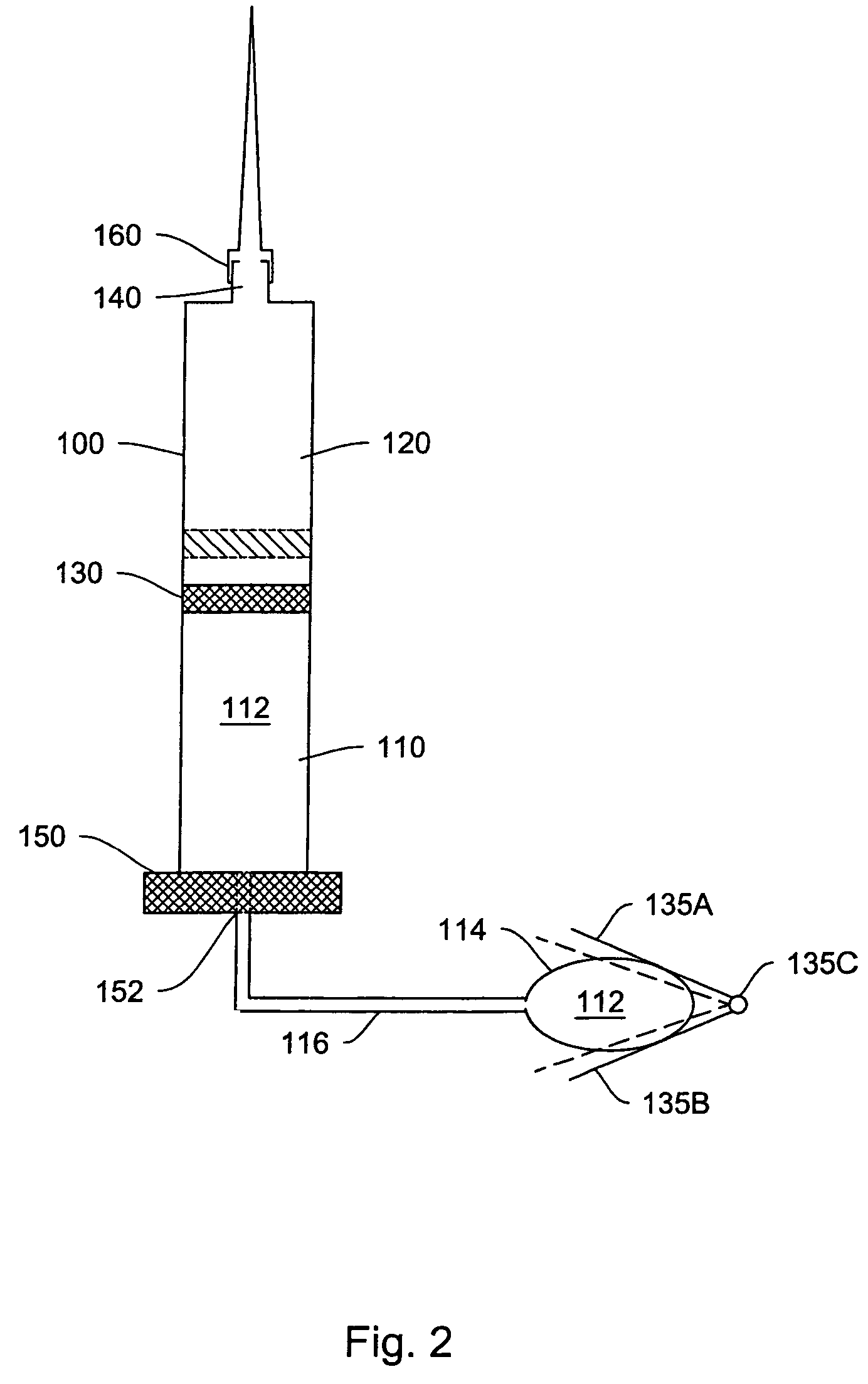
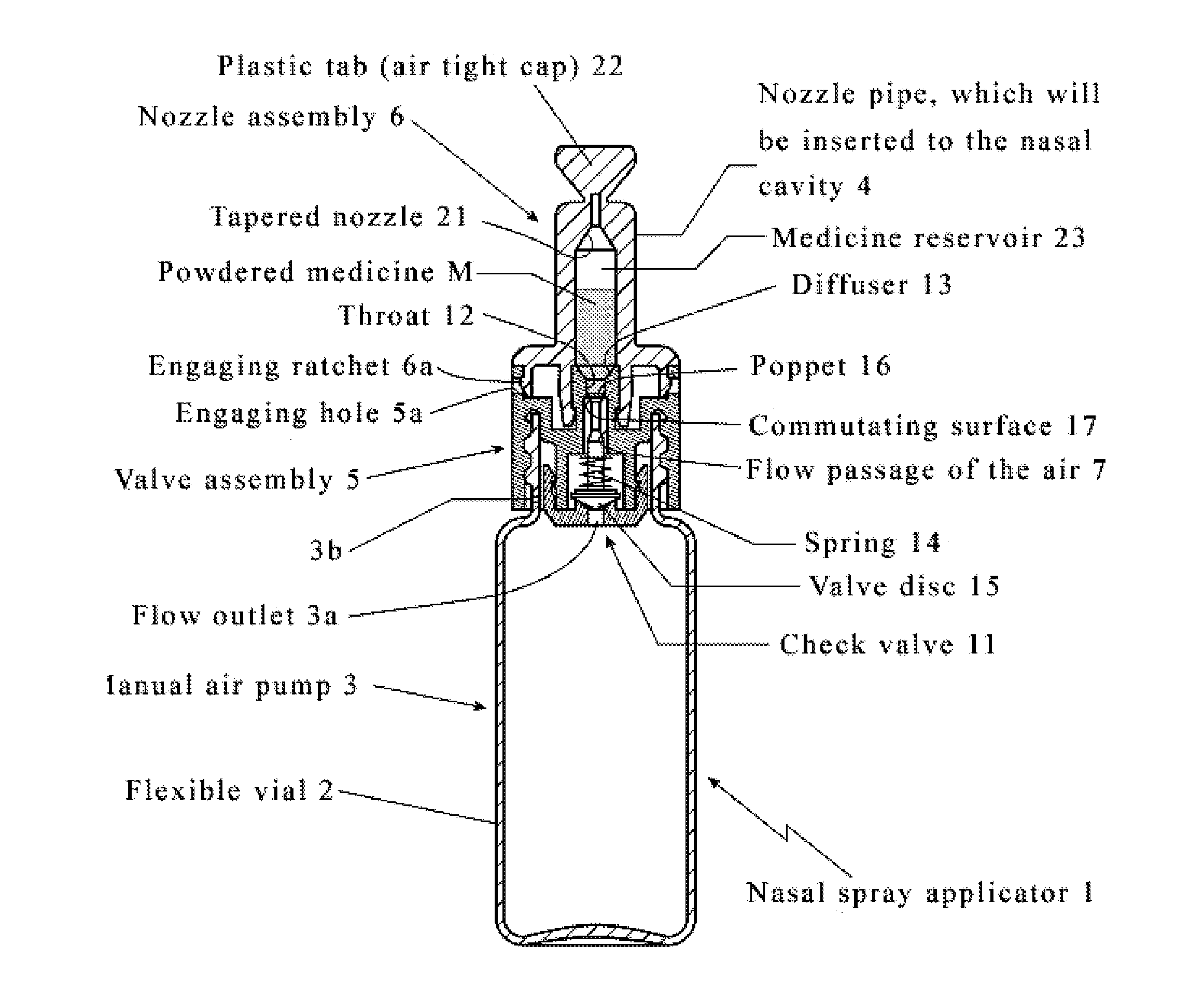
Intranasal granisetron and nasal applicator
Formulations and methods of manufacture are provided for granisetron dry powder compositions suitable for intranasal administration. Also provided are methods of use for preventing or controlling emesis and other diseases and disorders and devices, compositions, and methods for nasal delivery of therapeutic formulations. Devices for delivery of dry powder formulations are also provided. Devices can be single-use devices.
Owner:SHIN NIPPON BIOMEDICAL LAB
Method for treating urinary incontinence
Owner:FIRST QUALITY HYGIENIC
Method and apparatus for processing dermal tissue
ActiveUS20060271070A1Inexpensive and disposable and easy to useGood cosmetic effectDiagnosticsSurgerySingle-Use DeviceWound site
A portable and disposable device is provided for processing harvested dermal tissue. The device includes a housing presenting a handle having a gripping surface and a cutting head attached to the handle. A cutting assembly is supported by the cutting head and includes a plurality of spaced cutting blades that are rotatable with respect to the housing. A receptacle is disposed downstream of the cutting assembly and receives the sliced tissue from the cutting blades. The device is thus operable to slice harvested tissue into strips, and further into fine particles, that can be used for transplantation onto a wound site.
Owner:WRIGHT MEDICAL TECH
Method of treating urinary incontinence
A method for treating urinary incontinence includes the steps of inserting into the woman's vagina a first disposable device, removing the first disposable device, and inserting a second disposable device substantially identical to the first disposable device. The disposable devices have a working portion with opposed faces to provide support to an associated urinary system and an anchoring portion to maintain the disposable device in place during use. The anchoring portion has at least one member extending beyond at least one end of the working portion
Owner:FIRST QUALITY HYGIENIC
Disposable set for use with an endoscope
A disposable set for use with an endoscope is described. The set comprises a dispenser provided with a longitudinal transit passage adapted for passing the endoscope therealong. The dispenser stores a disposable multilumen tubing fitted at a distal end thereof with a cap that is detachably connectable to an optical head of the endoscope. The dispenser is fitted with a protective sleeve adapted to cover the endoscope during the endoscopic procedure. The dispenser is adapted for receiving a lubricant and distributing thereof within the transit passage.
Owner:STRYKER GI
Method of treating urinary incontinence
A method for treating urinary incontinence includes the steps of inserting into the woman's vagina a first disposable device, removing the first disposable device, and inserting a second disposable device substantially identical to the first disposable device. The disposable devices have a working portion with opposed faces to provide support to an associated urinary system and, optionally, an anchoring portion to maintain the disposable device in place during use. The working portion also has. The working portion also has an insertion equivalent diameter ranging from about 10 to about 25 mm and a use equivalent diameter ranging from about 25 to about 35 mm under an expansion pressure of about 20 to about 150 cm H2O.
Owner:FIRST QUALITY HYGIENIC
Method of treating urinary incontinence
A method for treating urinary incontinence includes the steps of inserting into the woman's vagina a first disposable device, removing the first disposable device, and inserting a second disposable device substantially identical to the first disposable device. The disposable devices have a generally cylindrical working portion with opposed faces to provide support to an associated urinary system and an anchoring portion to maintain the disposable device in place during use. The working portion also has an initial equivalent diameter ranging from about 20 to about 170 mm, an insertion equivalent diameter ranging from about 5 to about 25 mm, a use equivalent diameter ranging from about 20 to about 40 mm and a length ranging from about 20 to about 60 mm. The anchoring portion also has an initial equivalent diameter ranging from about 20 to about 60 mm, an insertion equivalent diameter ranging from about 10 to about 25 mm, a use equivalent diameter ranging from about 20 to about 60 mm and a length ranging from about 10 to about 50 mm.
Owner:FIRST QUALITY HYGIENIC
Method and apparatus for processing dermal tissue
Owner:APPLIED TISSUE TECH
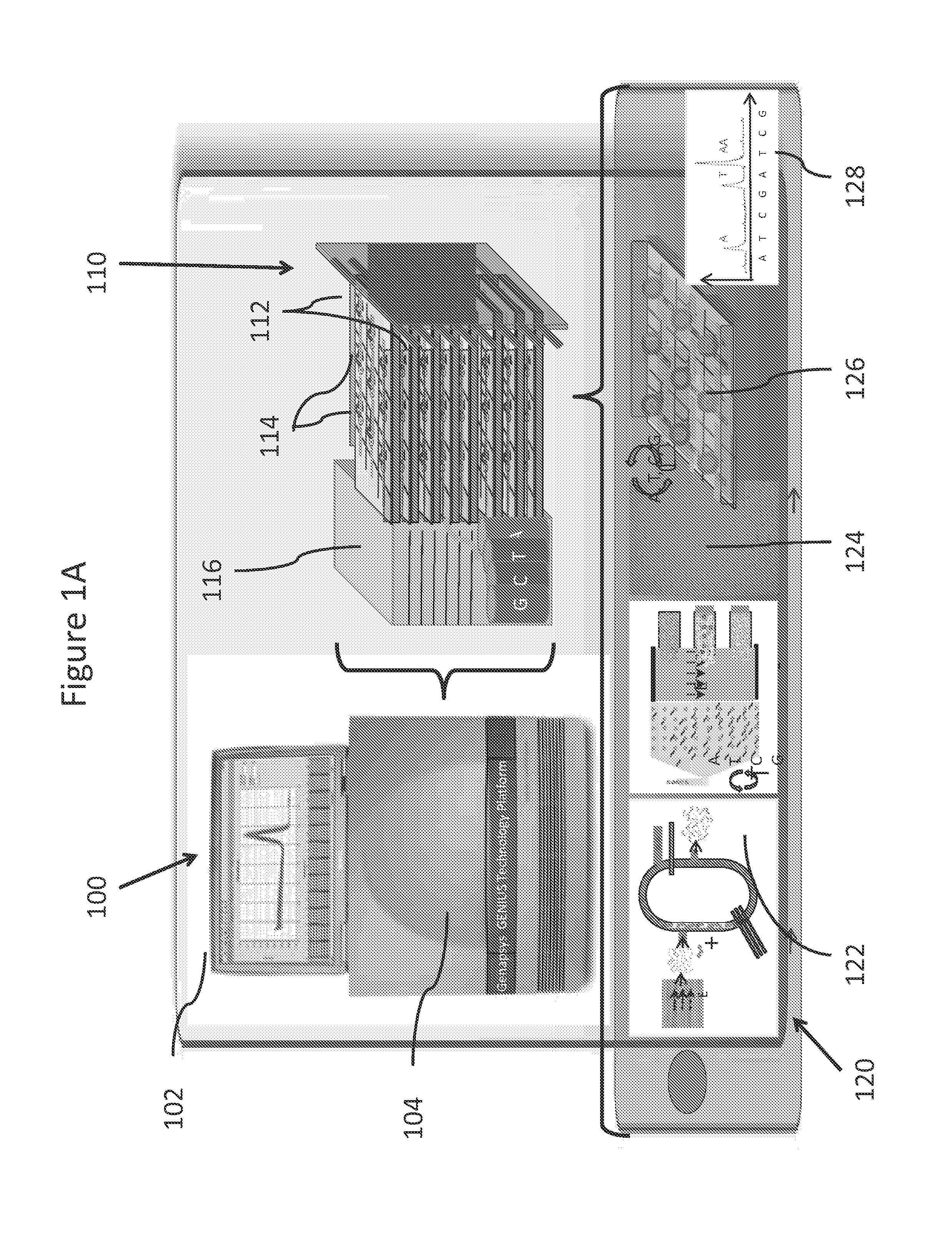
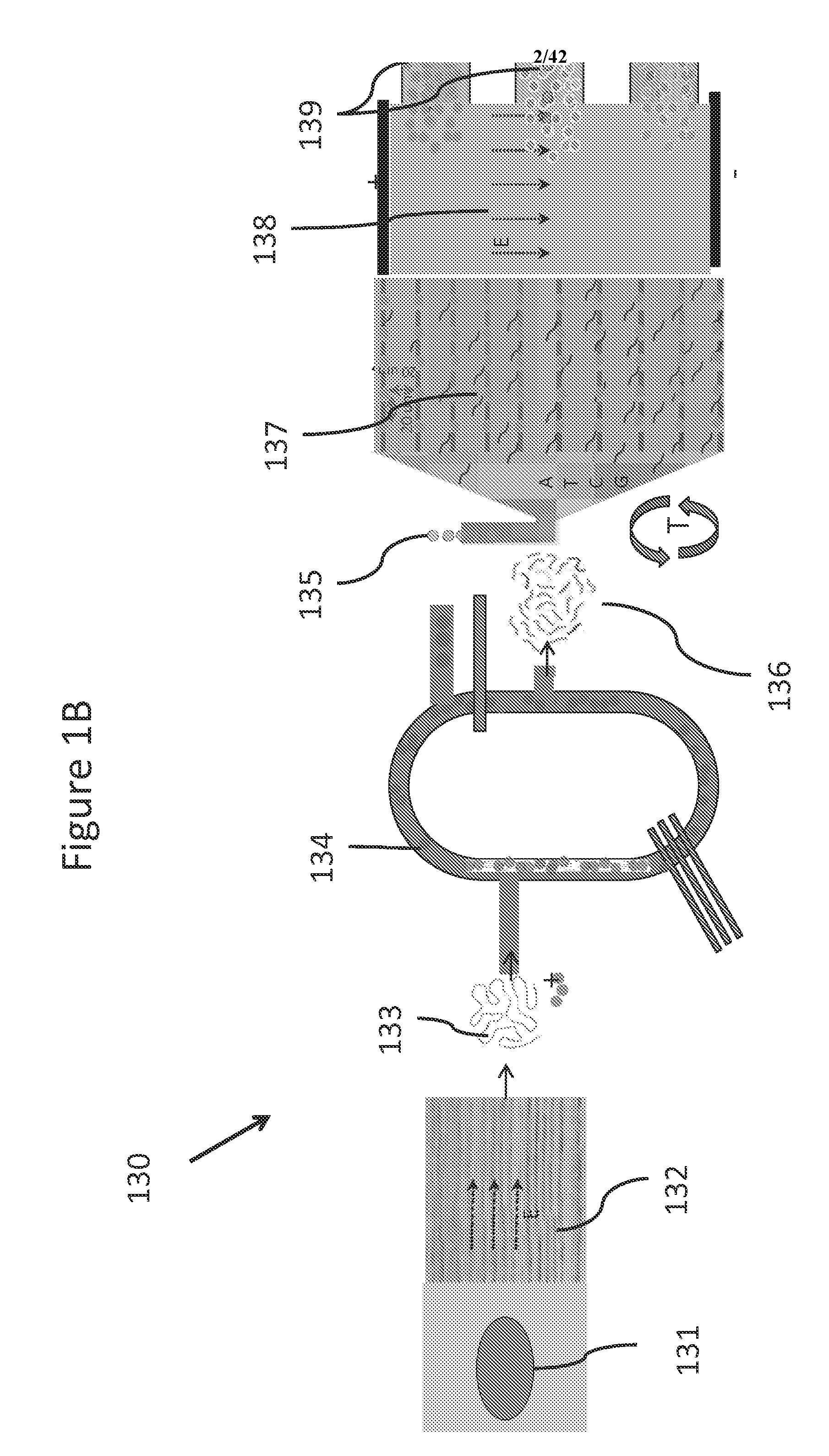
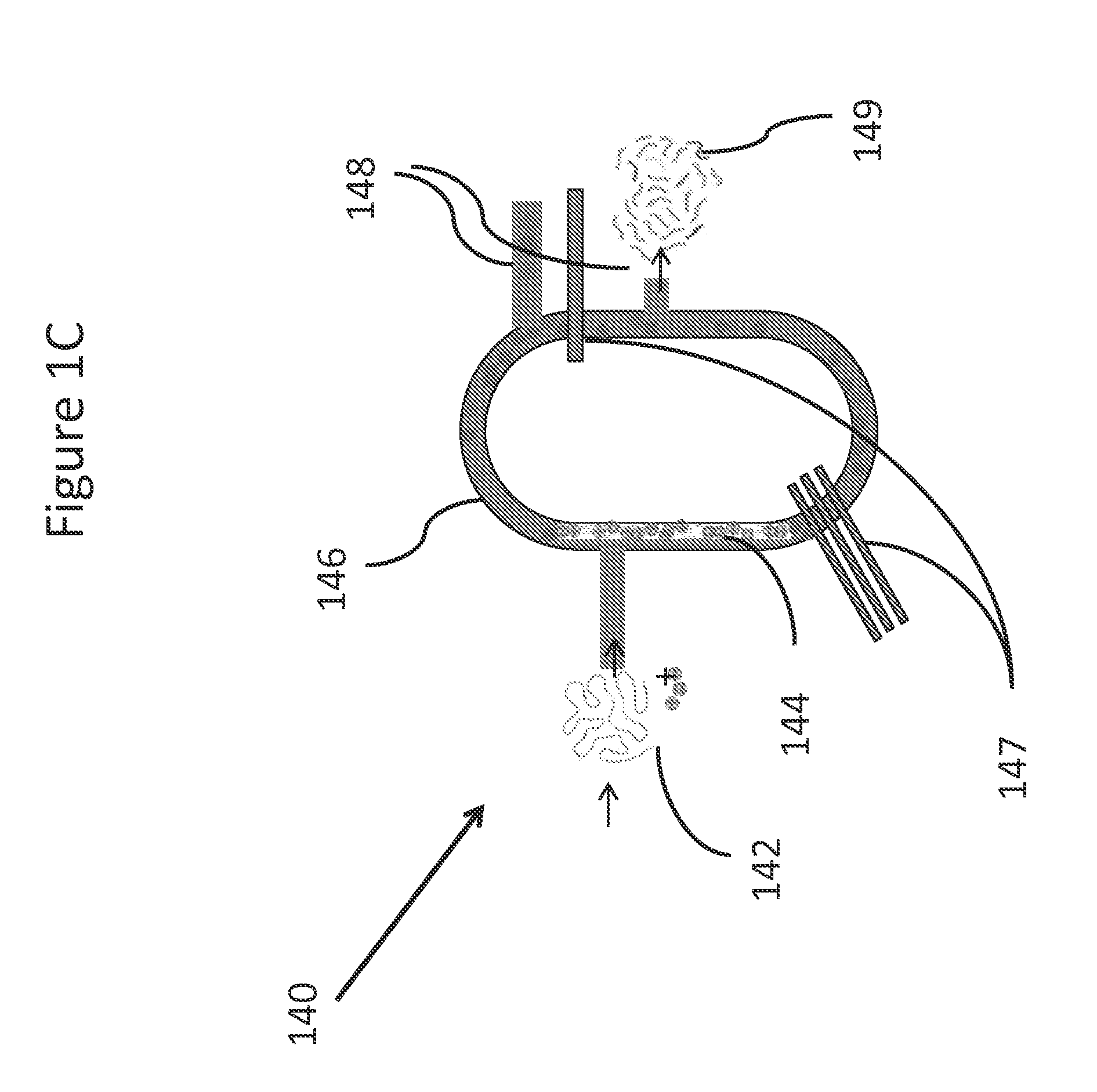
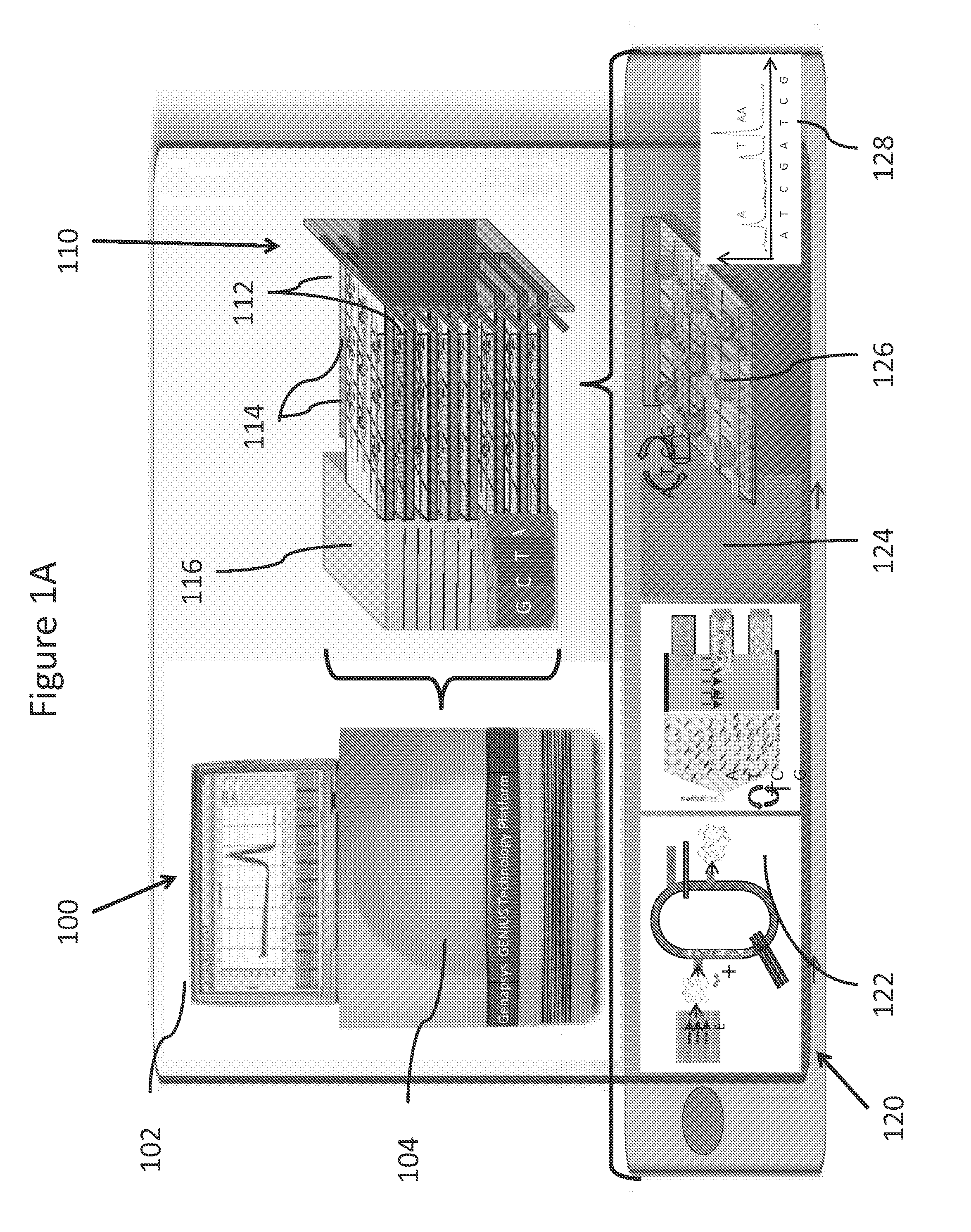
Systems and methods for genetic and biological analysis
ActiveUS20140057339A1Reduce phase errorReduction tendencyBioreactor/fermenter combinationsBiological substance pretreatmentsSingle-Use DevicePolynucleotide
The invention relate to systems and methods for sequencing polynucleotides, as well as detecting reactions and binding events involving other biological molecules. The systems and methods may employ chamber-free devices and nanosensors to detect or characterize such reactions in high-throughput. Because the system in many embodiments is reusable, the system can be subject to more sophisticated and improved engineering, as compared to single use devices.
Owner:SEQUENCING HEALTH INC
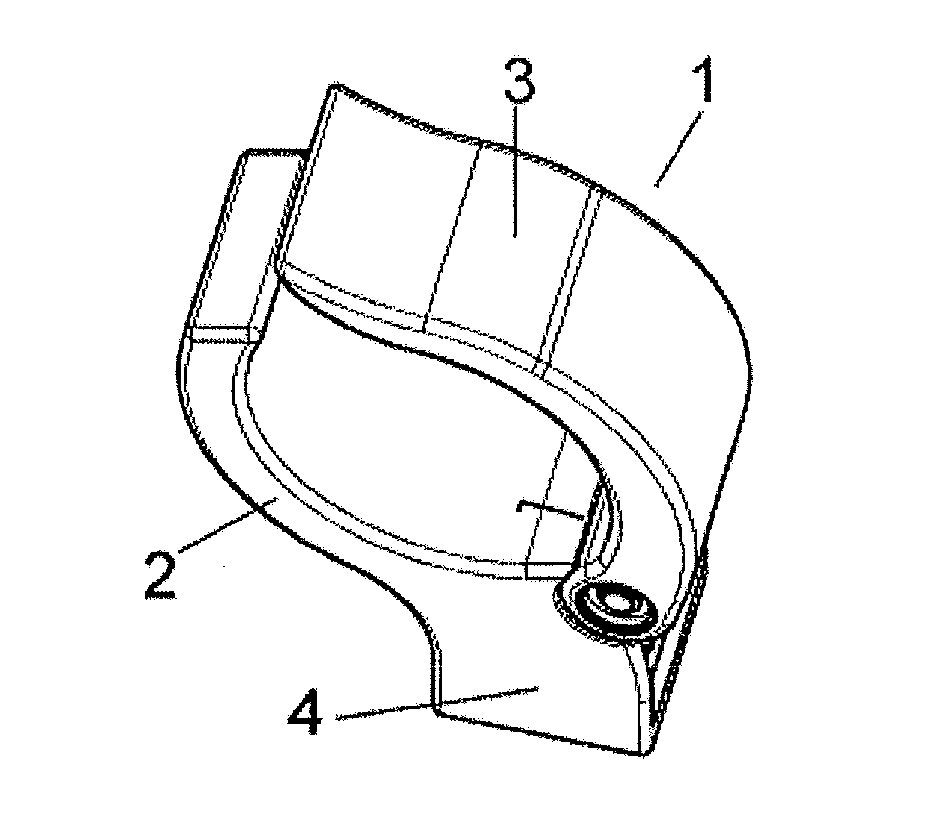
Manually openable clamping holder with sensor
The invention relates to a manually openable clamping holder (1) for releasably fixing a single-use medical device to a medical treatment apparatus. The clamping holder (1) has a sensor (9) for detecting the position of the clamping jaws (2, 3) of the clamping holder (1). With the clamping holder (1) the correct equipping of a medical treatment apparatus with the appropriate single-use device can be monitored economically and with simple operation.
Owner:FRESENIUS MEDICAL CARE DEUTSCHLAND GMBH
Method and apparatus for endoscopic ligament release
ActiveUS8672960B2Preventing ligaments and other anatomyReduce usageSurgeryCarpal tunnel releaseSingle-Use Device
The invention pertains to a method, apparatus, and system for cutting anatomic members, such as ligaments, in surgical procedures such as carpal tunnel release, plantar fasciotomy, gastroc release, cubital tunnel release, and tarsal tunnel release. The apparatus includes a retrograde knife and a guide tool for guiding the knife and a scope during surgery. Relevant features include a knife stop for preventing the knife from inadvertently raising out of the knife channel, indicators showing the proper orientation for the guide tool, a self dilating tip and channel design on the guide tool, a cover piece and / or pivotable panel system for preventing ligaments and other anatomy from getting caught in the guide tool, a pivot pin and groove system for stabilizing the knife and also assuring that the knife blade is not inadvertently raised out of the channel, and a use indicator for preventing re-use of a single use device.
Owner:SEG WAY ORTHOPAEDICS +1
Unitized photocatalytic air sterilization device
ActiveUS20110171080A1Improve sterilization efficiencyMaximize air flowCatalyst protectionWater/sewage treatment by irradiationSingle-Use DeviceHand held
A UV photocatalytic air purifier / sterilizer in which the multiple limited-lifetime components (such as the UV light, UV light electronics, and catalytic portion) of a photocatalytic UV air purifier / sterilizer are packaged together to form a single, handheld, unitized package, designed for easy insertion and removal into an air purifier. The invention may be configured enable maximum air flow through the photocatalytic portions of the device, thus further improving air cleaning efficiency by allowing many volumes of room air to be recirculated through the device and cleaned / purified / sterilized over the course of a day. In a preferred embodiment, the device may package a series of stacked TiO2 coated parallel metal catalytic plates, a UV germicidal lamp with a fluorescent tube form factor, and a UV lamp ballast into a single disposable or recyclable unit. This disposable unit can be easily clipped into a motorized air purifier unit designed for rapid servicing.
Owner:LO YANG ZHEN
Saliva Glucose Monitoring System
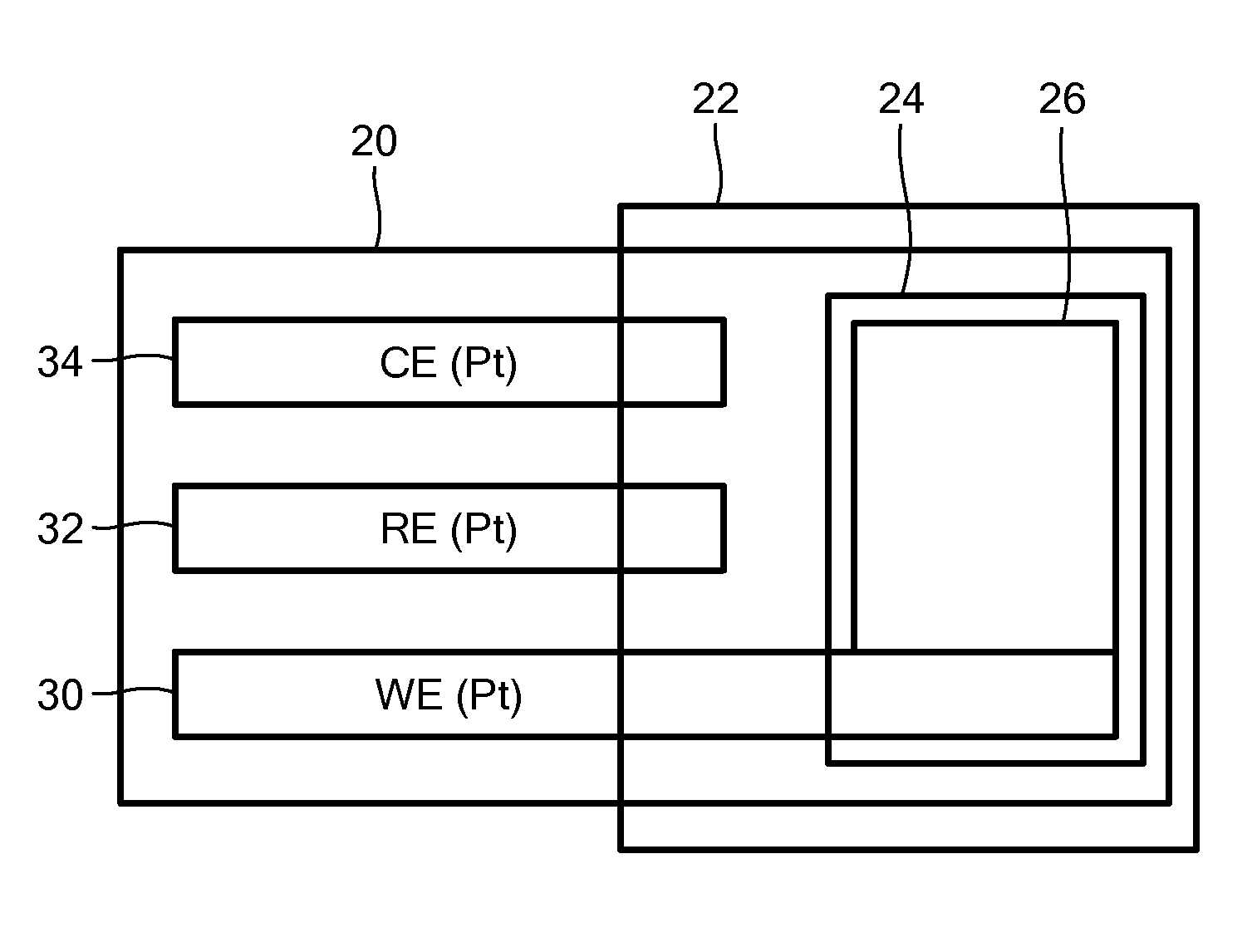
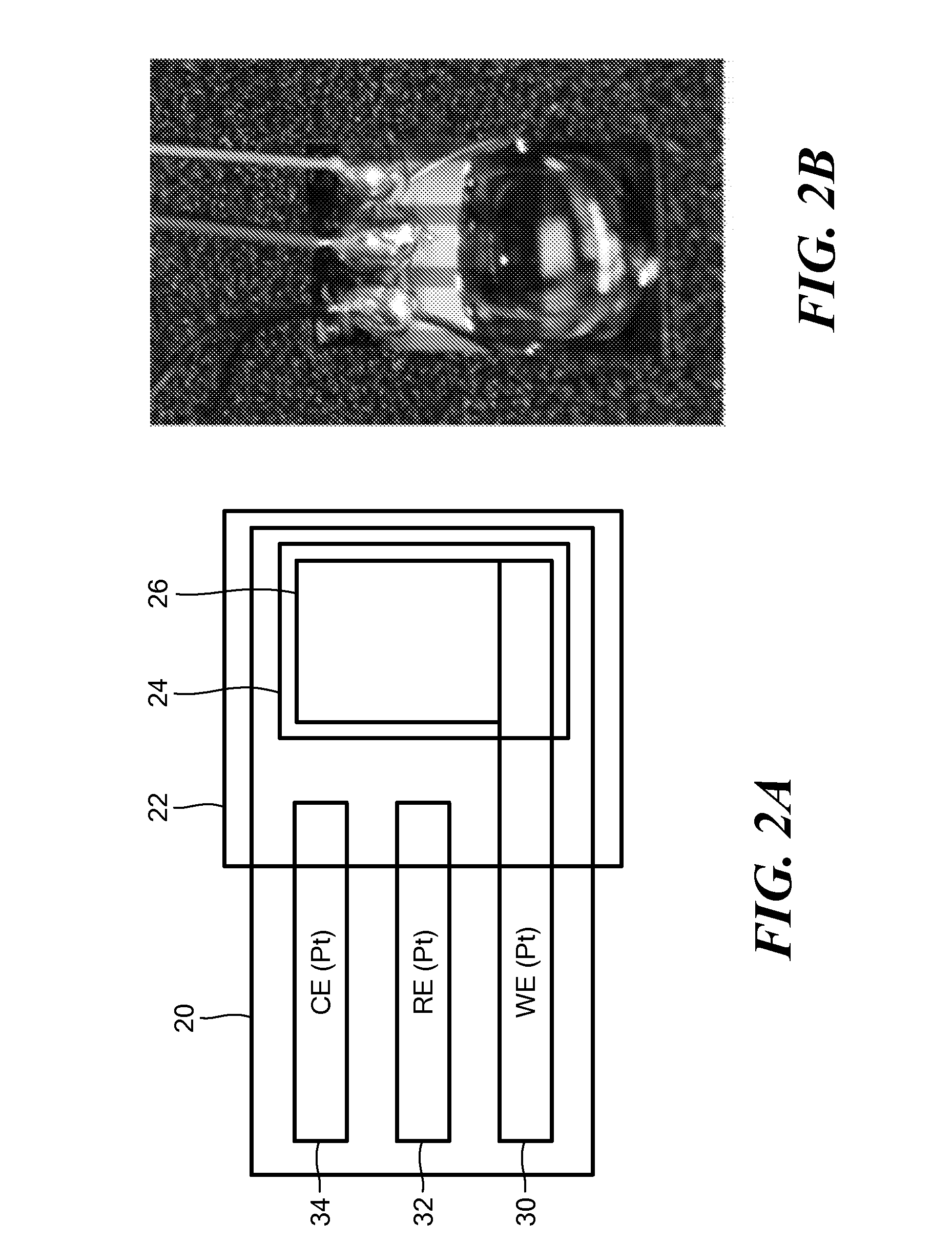
ActiveUS20140197042A1Exposure was also limitedProtective coatingImmobilised enzymesBioreactor/fermenter combinationsElectrochemical detectorClinical settings
A glucose sensor suitable for measuring glucose levels in human saliva is provided. Systems containing the glucose sensor and methods for making and using the sensor are also provided. The glucose sensor is highly sensitive and can detect glucose levels at least down to 5 ppm. Fabrication of the sensor involves depositing single-walled carbon nanotubes onto the surface of a working electrode in a 3 electrode electrochemical detector and functionalizing the nanotubes by depositing layers of polymers, metallic nanoparticles, and glucose oxidase enzyme onto the nanotubes. The sensor can be used as a disposable, single-use device or as part of an analytical system, such as a microfluidics system, for the analysis of multiple analytes. The sensor enables the diagnosis and monitoring of diabetes to be performed without pain or the need for finger pricks in a home or clinical setting.
Owner:NORTHEASTERN UNIV
Systems and methods for genetic and biological analysis
ActiveUS20140235457A1Reduction tendencyReduce errorsBioreactor/fermenter combinationsBiological substance pretreatmentsBio moleculesSingle-Use Device
The invention relate to systems and methods for sequencing polynucleotides, as well as detecting reactions and binding events involving other biological molecules. The systems and methods may employ chamber-free devices and nanosensors to detect or characterize such reactions in high-throughput. Because the system in many embodiments is reusable, the system can be subject to more sophisticated and improved engineering, as compared to single use devices.
Owner:SEQUENCING HEALTH INC
Testing system for determining hypoxia induced cellular damage
InactiveUS20130052675A1Quick testEasy to useBioreactor/fermenter combinationsBiological substance pretreatmentsLactate dehydrogenaseCell damage
The present invention relates to a testing system for assessing hypoxia induced cellular damage in a mammal including human, comprising a disposable device having a sample inlet and a collection chamber separated by a separation device wherein the collection chamber is connected to at least two, a first and a second, visible detection compartments, whereof at least one is arranged with chemical means for direct visual detection, said first detection compartment being arranged to determine whether level of hemoglobin (Hb) in a sample of body fluid taken from said mammal exceeds a predetermined threshold value, and said second detection compartment being arranged to evaluate level of total amount of lactate dehydrogenase (LDH) in said sample.
Owner:CALMARK SWEDEN AB
Method and apparatus for endoscopic ligament release
The invention pertains to a method, apparatus, and system for cutting anatomic members, such as ligaments, in surgical procedures such as carpal tunnel release, plantar fasciotomy, gastroc release, cubital tunnel release, and tarsal tunnel release. The apparatus includes a retrograde knife and a guide tool for guiding the knife and a scope during surgery. Relevant features include a knife stop for preventing the knife from inadvertently raising out of the knife channel, indicators showing the proper orientation for the guide tool, a self dilating tip and channel design on the guide tool, a cover piece and / or pivotable panel system for preventing ligaments and other anatomy from getting caught in the guide tool, a pivot pin and groove system for stabilizing the knife and also assuring that the knife blade is not inadvertently raised out of the channel, and a use indicator for preventing re-use of a single use device.
Owner:SEG WAY ORTHOPAEDICS +1
Imaging system with disposable part
InactiveUS20120130160A1Improve securityClear messageBronchoscopesLaryngoscopesComputer moduleDisplay device
An imaging system comprising a disposable part (1) comprising a camera device (5), said disposable part arranged to be suitable for allowing said camera device to be inserted into a body cavity, a reusable control module (8) in communication with said disposable part, said reusable control module comprising a display which displays image signals from the camera device in the disposable part, a timing device which generates time of use data of the disposable part, said time of use data representing the amount of time which the disposable part is in use, and said imaging system being arranged to prevent use of the disposable part if the time of use of the disposable part reaches a predefined level. The disposable part further comprises a non-volatile memory component which stores time of use data generated by said timing device and in that said reusable control module is arranged to continuously monitor the time of use data stored in the non-volatile memory component of the disposable part and to stop displaying image signals from the camera in the disposable part when the time of use data stored in the non-volatile memory component reaches said predefined level. In this way an imaging system is provided which is able to prevent using a disposable part which has been used on one patient on another patient.
Owner:AMBU AS
Disposable device for treatment of infections of human limbs
ActiveUS20110208189A1Improve efficiencyHigh cost-effectivenessInternal osteosythesisSurgical furnitureSingle-Use DeviceBiomedical engineering
A disposable device for treatment of infections of human limbs, particularly limbs having long bones susceptible to stabilization by intramedullary nailing. The device includes a tubular member made of a relatively rigid and biologically compatible material, having pores for impregnation with drugs or medicaments for infection treatment prior to or during insertion thereof in the stabilization site. An assembly for treatment of human limb infections including such device.
Owner:TECRES SPA
Systems and methods for genetic and biological analysis
ActiveUS9274077B2Reduction tendencyReduce errorsMicrobiological testing/measurementMaterial analysis by electric/magnetic meansBio moleculesSingle-Use Device
The invention relate to systems and methods for sequencing polynucleotides, as well as detecting reactions and binding events involving other biological molecules. The systems and methods may employ chamber-free devices and nanosensors to detect or characterize such reactions in high-throughput. Because the system in many embodiments is reusable, the system can be subject to more sophisticated and improved engineering, as compared to single use devices.
Owner:SEQUENCING HEALTH INC
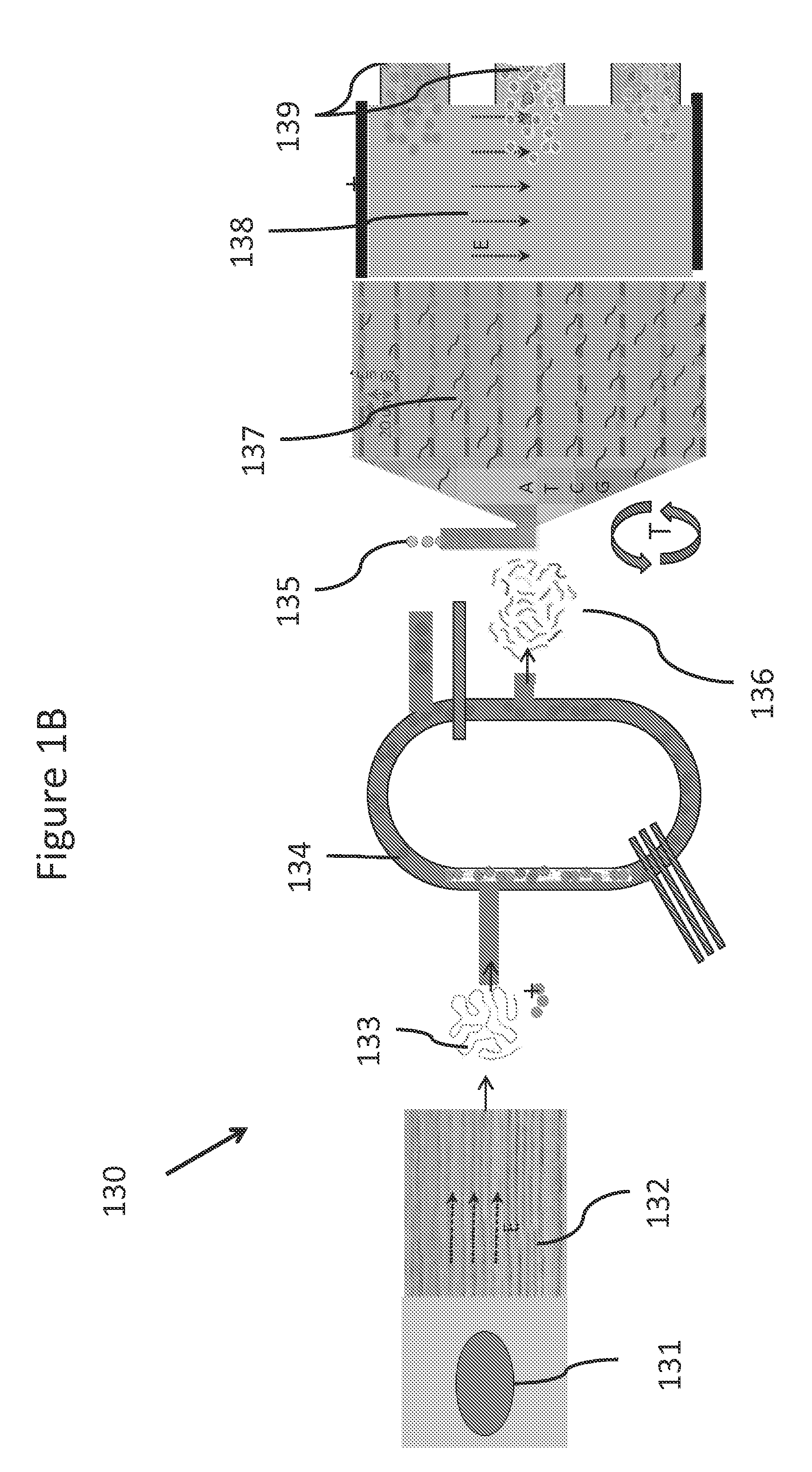
Disposable set for separations of blood into its components
InactiveUS20060226088A1Reduces direct collectionReduce processing costsWater/sewage treatment by centrifugal separationDiagnosticsWhole blood productUltrasonic sensor
Described herein is a continuous-flow centrifuge (CFC) which may be embodied in an automated system for collecting and separating whole blood into its components. The collection and separation system includes a console and a disposable set. Various blood processing procedures which produce specific blood products may be implemented by using a specific disposable set for each process. The disposable set may include a manifold, a CFC, and various components attached by tubing. These components may include one or more solution bags, blood product bags, bacterial filters, leukofilters, and a donor blood collection tube with access needle. The manifold and CFC disk may be included in a cassette that mounts onto the front panel of the console. The console may contain valve actuators, pressure transducers, ultrasonic sensors, a roller pump assembly, a CFC drive system, optical sensors, electronics, software, user interface components, a bar code reader and data acquisition components.
Owner:TERUMO MEDICAL CORP
Method for the assessment of particles and a system and device for use in the method
ActiveUS20070207551A1Small amountSave on transportationExhaust apparatusElement comparisonSingle-Use DeviceImage presentation
The present invention relates to a method and a device for the assessment of at least one parameter of particles in a liquid analyte material, comprising providing a device comprising a sample compartment comprising an exposing domain, an inlet through which a volume of a liquid sample representing the analyte material can be introduced, and a flow system comprising at least a channel allowing at least a portion of the volume of the liquid sample to flow within the device. A volume of the liquid sample is introduced in the device through the inlet of the disposable device, passing at least a portion of the volume of the liquid sample through the flow system of the device into the exposing domain of the sample compartment, arranging the device in relation to detection device comprising a detection means for quantitatively detecting spatial image data and processing means for processing the detected image presentation detecting electromagnetic signals from the sample in the exposing domain of the device in the detection device forming, in the detection device, a spatial image representation of the exposing domain, and processing the detected image presentation obtaining the assessment of the at least one parameter.
Owner:CHEMOMETEC AS
Disposable device for the continuous centrifugal separation of a physiological fluid
ActiveUS8070664B2Reduced Tolerance RequirementsReduce amount of shrinkageCentrifugesPhysiological fluidSingle-Use Device
The invention relates to disposable device for the continuous centrifugal separation of a physiological fluid. The inventive device comprises: a fixed axial element; a centrifugation chamber which is mounted such that it can rotate around the axis of said element; an inlet channel for the blood to be centrifuged, the dispensing port of which is located close to the base of the chamber; and an outlet passage for a separated constituent, the inlet port of which is located close to the other end of the chamber in a concentrated area of one of the separated constituents having the lowest mass density in order for same to be removed continuously. The above-mentioned chamber takes the form of a long tube. The fixed axial element comprises a second outlet passage for a second of the separated constituents, the inlet port of which is located close to the end of the chamber opposite the above-mentioned base in a concentrated area of said second separated constituent having the highest mass density in order for same to be removed continuously.
Owner:SORIN GRP ITAL SRL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com