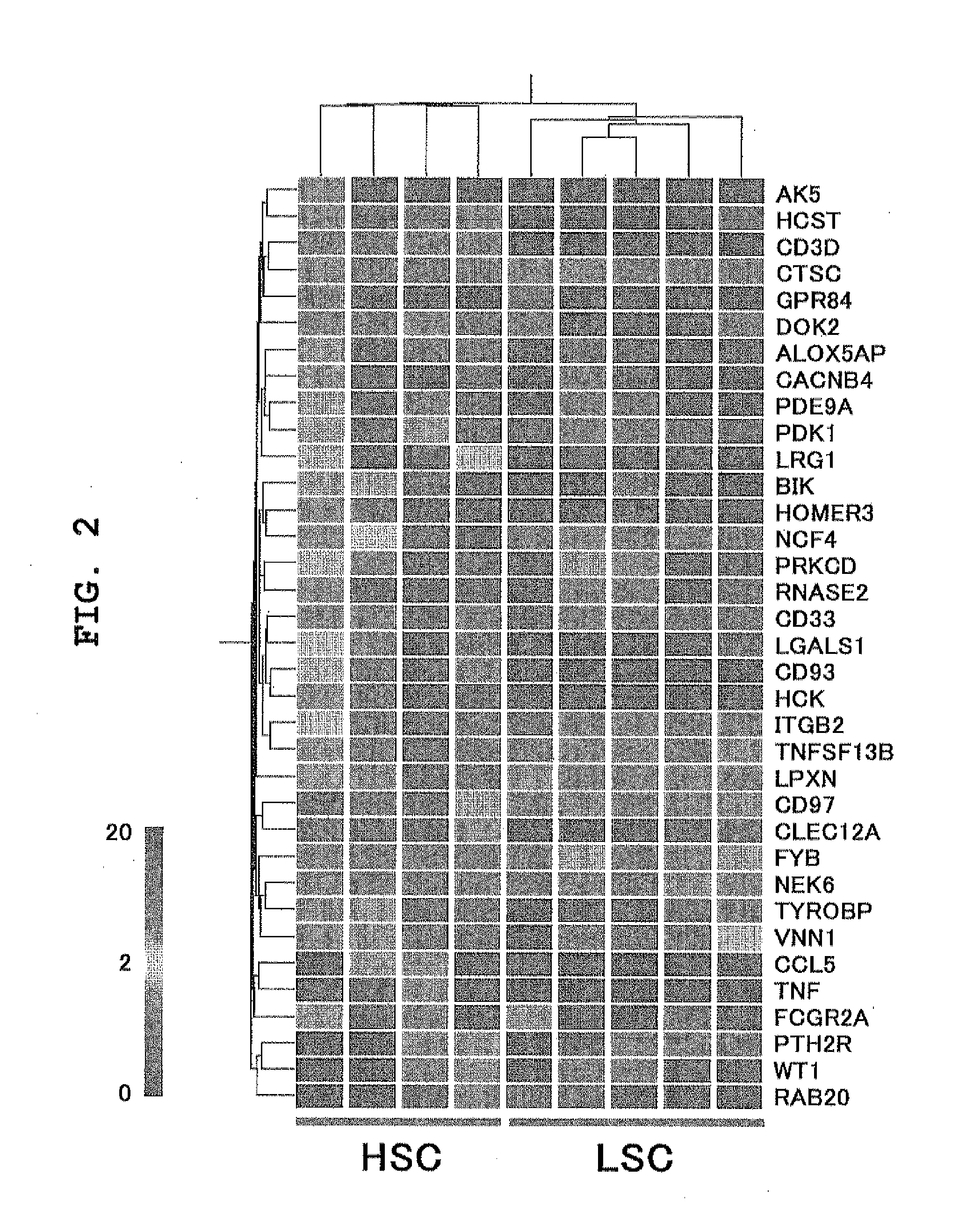
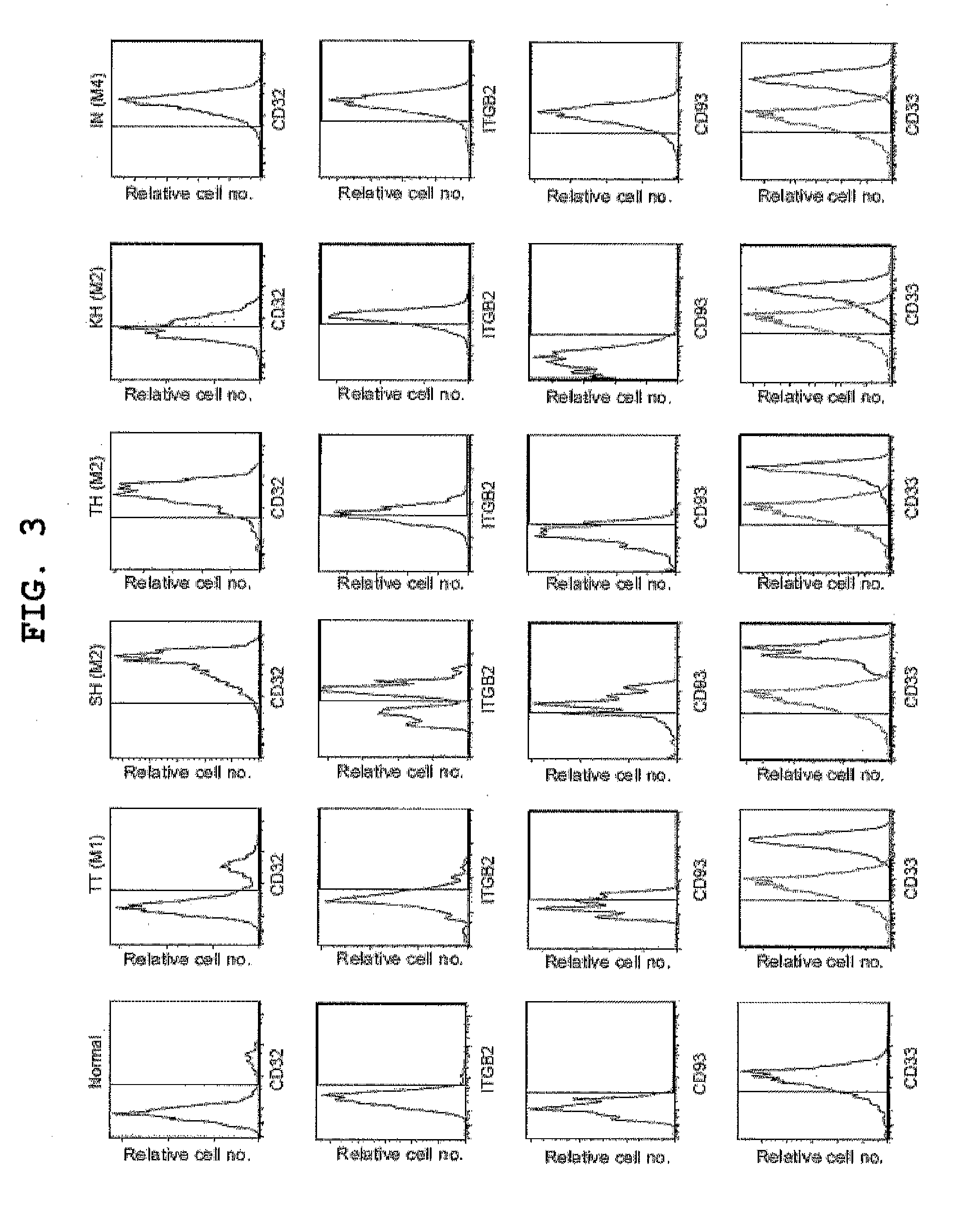
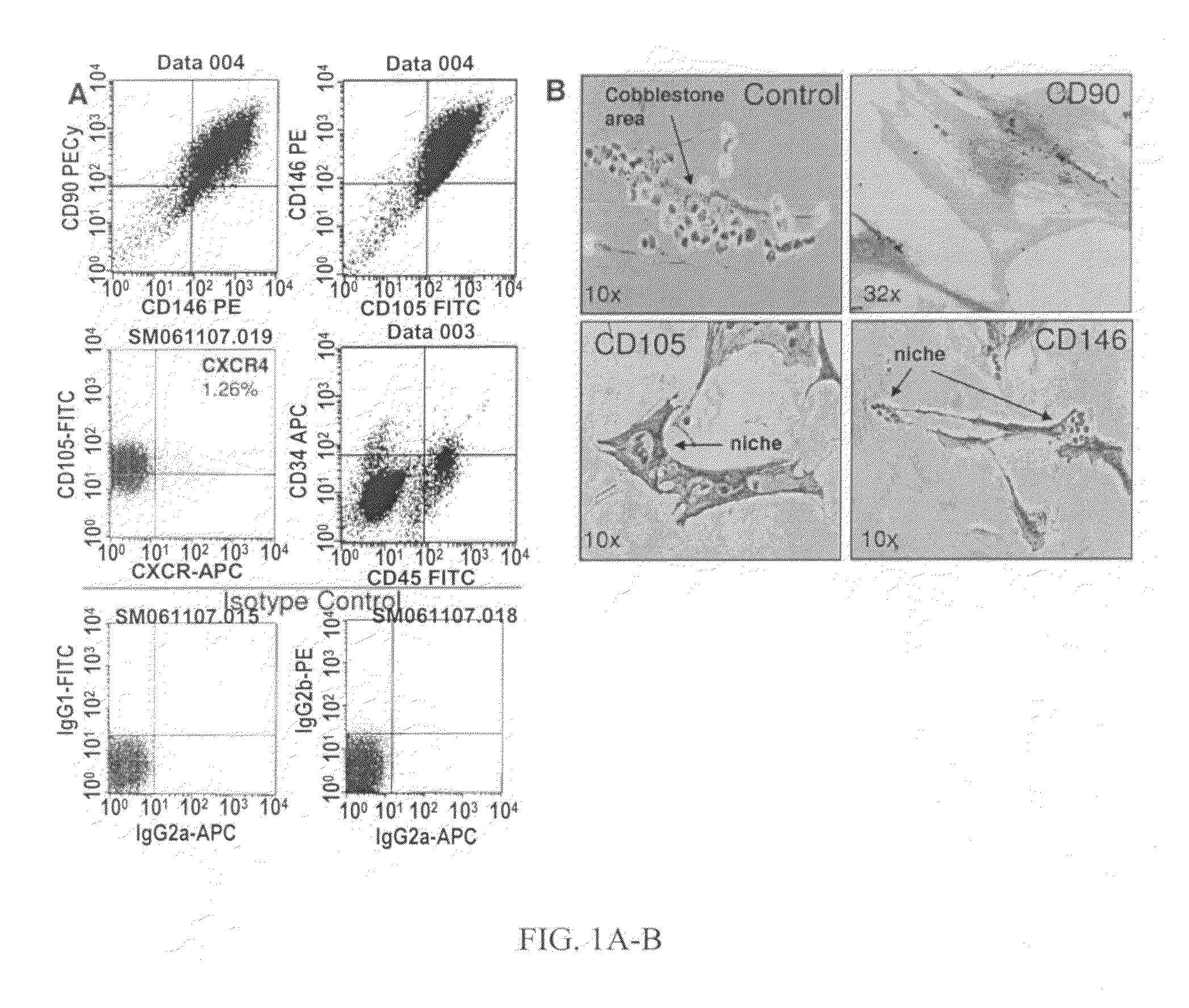
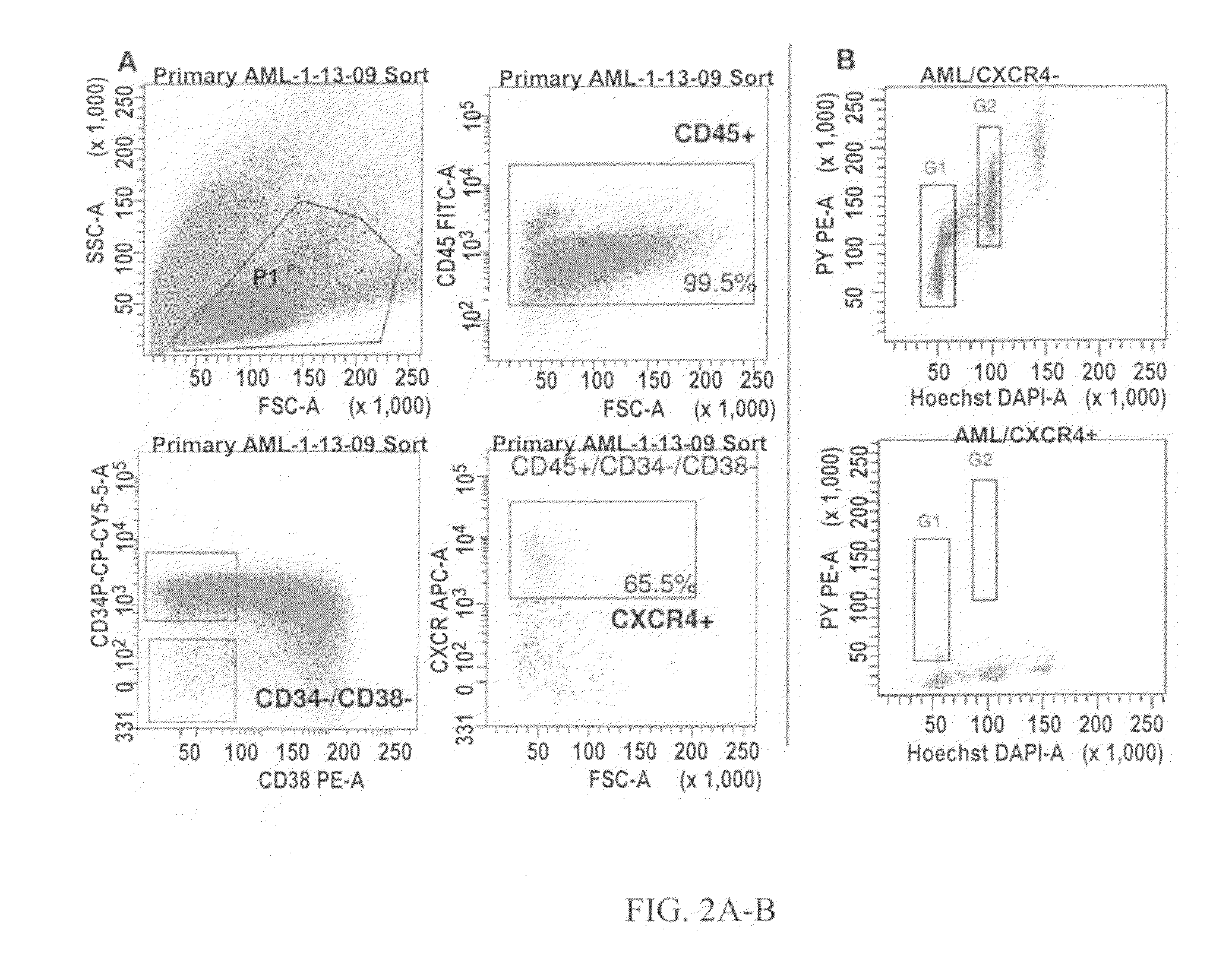
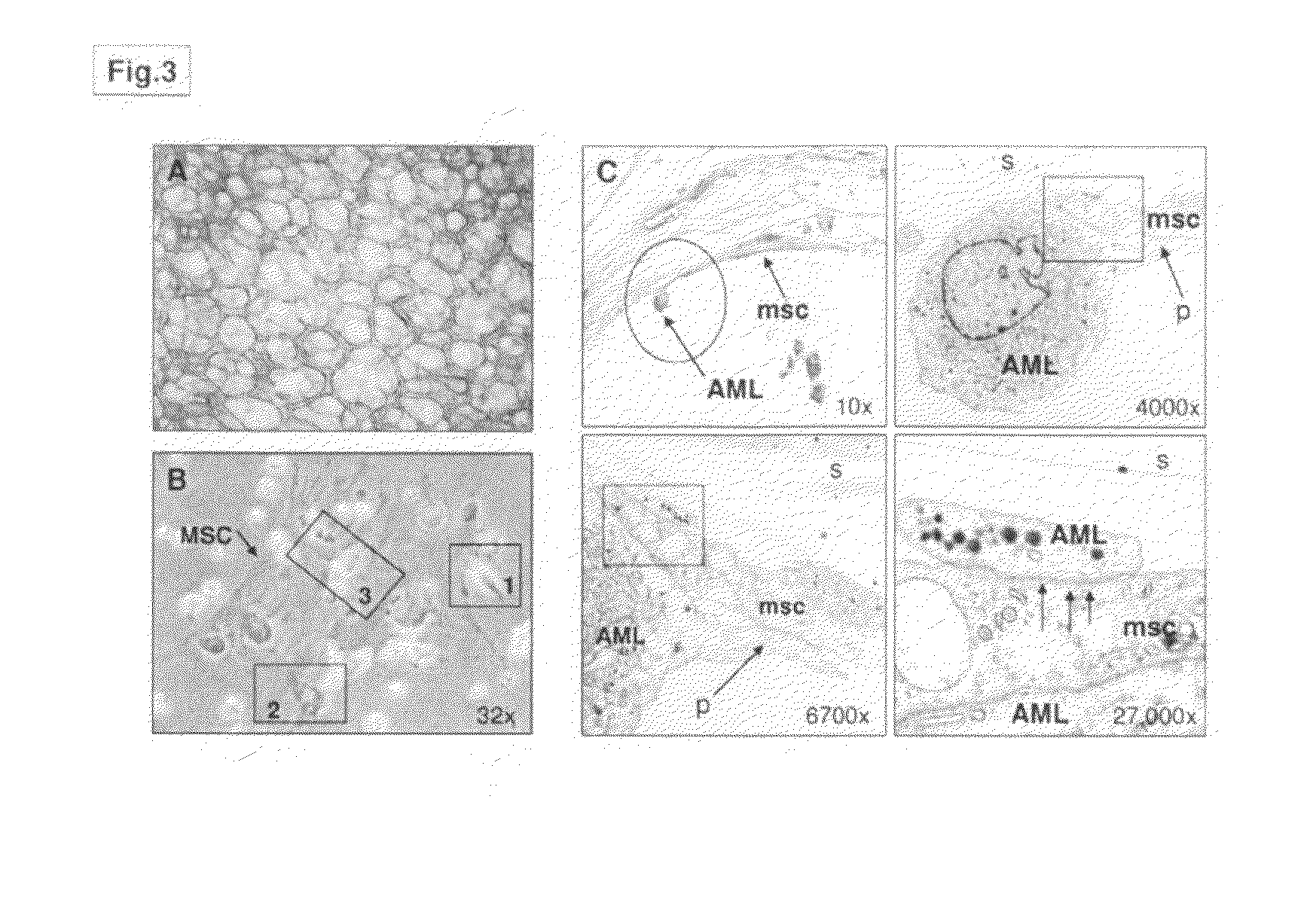
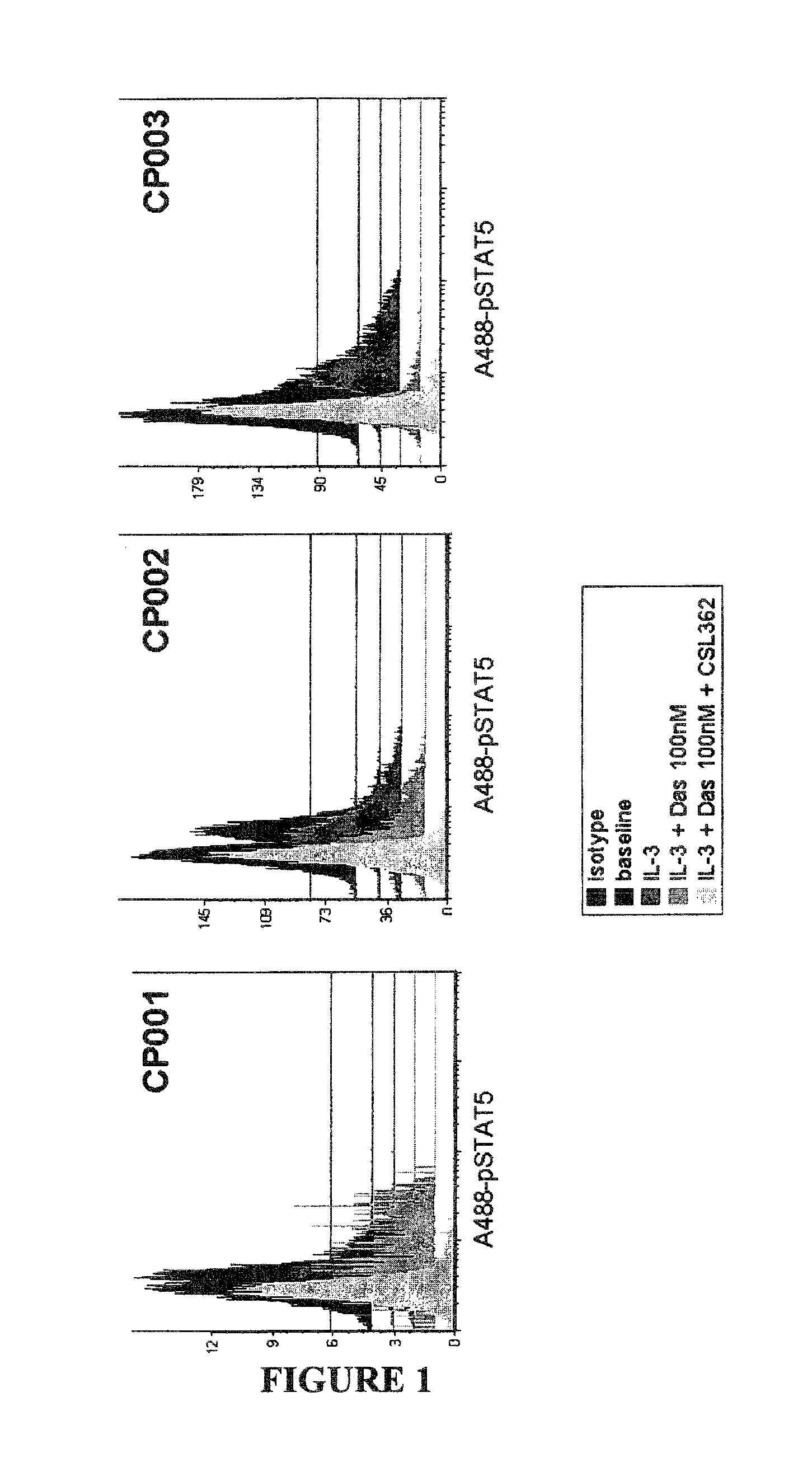
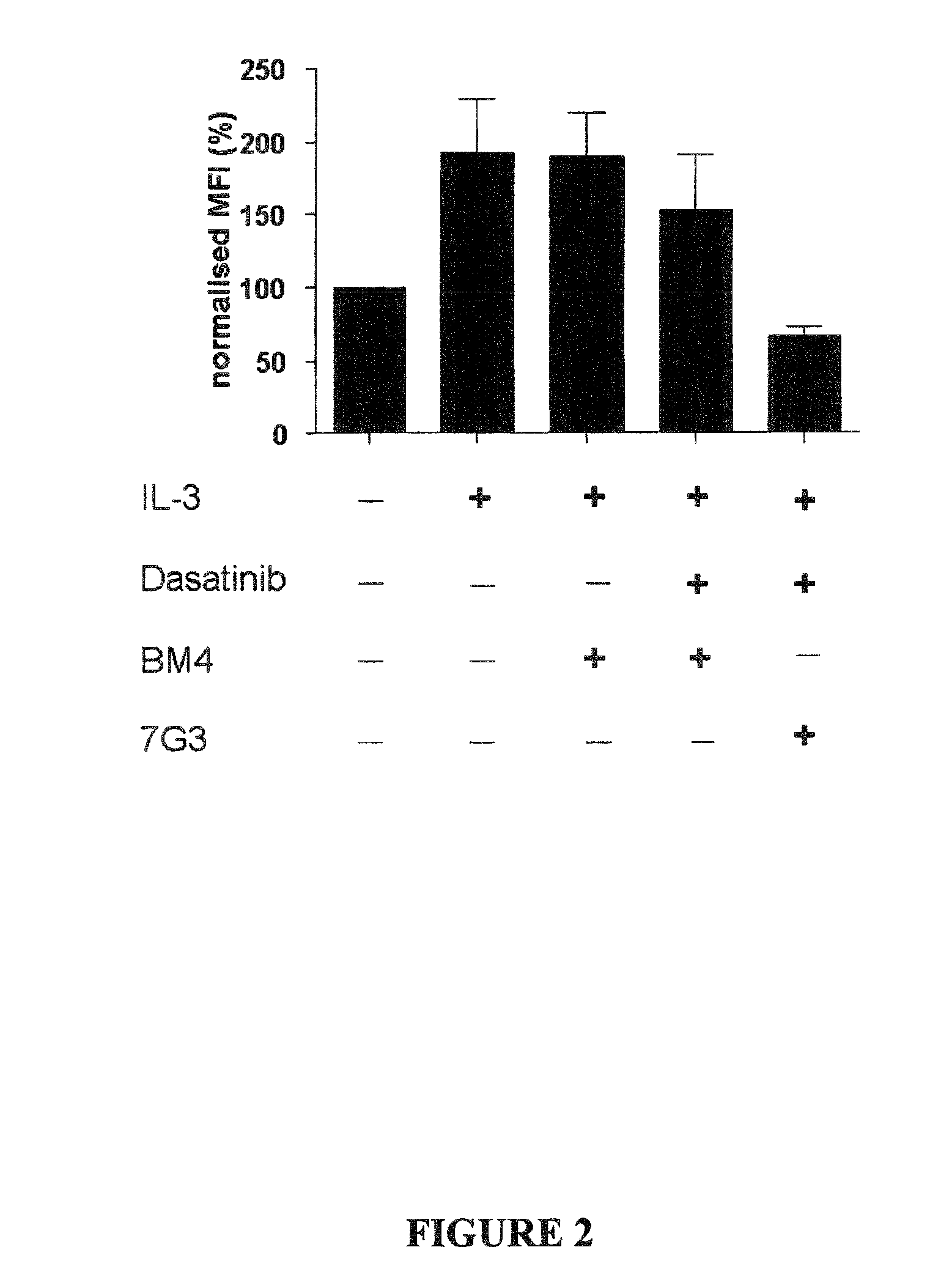
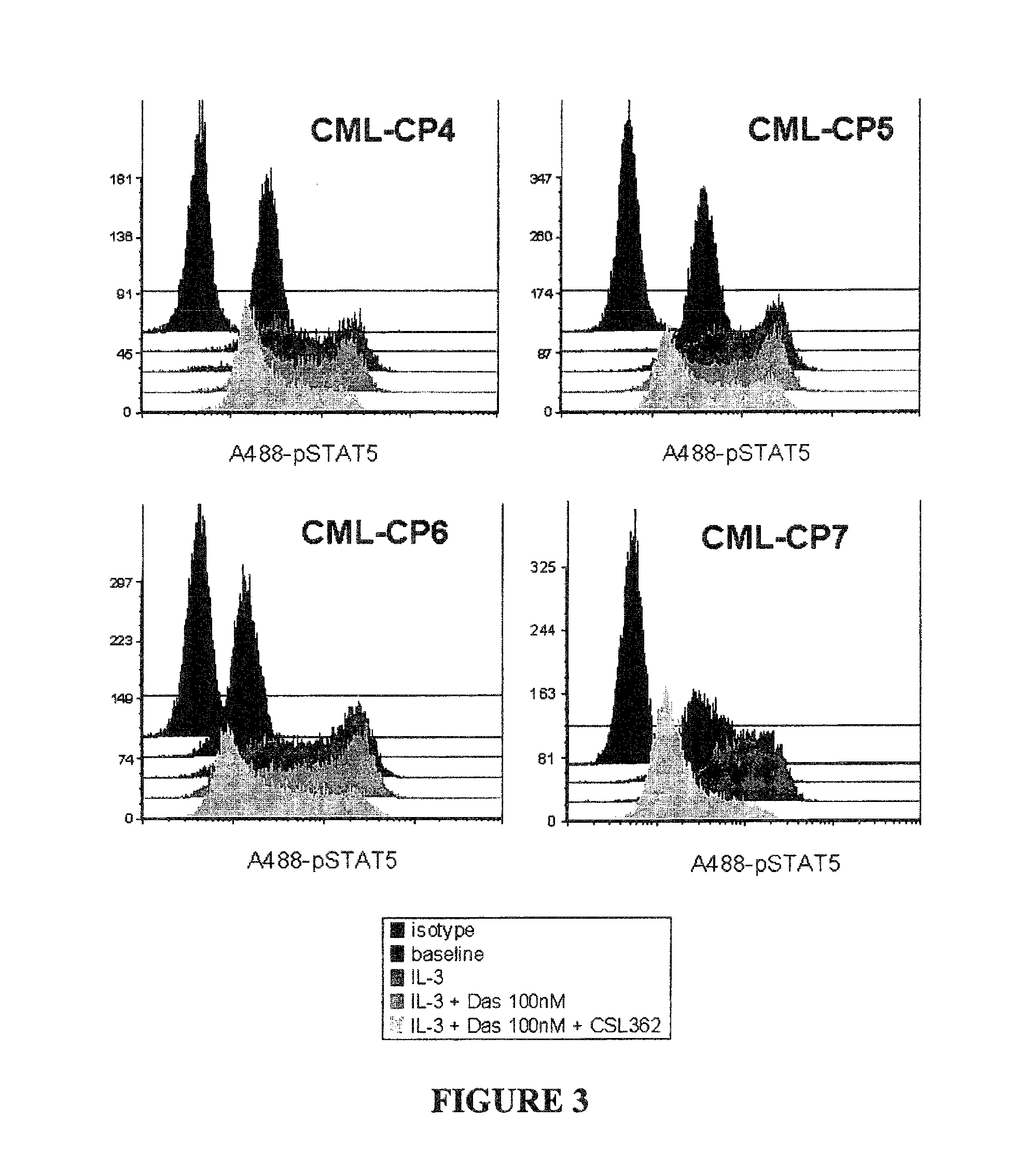
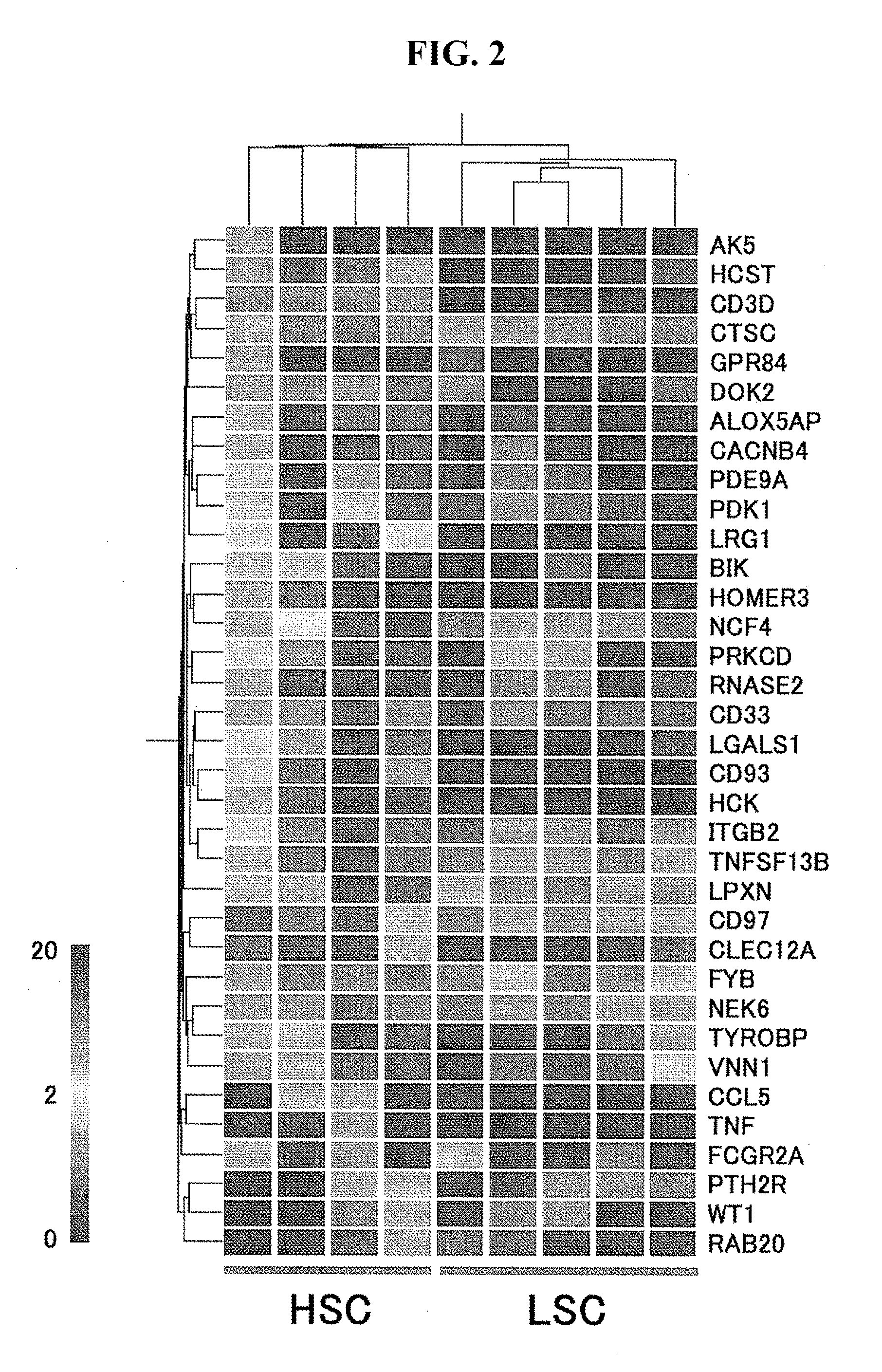
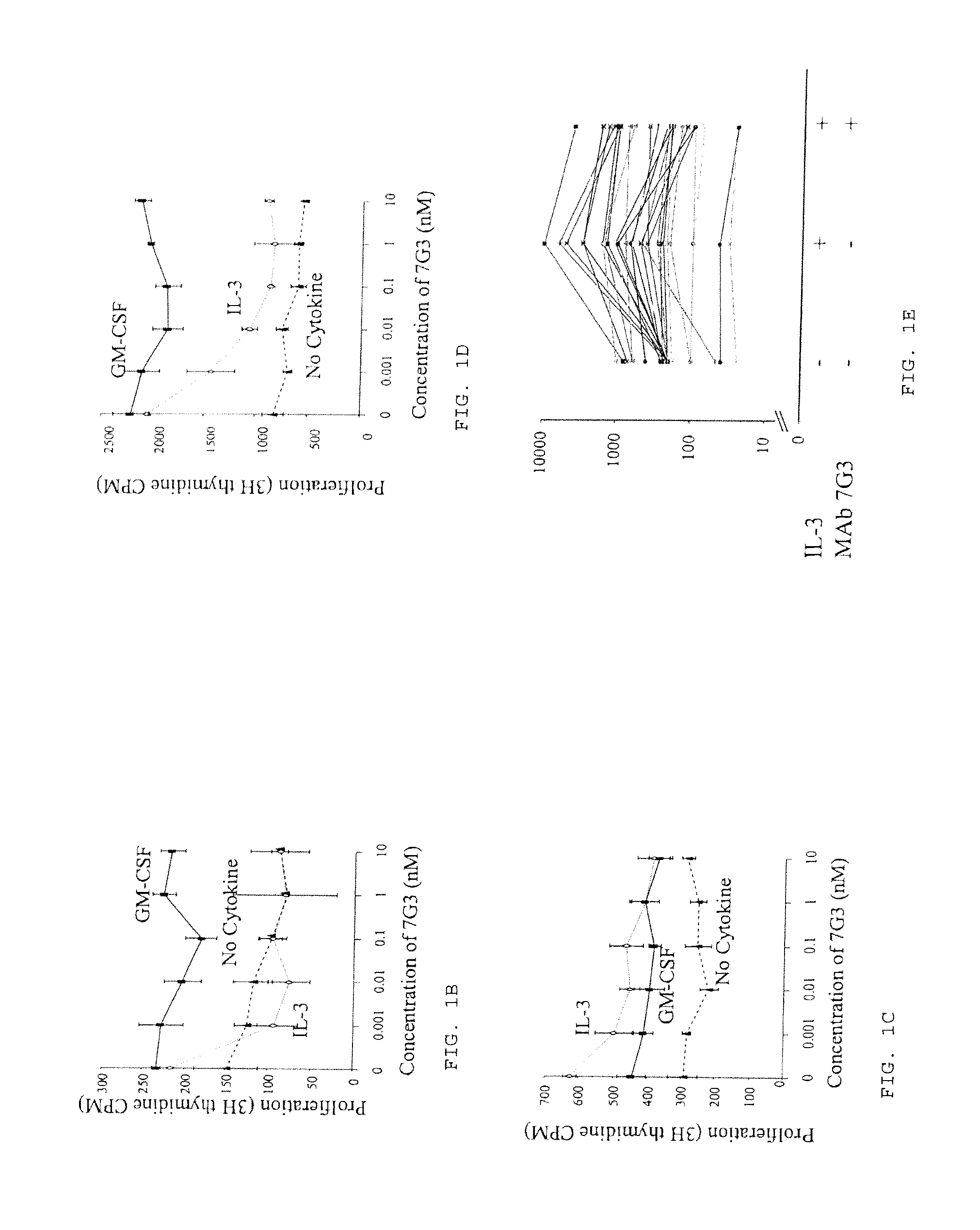
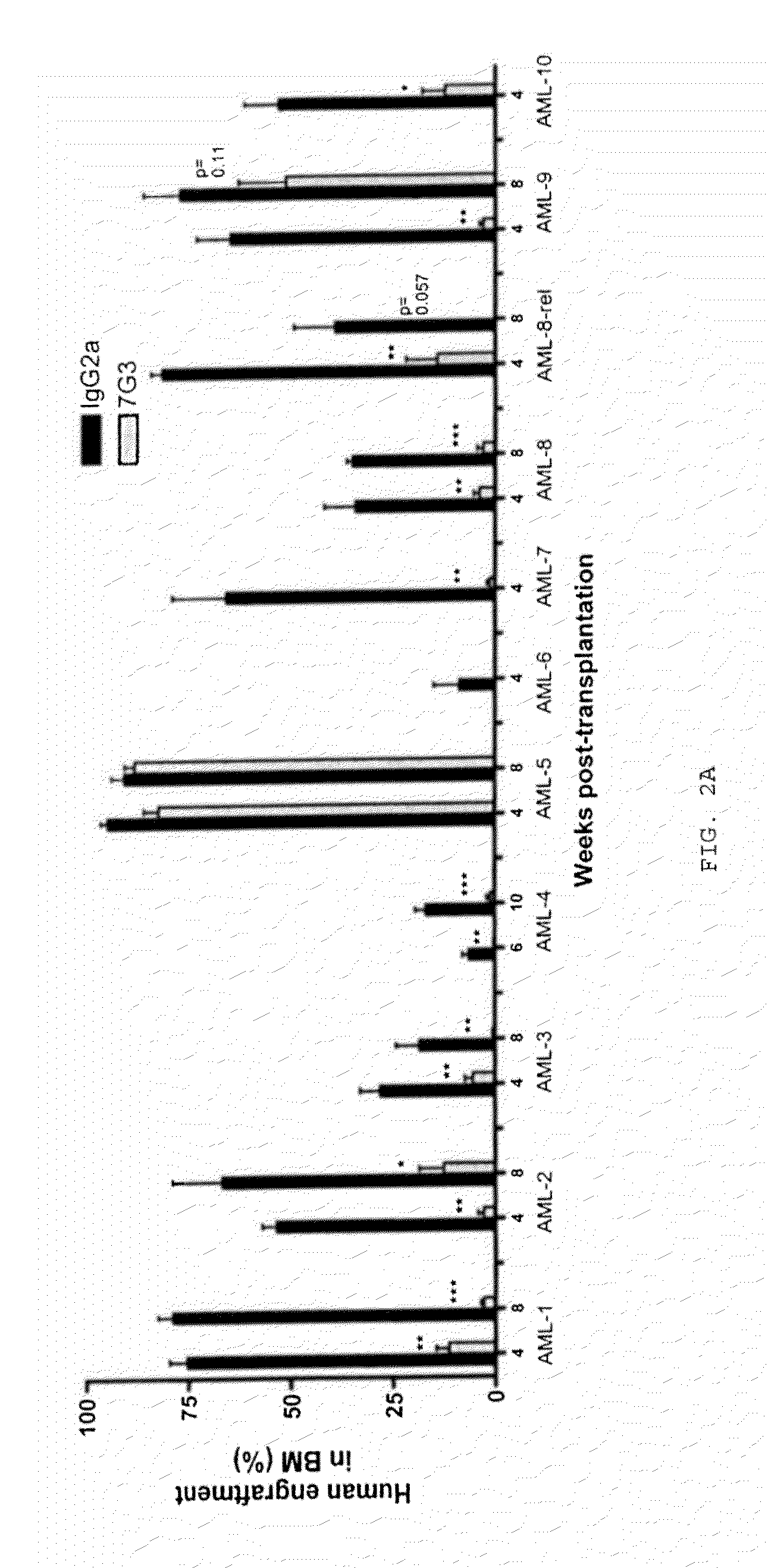
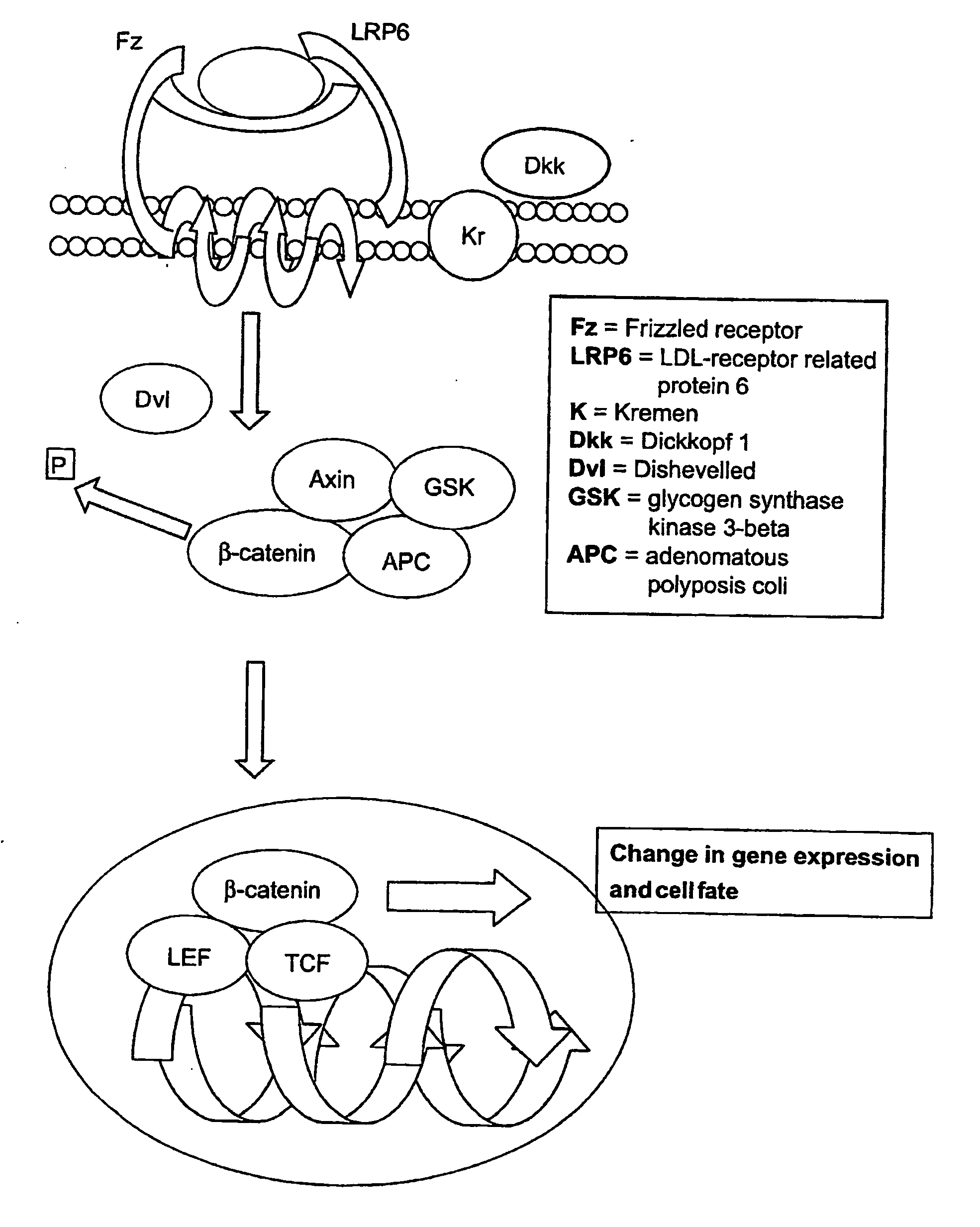
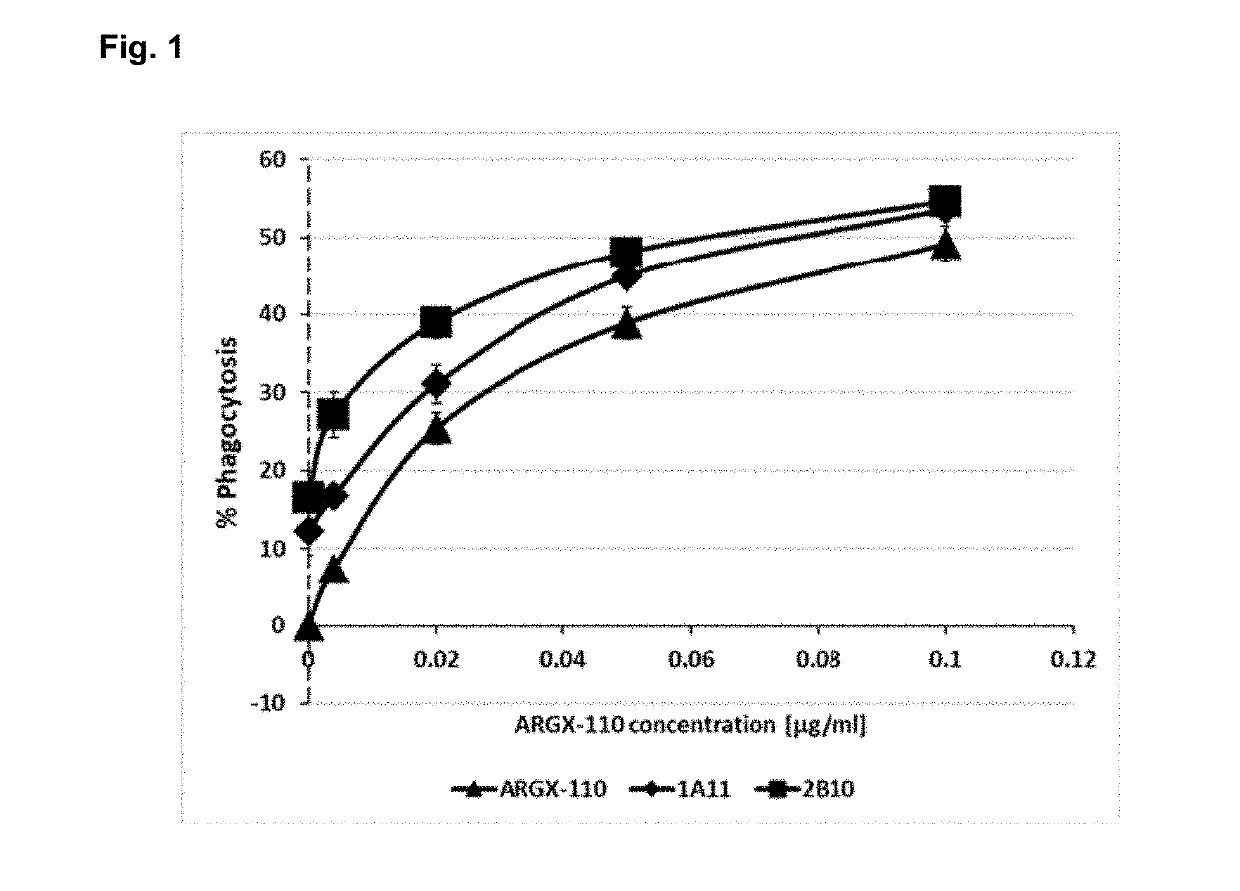
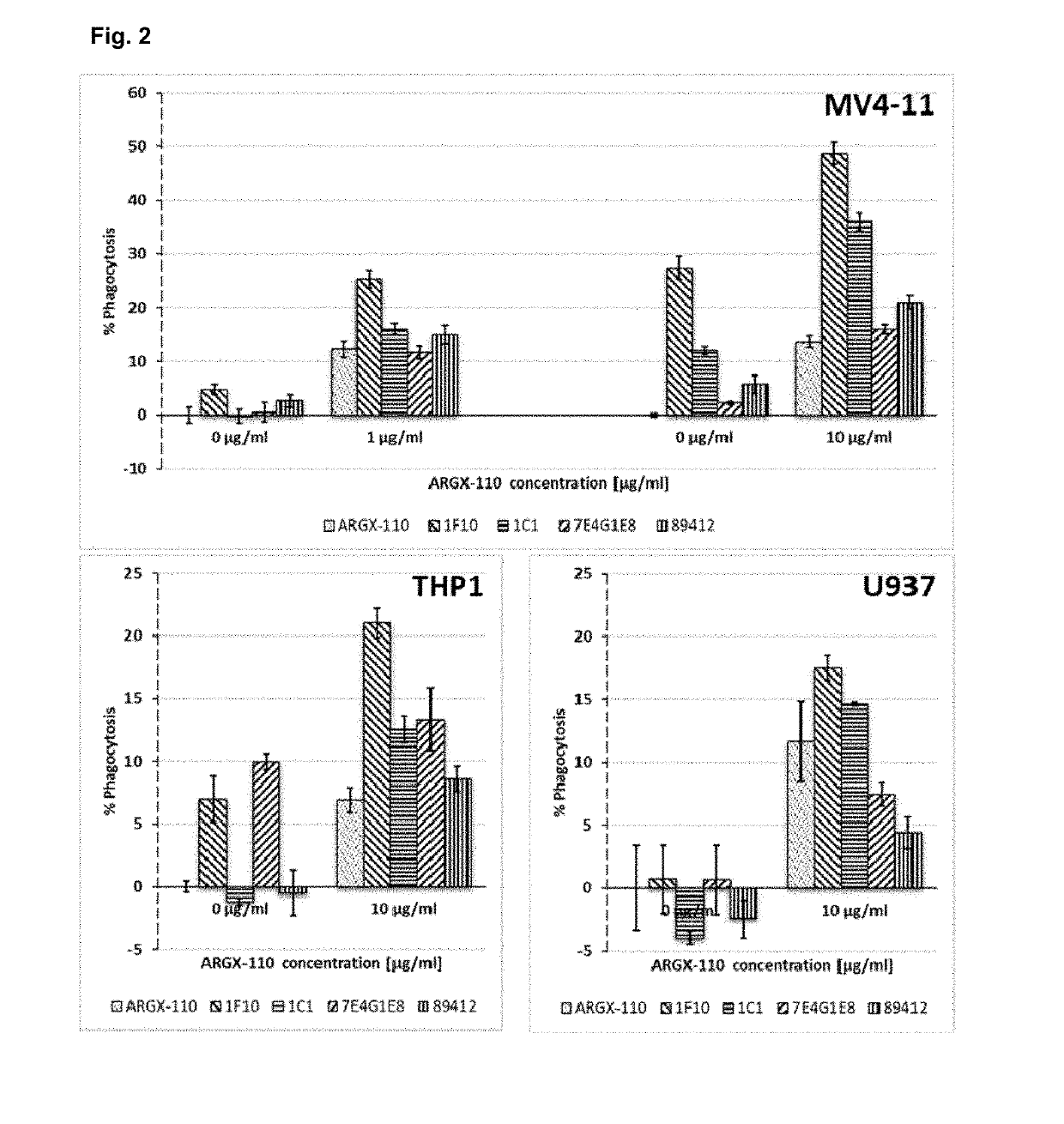
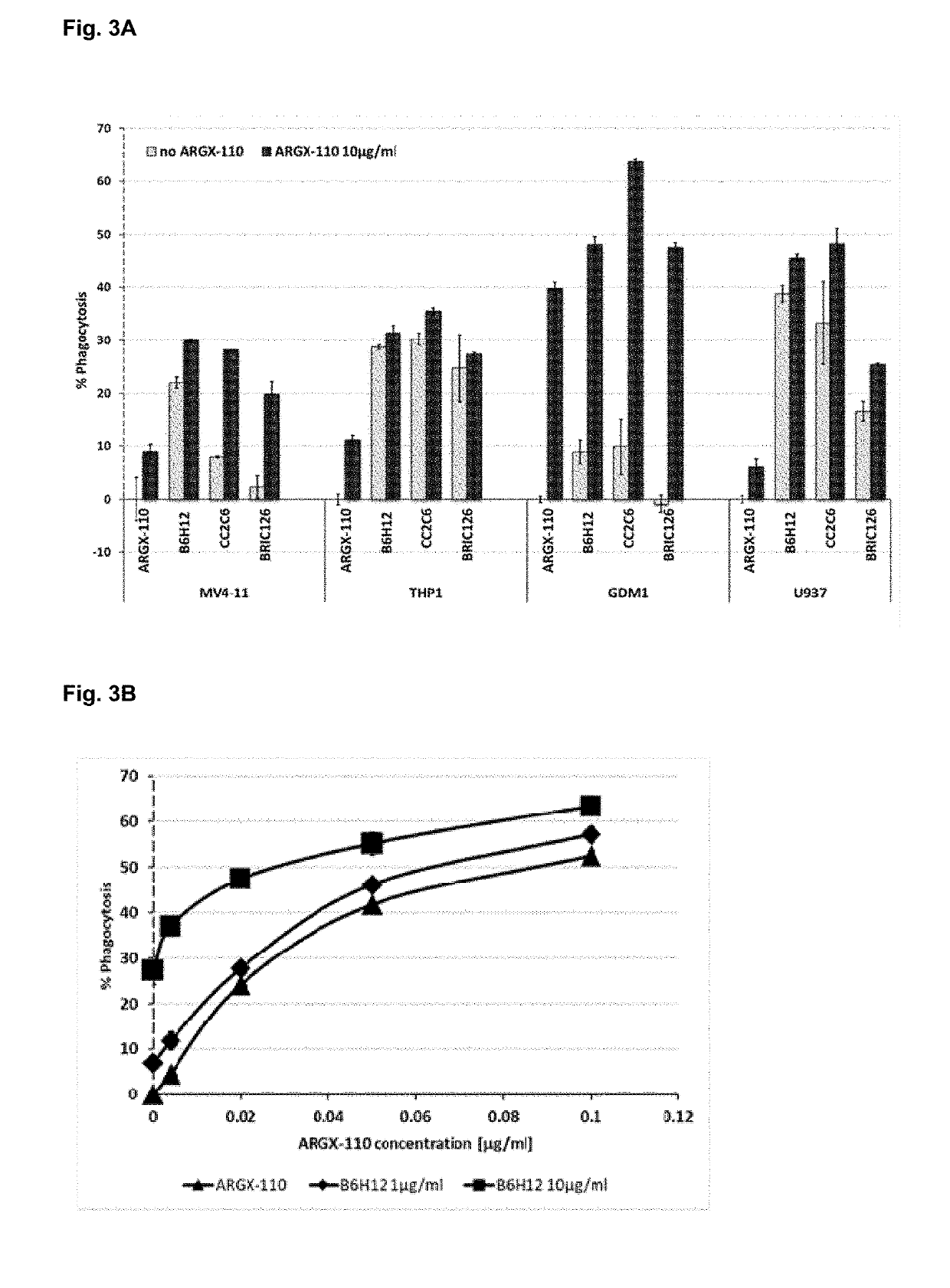
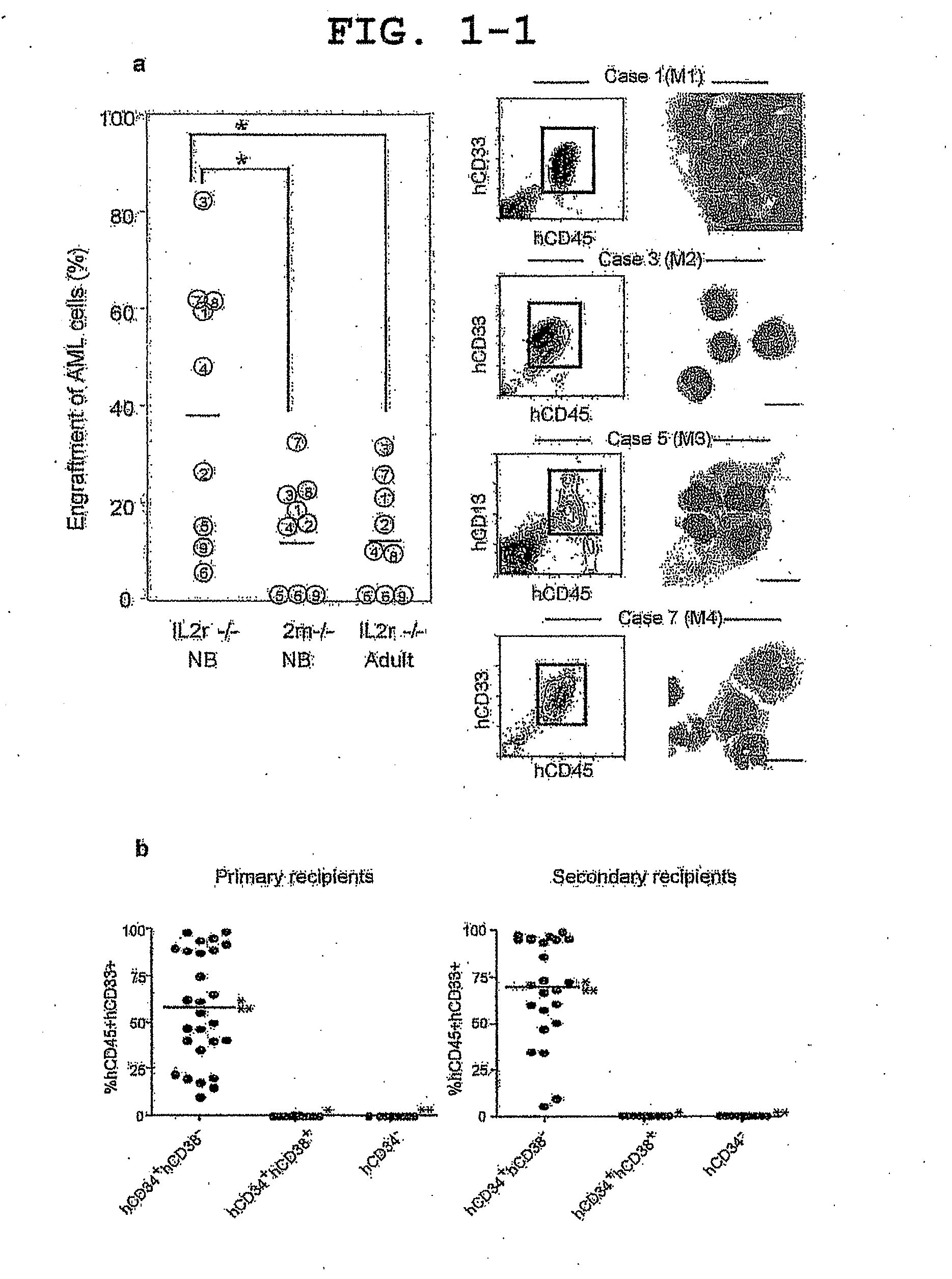
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
30 results about "Leukemic Stem Cell" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
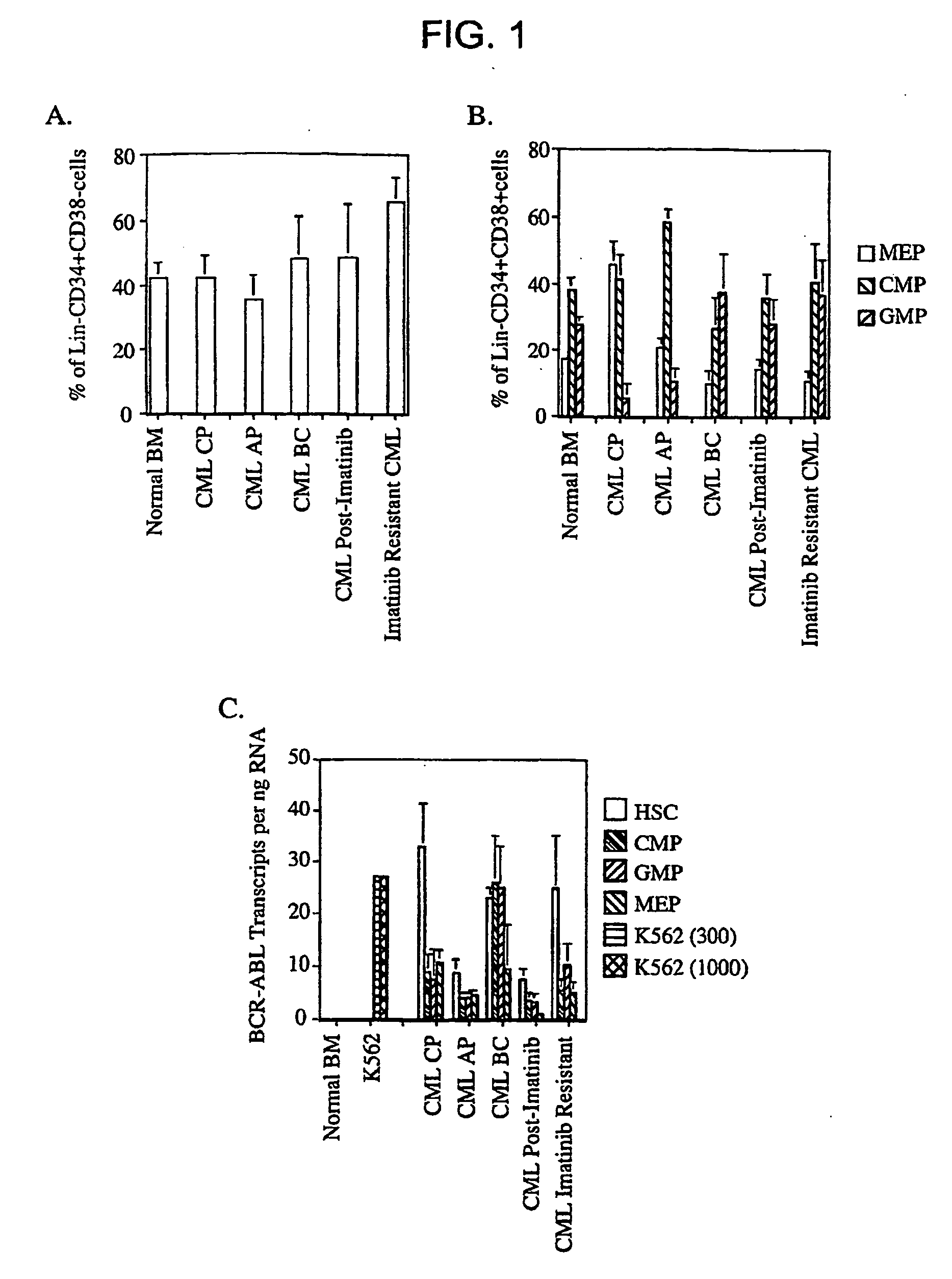
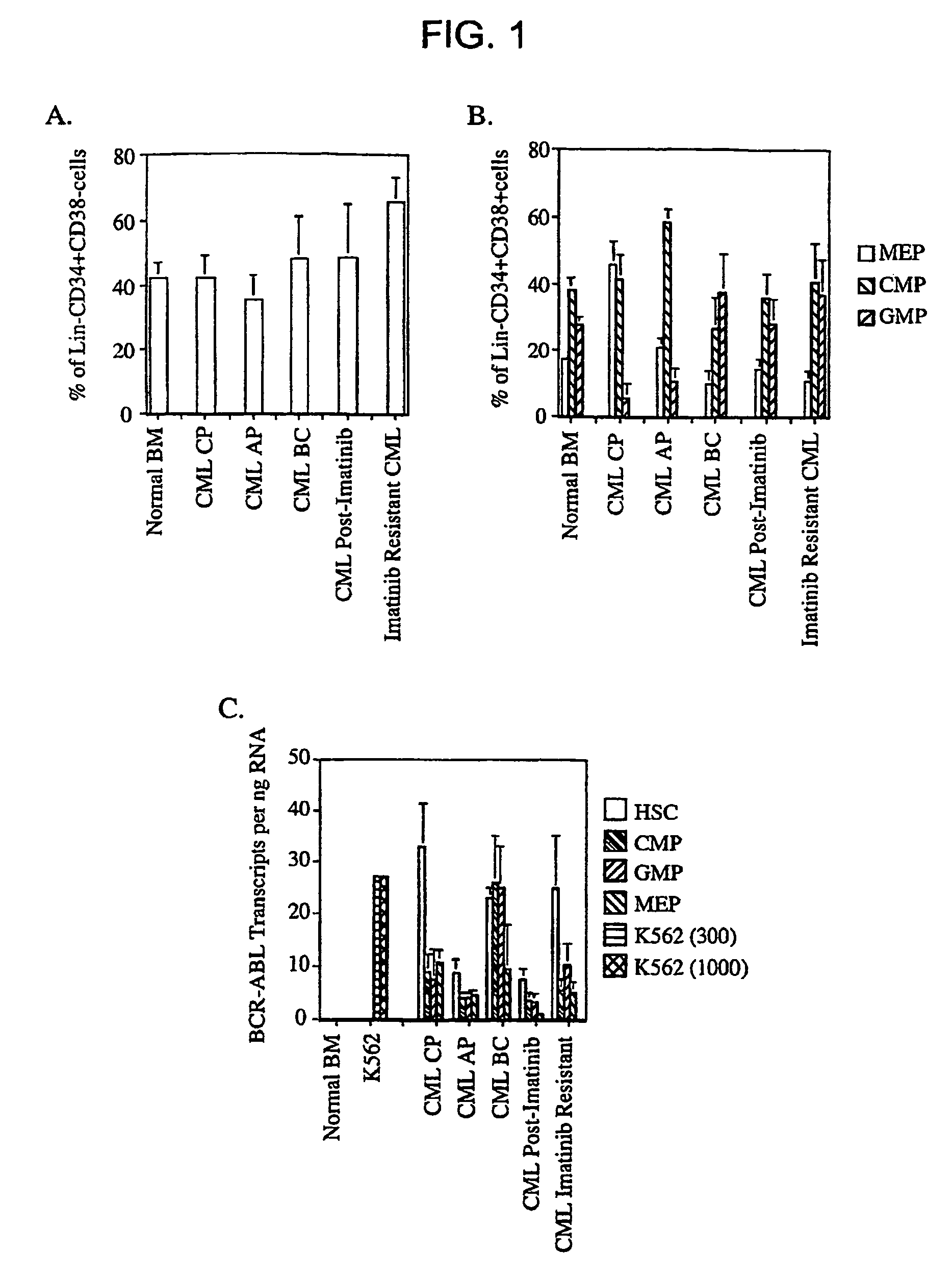
The leukemic stem cell. LSCs can be isolated based on their cell surface markers using the currently available cell-sorting technologies. Once the leukemic stem cells have been isolated, they can be analyzed using the same techniques applied in any type of cancer cell to understand the specific mutations and pathways that propel growth and survival.
Leukemia stem cell markers
InactiveUS20120070450A1Prevent relapseAnimal cellsOrganic active ingredientsHematopoietic cellAnalyte
The invention provides a test method for predicting the initial onset or a recurrence of acute myeloid leukemia (AML) comprising (1) measuring the expression level of human leukemic stem cell (LSC) marker genes in a biological sample collected from a subject for a transcription product or translation product of the gene as an analyte and (2) comparing the expression level with a reference value; an LSC-targeting therapeutic agent for AML capable of suppressing the expression of a gene selected from among LSC marker genes or a substance capable of suppressing the activity of a translation product of the gene; a method for producing a sample containing hematopoietic cells for autologous transplantation or allogeneic transplantation for AML patients comprising obtaining an LSC-purged sample with at least 1 kind of LSC marker as an index; and a method of preventing or treating AML.
Owner:RIKEN
Human bone marrow microenvironments and uses thereof
The present invention is directed to an in vitro cultured permissive niche, or human bone marrow microenvironment, comprising a scaffold coated with human mesenchymal stem cells and a culture medium, wherein the stem cells are viable and proliferate in culture and the niche is permissive for the establishment of introduced hematopoietic or leukemic cell populations. The present invention is also directed to establishment of a permissive niche in a non-human animal model comprising a scaffold coated with human mesenchymal stem cells introduced into the animal ectopically, wherein the niche and the model are permissive for the establishment of introduced hematopoietic or leukemic cell populations. The implanted scaffold forms an ectopic human bone marrow microenvironment to study the mesenchymal leukemic stem cell niche. In addition, the present invention is directed to methods of using the in vitro cultured human bone marrow microenvironment and the non-human animal model to evaluate an agent for anti-leukemic properties.
Owner:THE FEINSTEIN INST FOR MEDICAL RES
Method of treatment of philadelphia chromosome positive leukaemia
InactiveUS20120244116A1Organic active ingredientsPeptide/protein ingredientsBcr-Abl tyrosine-kinase inhibitorLestaurtinib
The invention provides a method for the treatment of Ph+ leukemia in a patient comprising administering to the patient (i) a BCR-ABL tyrosine kinase inhibitor, and (ii) an agent which selectively binds to a cell surface receptor expressed on Ph+ leukemic stem cells. The invention further provides for the use of (i) and (ii) in, or in the manufacture of a medicament for, the treatment of Ph+ leukemia in a patient; and a composition for the treatment of Ph+ leukemia in a patient comprising (i) and (ii); and kits comprising (i) and (ii). In some embodiments, the tyrosine kinase inhibitor is or is not imatinib; or is selected from the group consisting of dasatinib, nilotinib, bosutinib, axitinib, cediranib, crizotinib, damnacanthal, gefitinib, lapatinib, lestaurtinib, neratinib, semaxanib, sunitinib, toceranib, tyrphostins, vandetanib, vatalanib, INNO-406, AP24534, XL228, PHA-739358, MK-0457, SGX393 and DC2036; or is selected from the group consisting of dasatinib and nilotinib. In some embodiments, the agent binds to a receptor involved in signalling by at least one of IL-3, G-CSF and GM-CSF. In some embodiments, the agent is a mutein selected from the group consisting of IL-3 muteins, G-CSF muteins and GM-CSF muteins. In some embodiments, the mutein is an IL-3 mutein. In some embodiments, the agent is a soluble receptor which is capable of binding to IL-3.
Owner:CSL LTD
Leukemia stem cell markers
InactiveUS20140274788A1Prevent relapseOrganic active ingredientsMicrobiological testing/measurementHematopoietic cellAnalyte
Owner:RIKEN +1
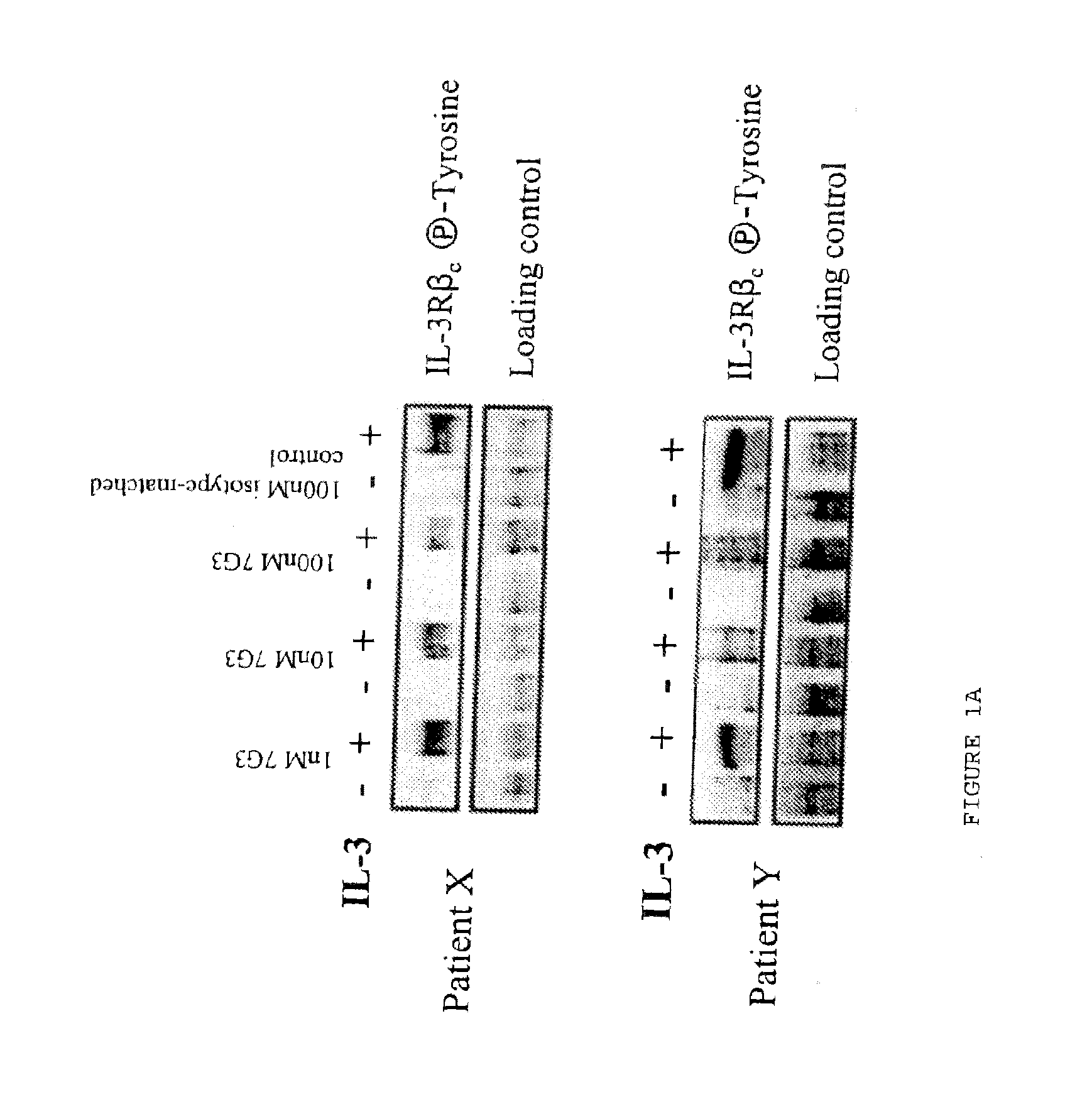
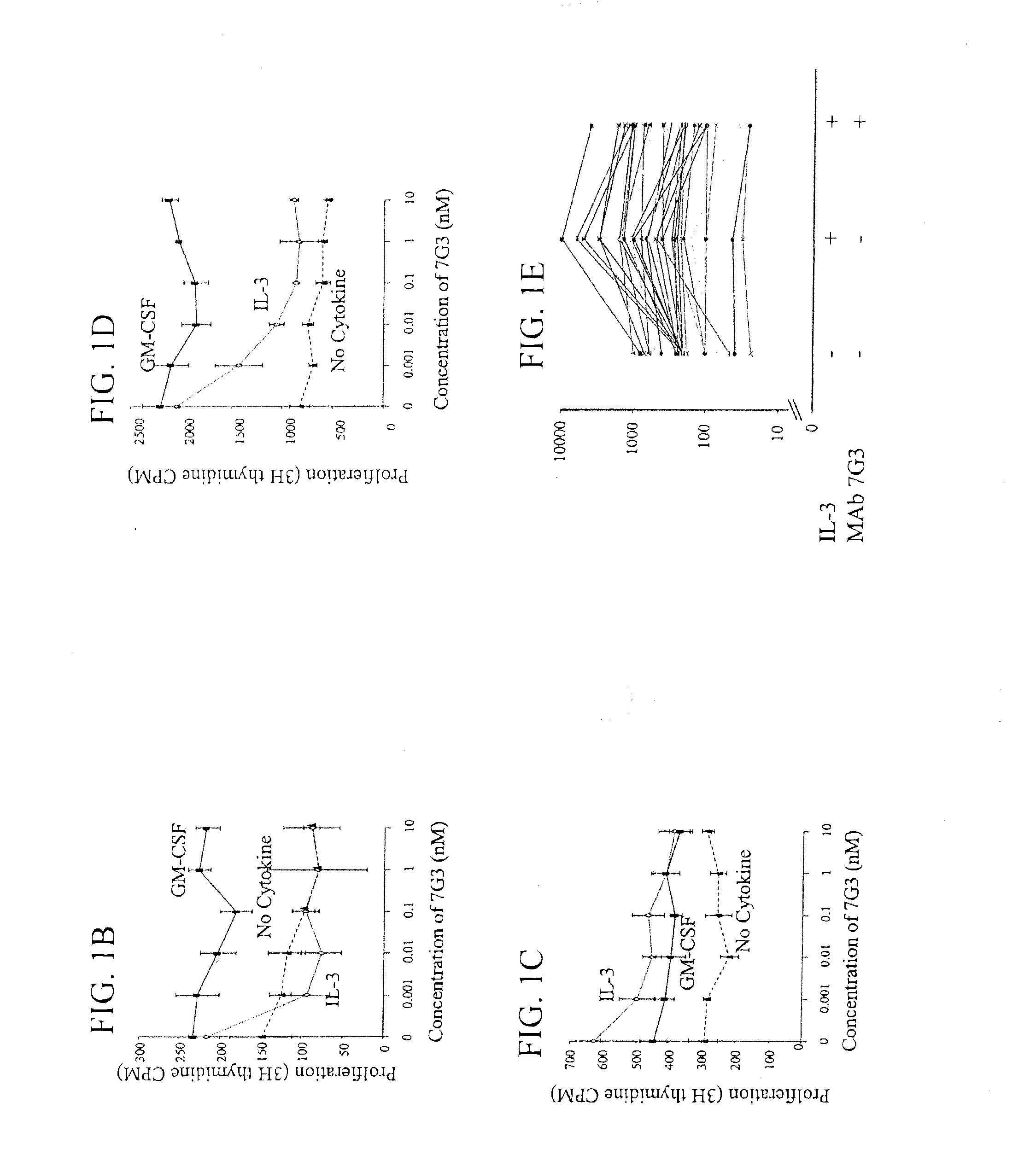
Method of inhibition of leukemic stem cells
InactiveUS20110052574A1Enhanced Fc effector functionFunction increaseOrganic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsAntigen bindingLeukemic Stem Cell
Owner:CSL LTD +1
Method of inhibition of leukemic stem cells
InactiveUS20130230510A1Function increaseOrganic active ingredientsAntibody ingredientsAntigen bindingLeukemic Stem Cell
A method for inhibition of leukemic stem cells expressing IL-3R.alpha.; (CD 123), comprises contacting the cells with an antigen binding molecule comprising a Fc region or a modified Fc region having enhanced Fc effector function, wherein the antigen binding molecule binds selectively to IL-3R.alpha. (CD123). The invention includes the treatment of a hematologic cancer condition in a patient by administration to the patient of an effective amount of the antigen binding molecule.
Owner:UNIV HEALTH NETWORK +1
Identification, Isolation and Elimination of Cancer Stem Cells
InactiveUS20080020407A1Biological material analysisArtificial cell constructsCell specificBiological activation
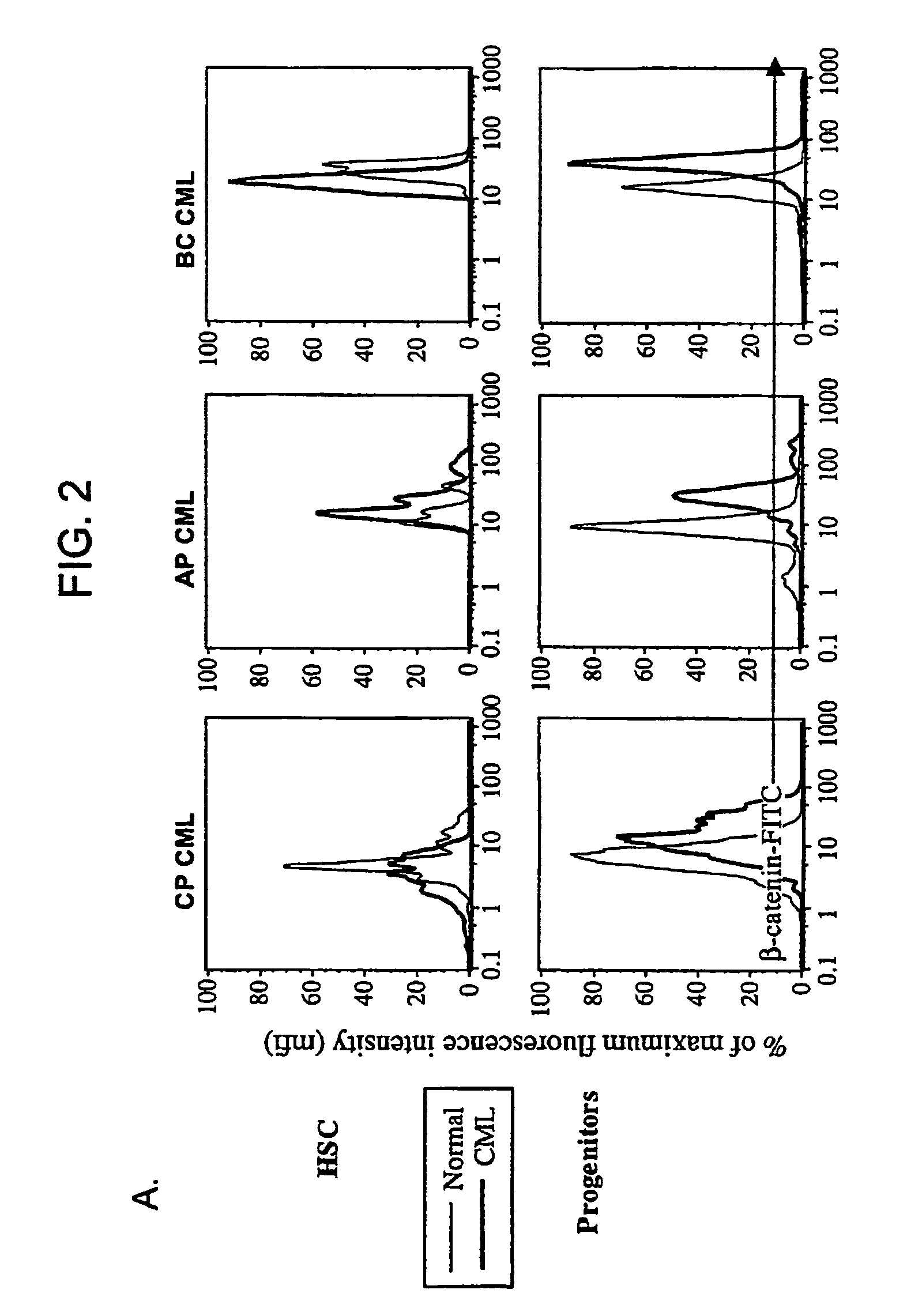
Isolated populations of leukemic stem cells are provided. The cells are useful for experimental evaluation, and as a source of lineage and cell specific products, and as targets for the discovery of factors or molecules that can affect them. Detection of leukemic stem cells is useful in predicting disease progression, relapse, and development of drug resistance. Proliferation of LSC may be inhibited through interfering with activation of the beatenin pathway. Methods are provided for the clinical staging of pre-leukemia and leukemias by differential analysis of hematologic samples for the distribution of one or more hematopoietic stem or progenitor cell subsets.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Identification, isolation and elimination of cancer stem cells
Isolated populations of leukemic stem cells are provided. The cells are useful for experimental evaluation, and as a source of lineage and cell specific products, and as targets for the discovery of factors or molecules that can affect them. Detection of leukemic stem cells is useful in predicting disease progression, relapse, and development of drug resistance. Proliferation of LSC may be inhibited through interfering with activation of the bcatenin pathway. Methods are provided for the clinical staging of pre-leukemia and leukemias by differential analysis of hematologic samples for the distribution of one or more hematopoietic stem or progenitor cell subsets.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Methods and compositions for identifying leukemic stem cells
ActiveUS20180120321A1Improve the level ofMicrobiological testing/measurementBiological material analysisLeukemic Stem CellMolecular biology
Owner:CHARLOTTE MECKLENBURG HOSPITAL AUTHORITY
Method of treatment of philadelphia chromosome positive leukemia
InactiveUS20150093355A1Organic active ingredientsPeptide/protein ingredientsBcr-Abl tyrosine-kinase inhibitorLestaurtinib
The invention provides a method for the treatment of Ph+ leukemia in a patient comprising administering to the patient (i) a BCR-ABL tyrosine kinase inhibitor, and (ii) an agent which selectively binds to a cell surface receptor expressed on Ph+ leukemic stem cells. The invention further provides for the use of (i) and (ii) in, or in the manufacture of a medicament for, the treatment of Ph+ leukemia in a patient; and a composition for the treatment of Ph+ leukemia in a patient comprising (i) and (ii); and kits comprising (i) and (ii). In some embodiments, the tyrosine kinase inhibitor is or is not imatinib; or is selected from the group consisting of dasatinib, nilotinib, bosutinib, axitinib, cediranib, crizotinib, damnacanthal, gefitinib, lapatinib, lestaurtinib, neratinib, semaxanib, sunitinib, toceranib, tyrphostins, vandetanib, vatalanib, INNO-406, AP24534, XL228, PHA-739358, MK-0457, SGX393 and DC2036; or is selected from the group consisting of dasatinib and nilotinib. In some embodiments, the agent binds to a receptor involved in signalling by at least one of IL-3, G-CSF and GM-CSF. In some embodiments, the agent is a mutein selected from the group consisting of IL-3 muteins, G-CSF muteins and GM-CSF muteins. In some embodiments, the mutein is an IL-3 mutein. In some embodiments, the agent is a soluble receptor which is capable of binding to IL-3.
Owner:CSL LTD
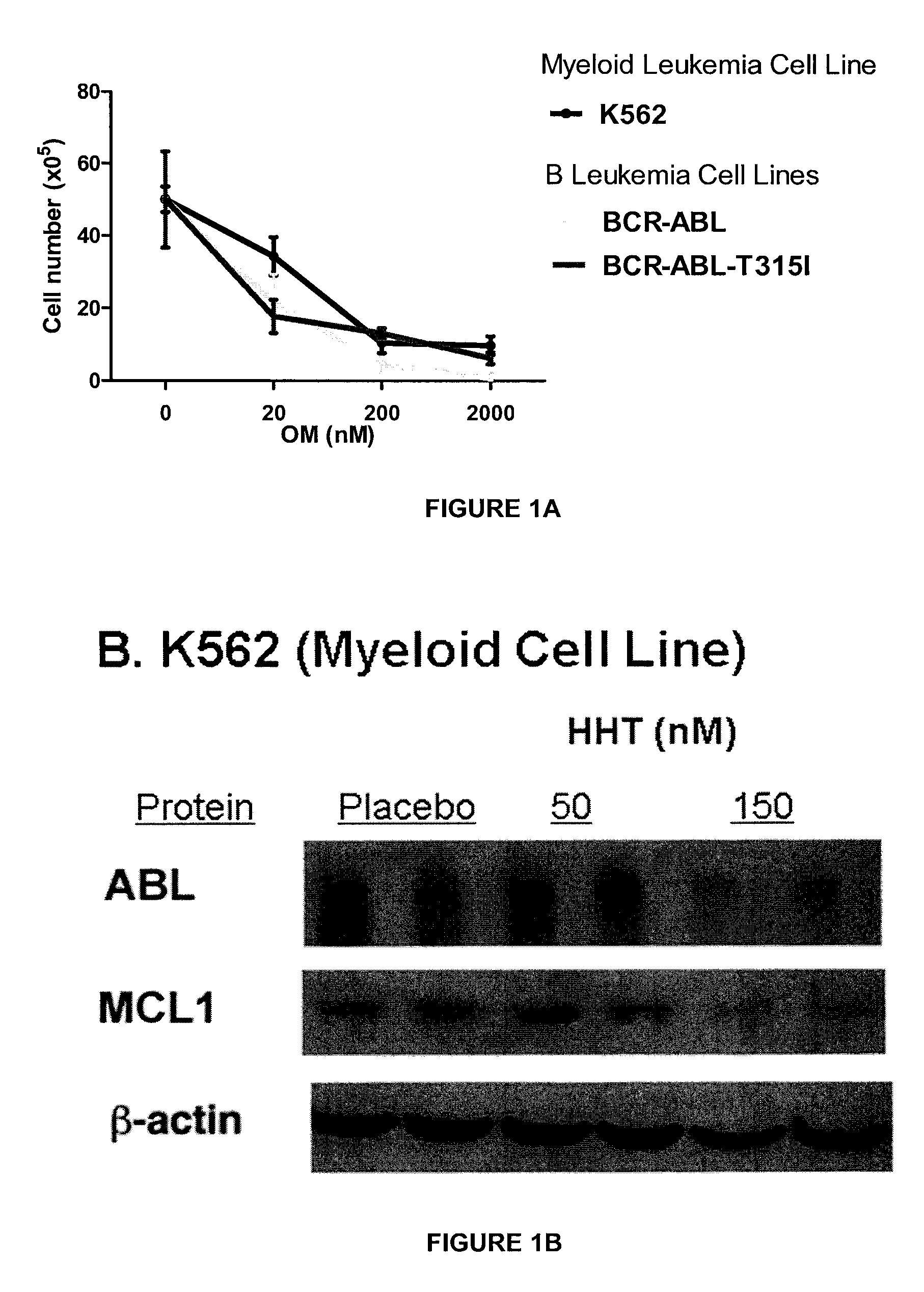
Use of mitochondrial activity inhibitors for the treatment of poor prognosis acute myeloid leukemia
ActiveCN109689051AOrganic active ingredientsOrganic chemistryHigh level expressionMitochondrial electron transport
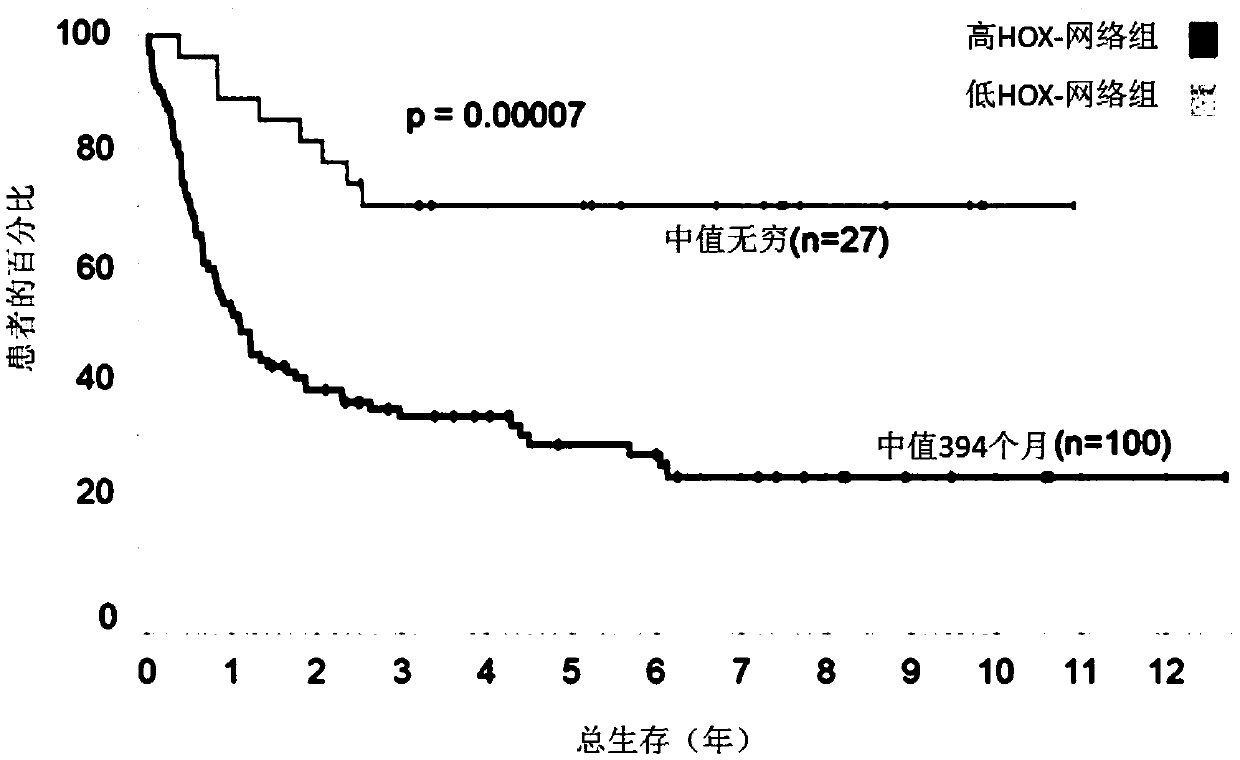
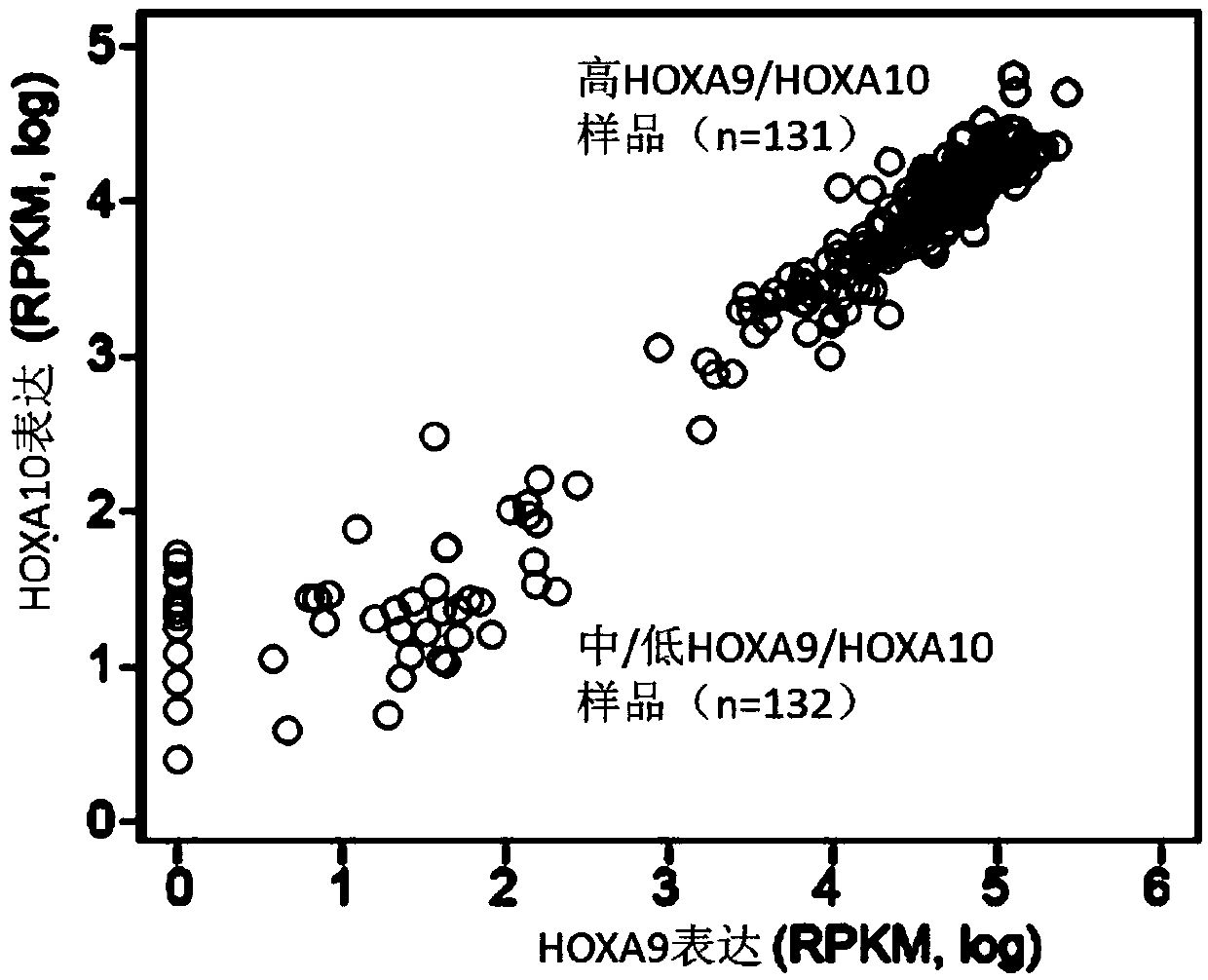
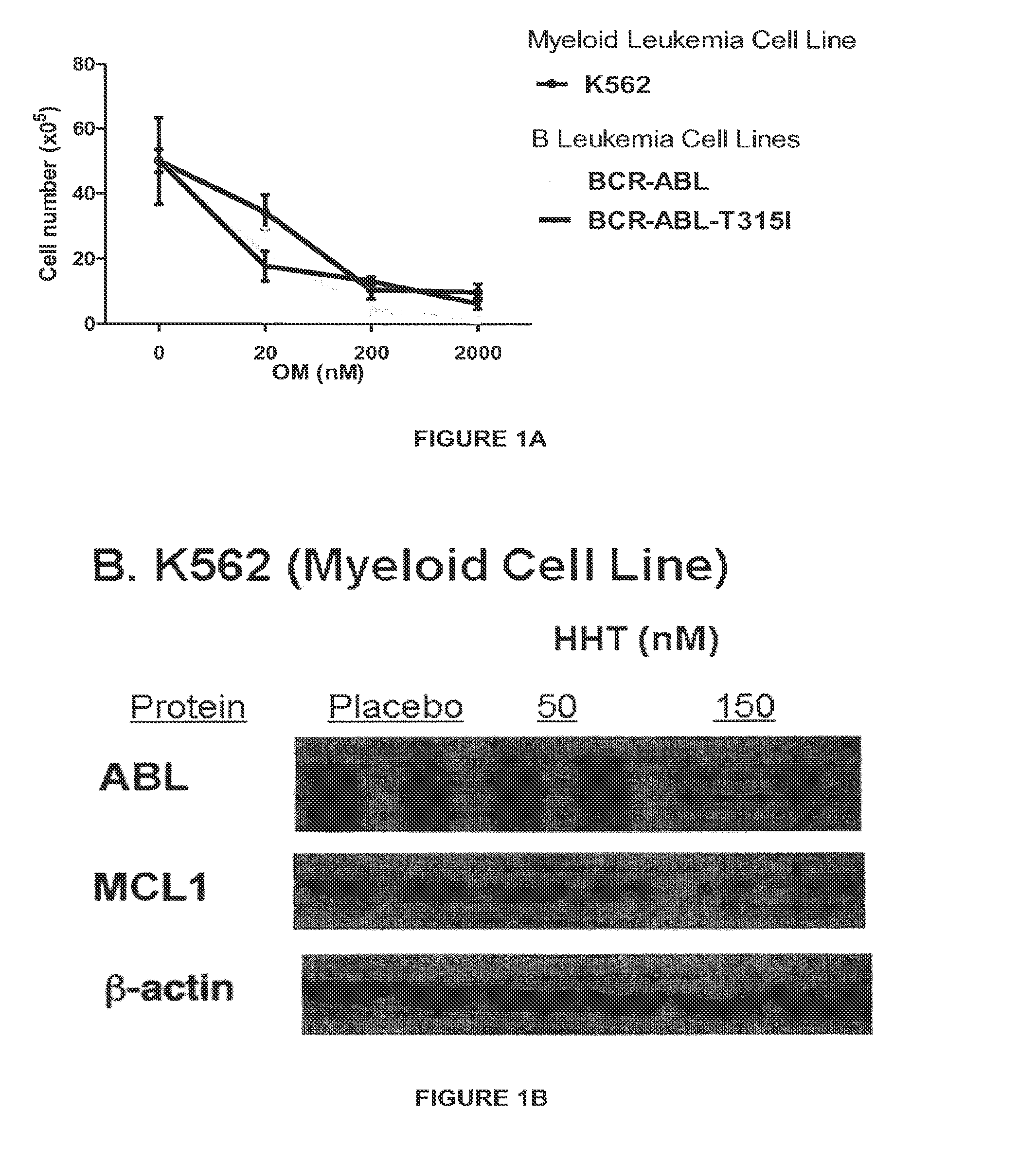
A method for treating acute myeloid leukemia (AML), such as poor risk AML, by administering to a subject in need thereof an effective amount of a mitochondrial activity inhibitor, for example a classA electron transport chain (ETC) complex I inhibitor such as Mubritinib or a pharmaceutically acceptable salt thereof, is disclosed. The AML to be treated may be characterized by certain features, such as high level of expression of one or more Homeobox (HOX)-network genes, high and / or low expression of specific genes, the presence of one or more cytogenetic or molecular risk factors such as intermediate cytogenetic risk, normal karyotype (NK), mutated NPM1, mutated CEBPA, mutated FLT3, mutated DNMT3A, mutated TET2, mutated IDH1, mutated IDH2, mutated RUNX1, mutated WT1, mutated SRSF2, intermediate cytogenetic risk with abnormal karyotype (intern(abnK)), trisomy 8 (+8) and / or abnormal chromosome (5 / 7), and / or a high leukemic stem cell (LSC) frequency.
Owner:UNIV DE MONTREAL
Cd70 combination therapy
ActiveUS20190241668A1Mortality rate is decreasedImprove survivalPharmaceutical delivery mechanismImmunoglobulins against cell receptors/antigens/surface-determinantsCombined Modality TherapyMalignancy
The present invention relates to combination therapies for the treatment of malignancy, particularly myeloid malignancy such as acute myeloid leukemia (AML). The combination therapies may include an antibody molecule that binds to CD70 and at least one antibody molecule that binds to a leukemic stem cell target. Preferred leukemic stem cell targets are TIM-3, IL1R3 / IL1RAP and CD47.
Owner:ARGENX BV
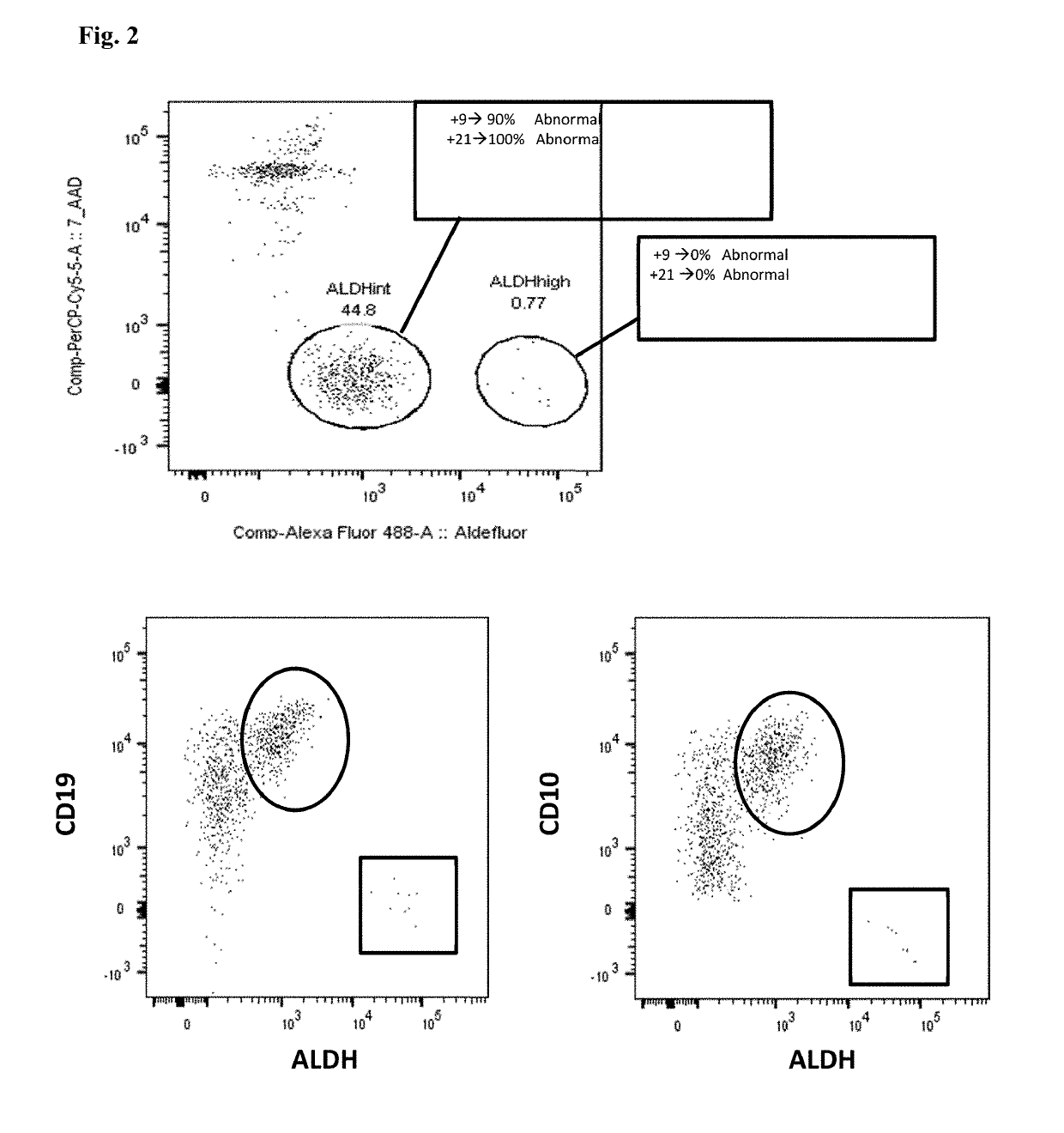
Leukemic stem cell markers
InactiveUS20160305945A1Guaranteed monitoring effectAdequate discriminationBiological particle analysisDisease diagnosisMyeloid leukemiaLeukemia
The present invention relates to a method for identifying Leukemic stem cells (LSC), based on measurement of the presence of a specific set of markers. The invention further relates to a container for use in performing the method. Also provided is application of the method for predicting response to therapy and / or chance of relapse of Acute Myeloid Leukemia, and application of the method in the context of screening for compounds that specifically eradicate Leukemic cells and not benign cells.
Owner:STICHTING VU VUMC
Prognosis and treatment of relapsing leukemia
InactiveUS20200080157A1Organic active ingredientsMicrobiological testing/measurementHematopoietic cellRelapse leukemia
Described are biomarkers and associated methods for determining a prognosis for a subject with leukemia and / or for detecting Leukemic Regenerating Cells (LRCs) or Hematopoietic Regenerating Cells. While chemotherapy may successfully deplete leukemic stem cells (LSCs), a molecularly distinct population of LRCs are observed following cytotoxic chemotherapy not seen in therapy naïve subjects with leukemia or healthy hematopoietic cell populations. Also described are methods for the treatment of leukemia that target LRCs in a subject in need thereof.
Owner:MCMASTER UNIV
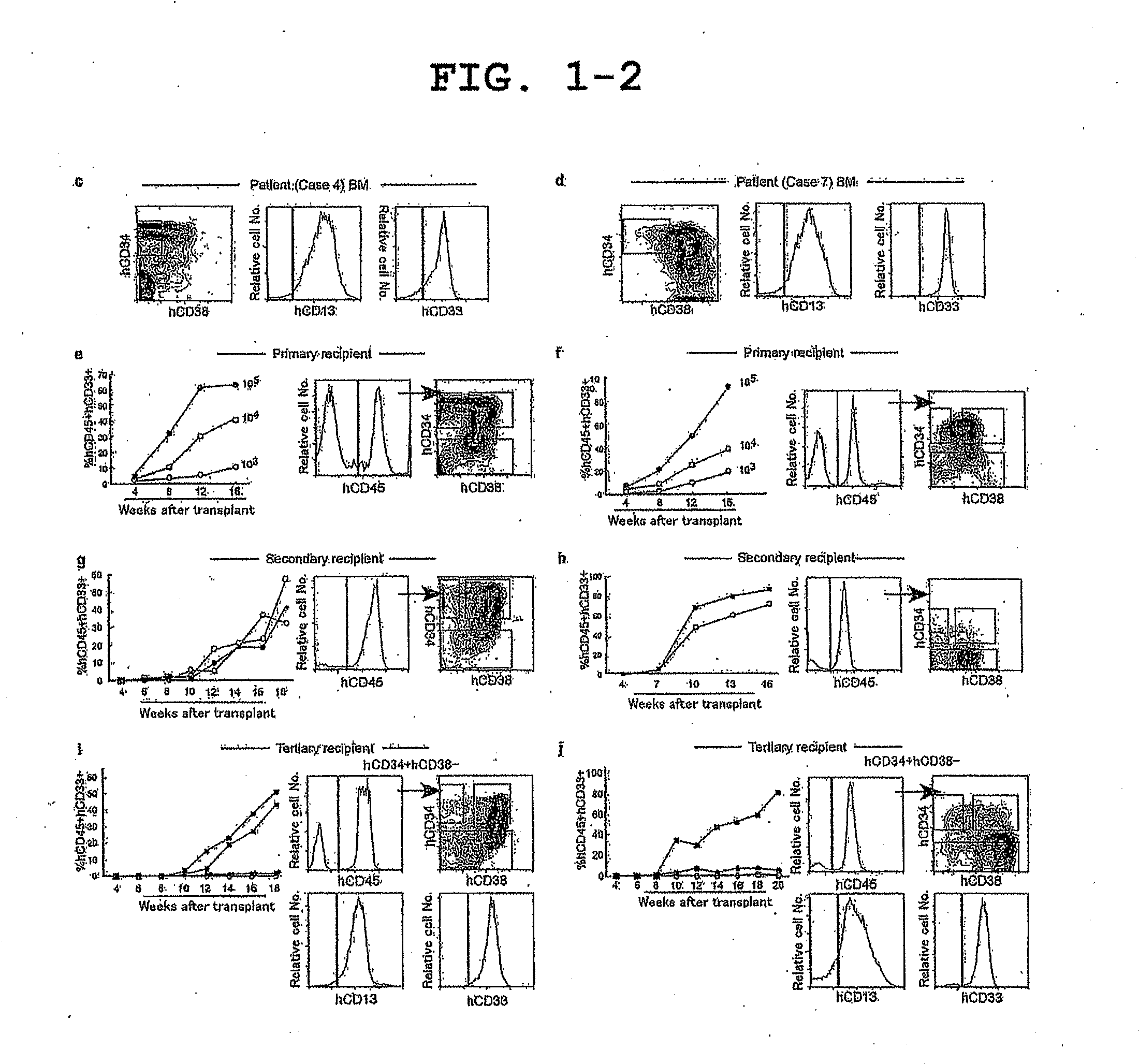
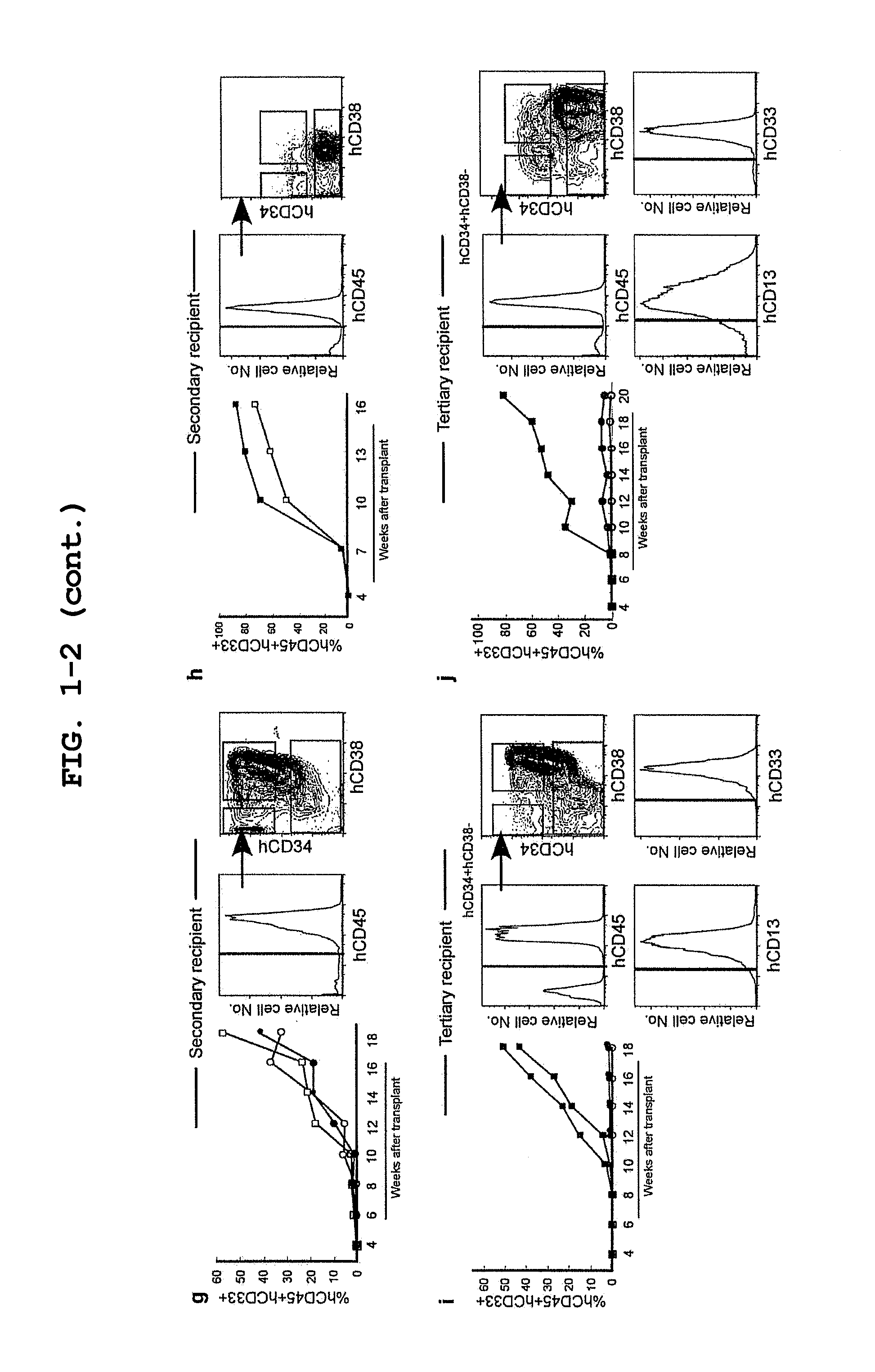
Mouse having human leukemic stem cell and leukemic non-stem cell amplified therein, and method for production thereof
ActiveUS20110307964A1RecurrenceMonitor effectivenessMicrobiological testing/measurementLibrary screeningLeukemic Stem CellLeukemia
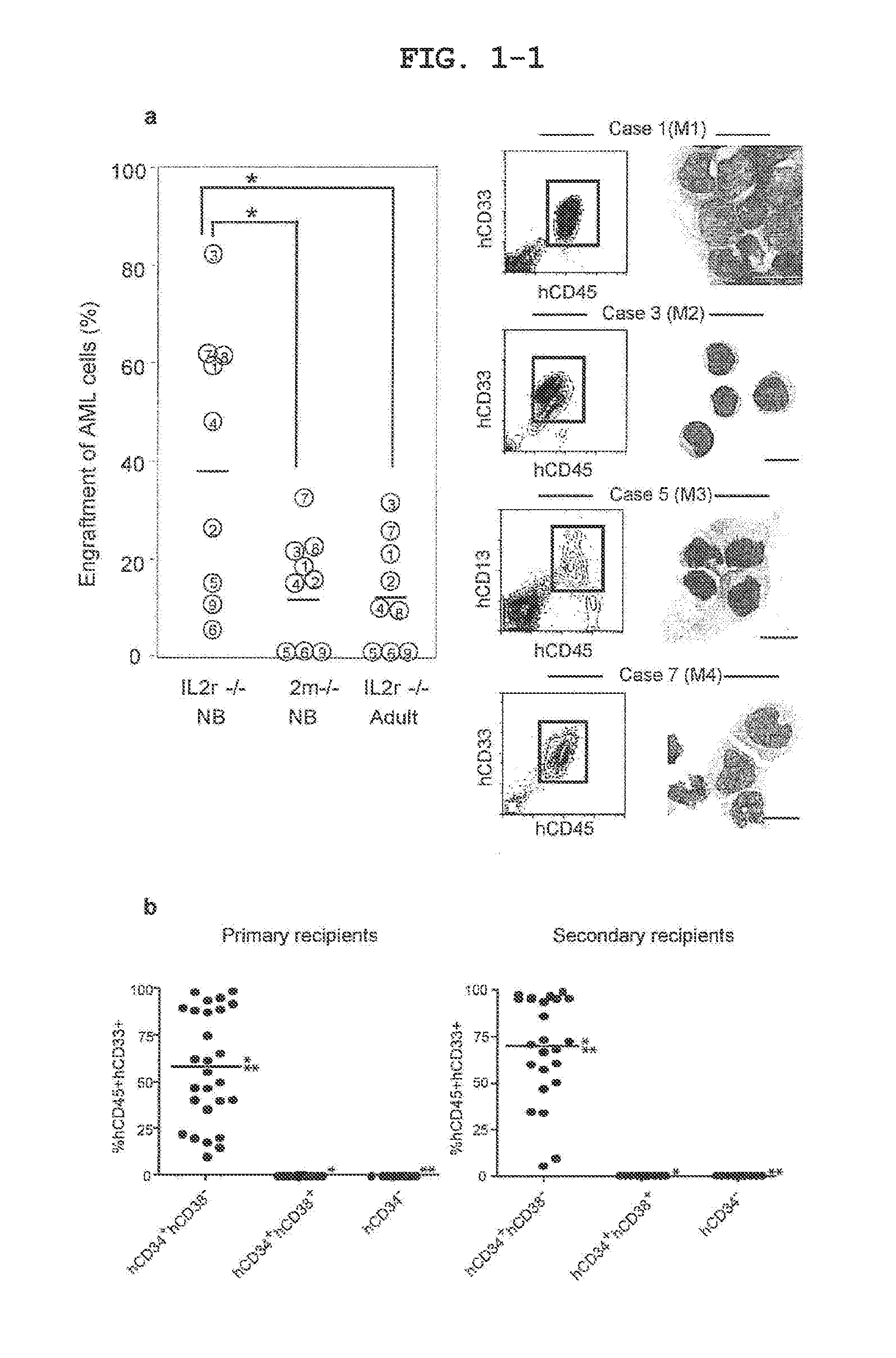
The invention relates to a method of selectively expanding human leukemic cells in a non-adult NOD / SCID / IL2rgnull mouse by transplanting a substance containing a leukemic stem cell derived from a human acute myelogenous leukemia patient to the mouse. In addition, the invention relates to screening for a medicament capable of eradicating leukemic stem cell (LSC), consideration of treatment methods suitable for individual patients, identification of a differentially expressed gene and the like, using a mouse with expanded human leukemic cells.
Owner:JACKSON LAB THE
Compounds for treating or inhibiting recurrence of acute myeloid leukemia
PendingUS20210299128A1Improve throughputLimit deliveryGroup 5/15 element organic compoundsOrganic chemistry methodsMyeloid leukemiaOncology
This invention relates to compounds for treating acute myeloid leukemia or inhibiting recurrence of acute myeloid leukemia and for inhibiting growth of and / or killing leukemic stem cells.
Owner:FLASH THERAPEUTICS LLC
Method of inhibition of leukemic stem cells
InactiveUS20150152185A1Function increaseOrganic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsAntigen bindingLeukemic Stem Cell
A method for inhibition of leukemic stem cells expressing IL-3R.alpha.; (CD 123), comprises contacting the cells with an antigen binding molecule comprising a Fc region or a modified Fc region having enhanced Fc effector function, wherein the antigen binding molecule binds selectively to IL-3R.alpha. (CD123). The invention includes the treatment of a hematologic cancer condition in a patient by administration to the patient of an effective amount of the antigen binding molecule.
Owner:CSL LTD +1
Methods and compositions for identifying leukemic stem cells
ActiveUS10222376B2Microbiological testing/measurementBiological material analysisLeukemic Stem CellMolecular biology
Owner:CHARLOTTE MECKLENBURG HOSPITAL AUTHORITY
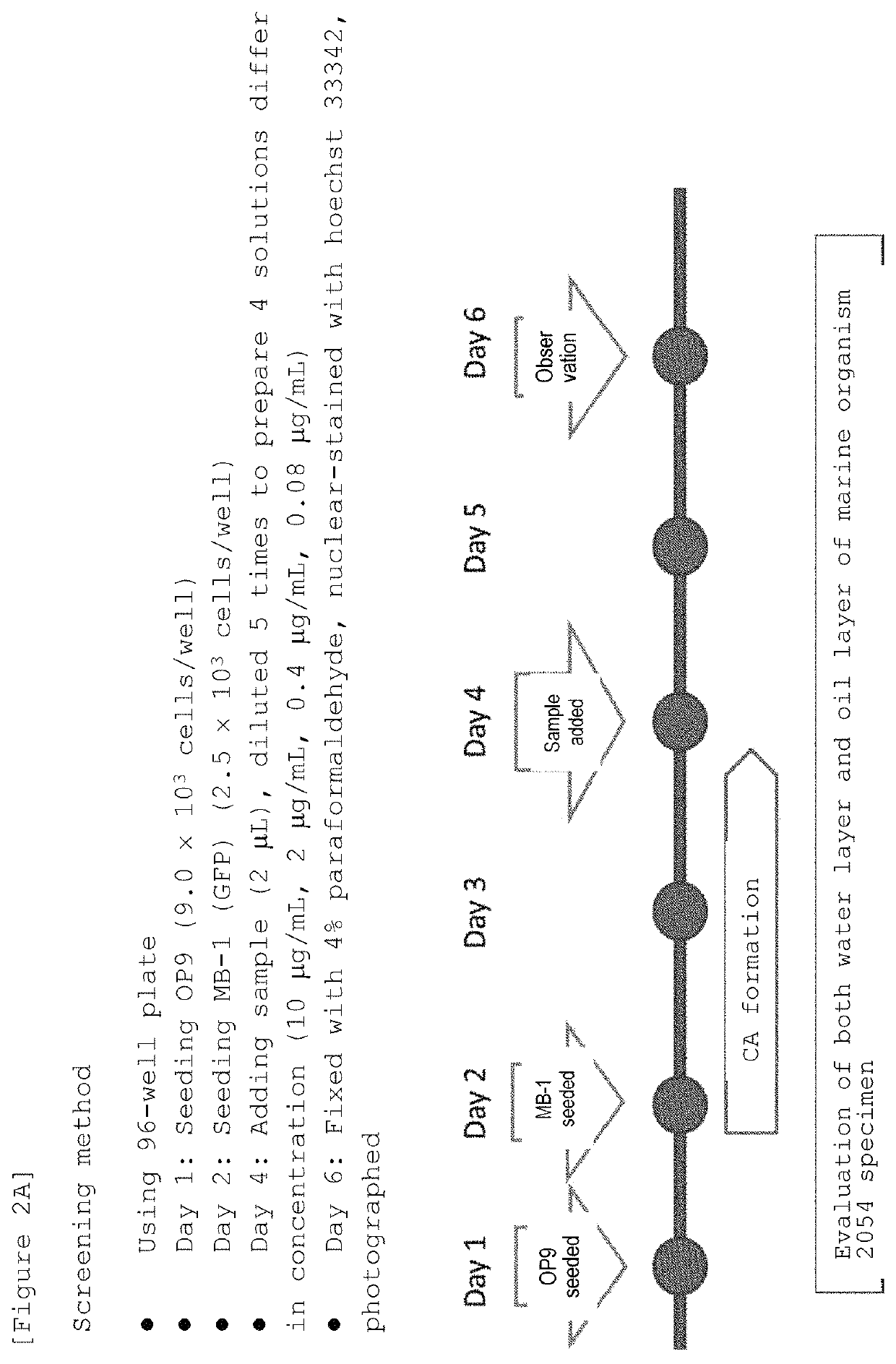
Marine organism-derived extract, compound, and medical composition having niche formation suppressing activity of leukemic stem cells
ActiveUS10933044B2Prevent relapseOrganic active ingredientsOrganic chemistryChemical compoundPharmaceutical drug
Owner:WASEDA UNIV +1
Method for leukaemia high-flux medicine screening
PendingCN111118099AGood curative effectLittle side effectsMicrobiological testing/measurementMaterial analysisOncologyChemo therapy
The invention provides a method for leukaemia high-flux medicine screening. The method comprises the following steps of performing separation, extraction, identification and culture on neoplastic hematologic disorder stem cells: performing acquisition on bone marrow samples, performing separation on mononuclear cells, performing separation on leukaemia stem cell groups through an immunomagnetic bead method; and performing subculturing on the cells after separation and purification of magnetic beads; and establishing a medicine library: according to a medicine description and pharmacokinetics,selecting the concentration of medicines, performing mixed culture on the medicines and the neoplastic hematologic disorder stem cells, and analyzing the restraining effect of the medicines on the neoplastic hematologic disorder stem cells. According to the method for leukaemia high-flux medicine screening disclosed by the invention, in accordance with a patient, an appropriate treatment scheme and opinions can be accurately given in an individualizing manner, a valuable treatment opportunity is strived for the recurrent and refractory patient, reagent risk is reduced, the medicine curative effect is increased to the maximum degree, and medicine side effects are reduced to the maximum degree; and besides, pharmaceutical combinations can be performed, in accordance with clinical frequently-used chemotherapeutics combinations, comprehensive marking is performed, and a reference is provided for a doctor in the respect of formulating clinical united medication for the patient.
Owner:HARVEST BIOTECH CO LTD
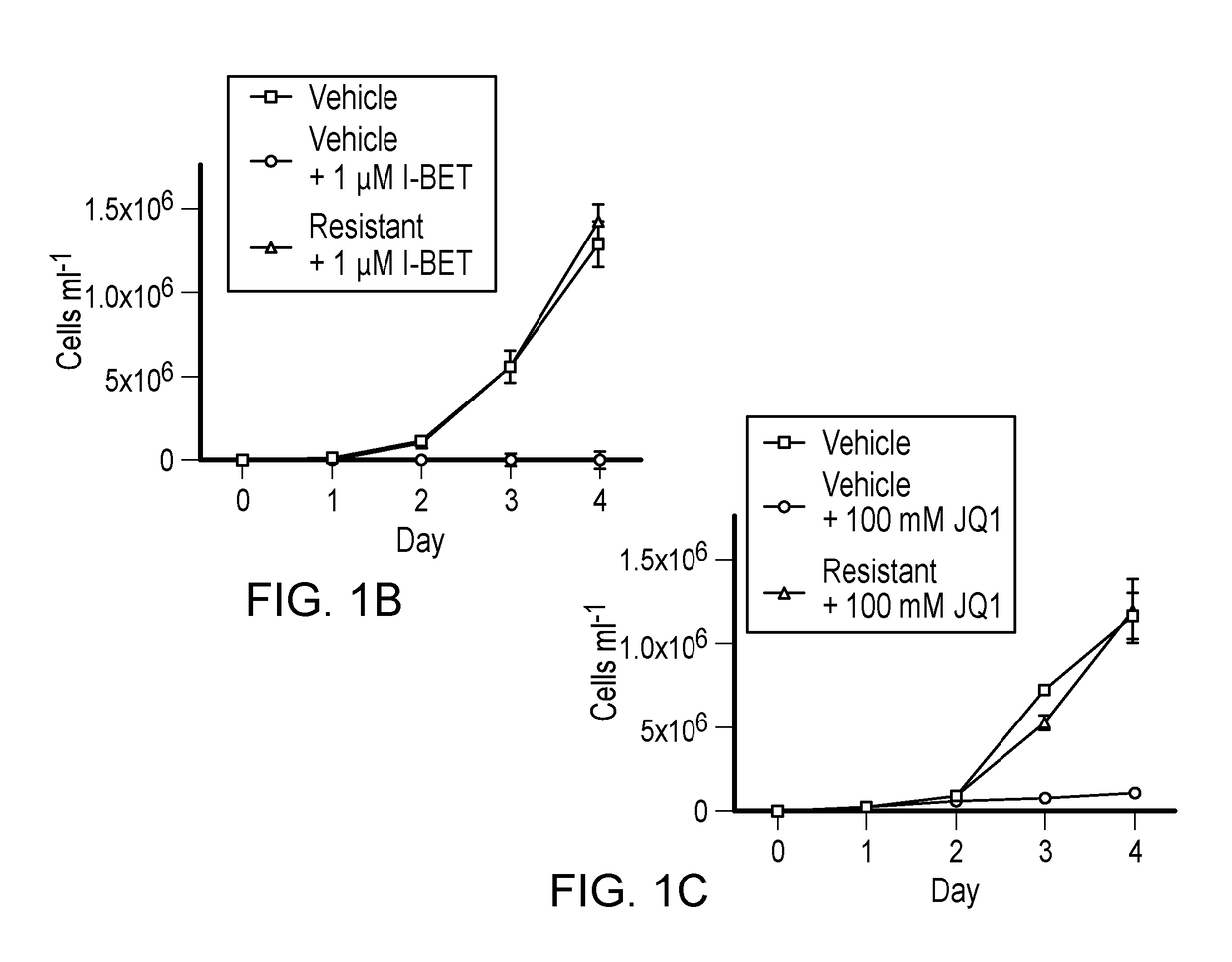
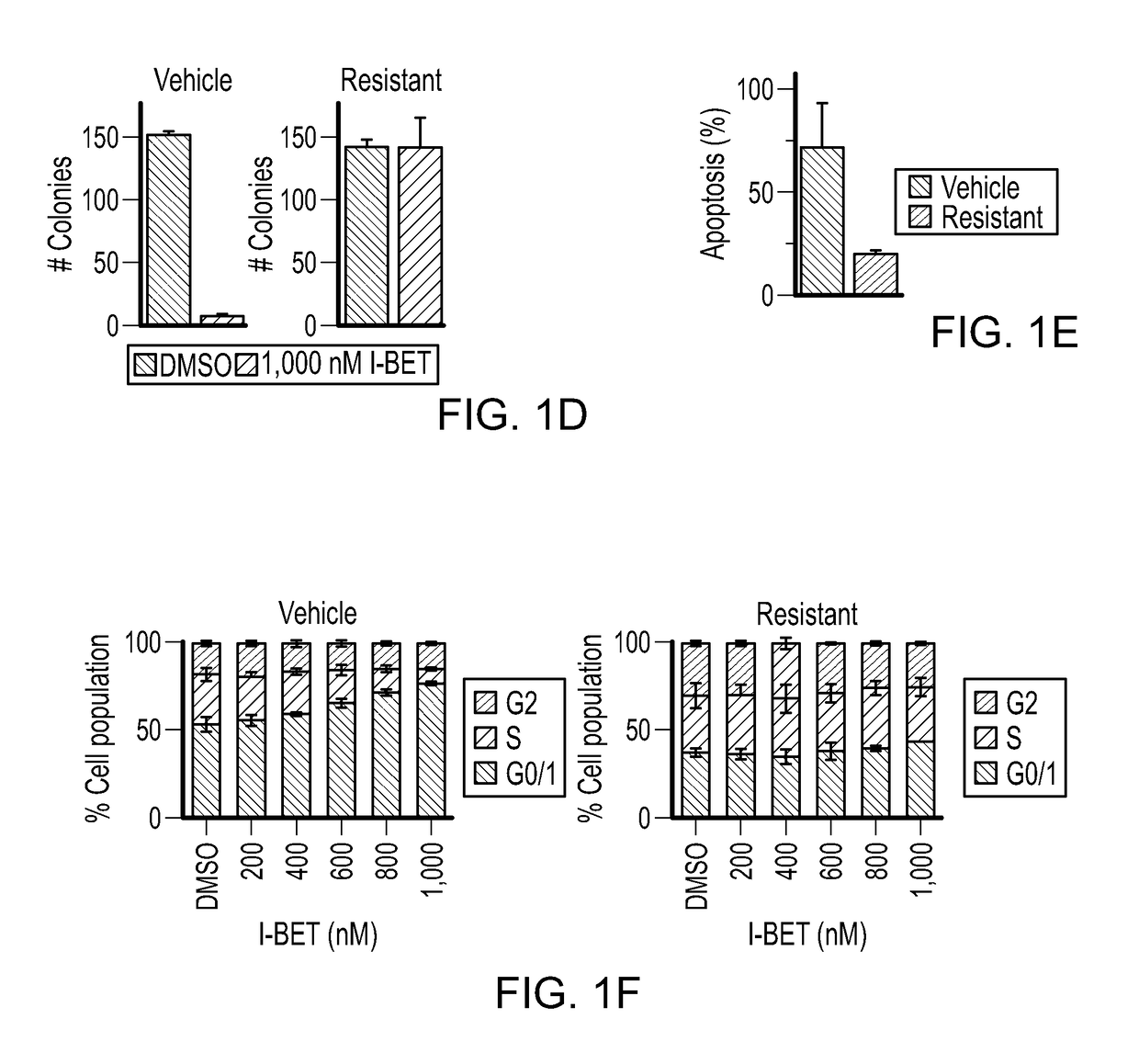
Leukaemia stem cell line, its method of production and uses thereof
InactiveUS9896663B2Improve concentrationCell culture active agentsBiological testingProgenitorStem cell line
Bromodomain and extra terminal protein (BET) resistant leukemic cell lines and methods for producing such cell lines are described as are methods for using such cell lines in screening assays to identify therapeutic agents. The cell lines can be generated from haematopoietic stem and progenitor cells (HSPCs) that are clonally enriched by serially exposing c-kit positive cells to a BET inhibitor.
Owner:PETER MACCALLUM CANCER INST
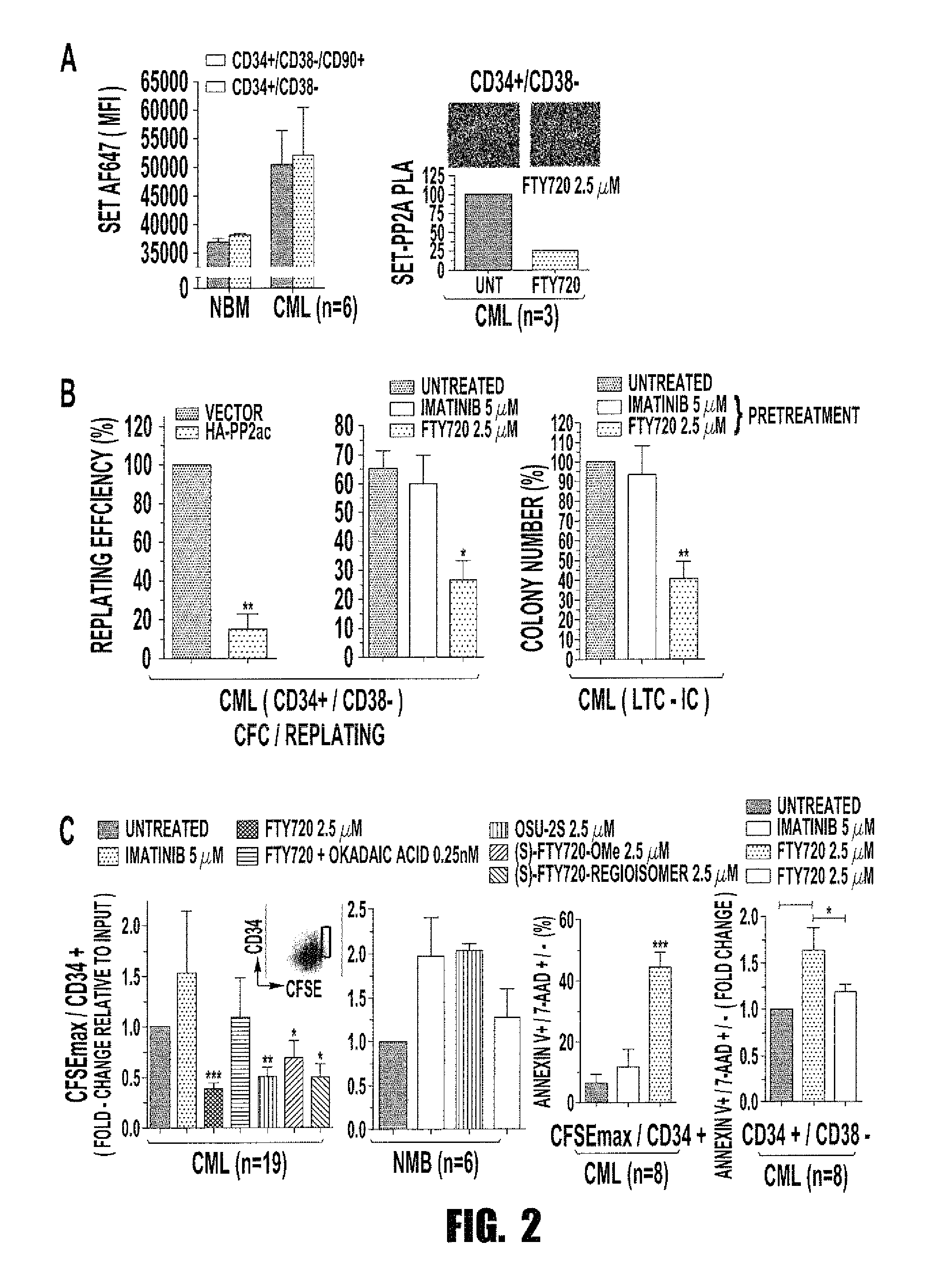
Inhibition of leukemic stem cells by pp2a activating agents
ActiveUS20150141502A1High activityLose activityBiocideAnimal repellantsAlkoxy groupLeukemic Hematopoietic Stem Cell
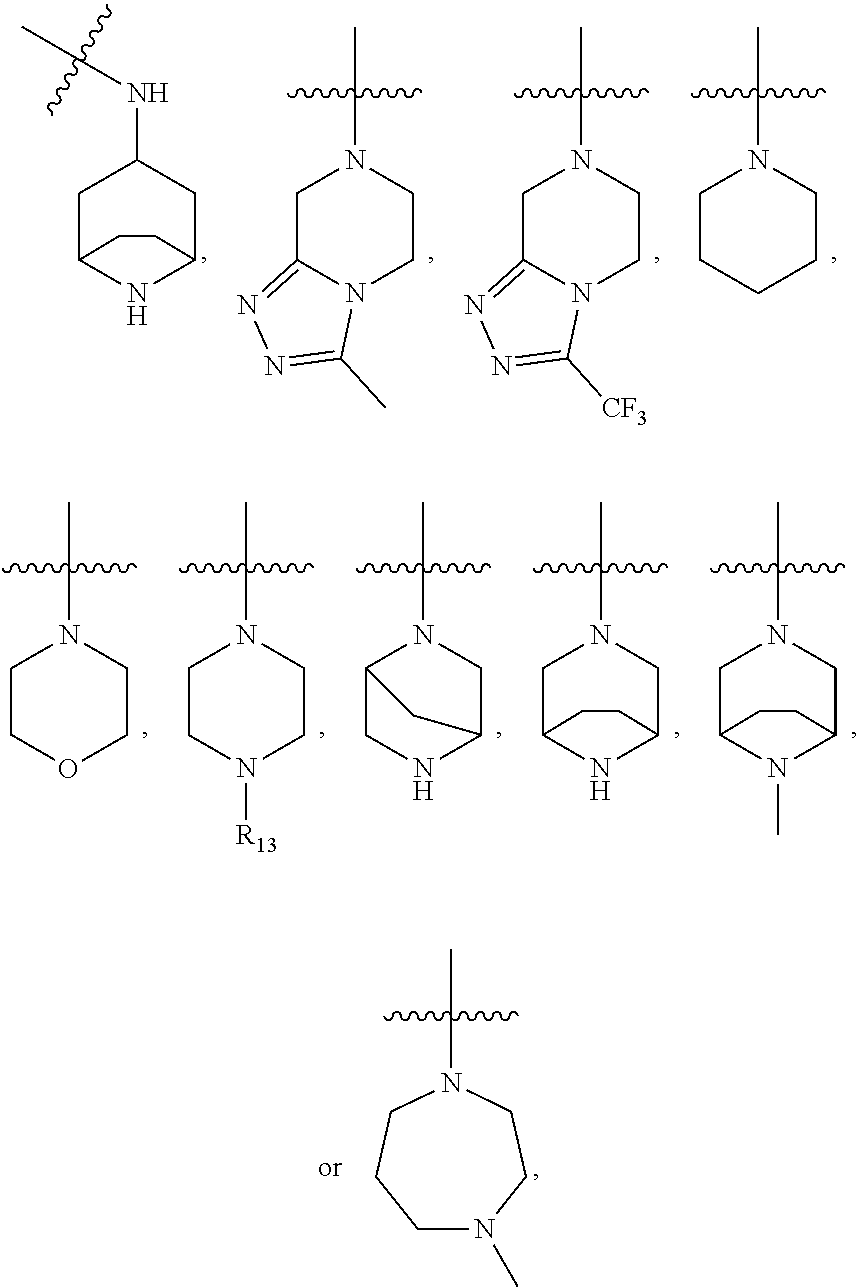
A method of inhibiting the growth of leukemic hematopoietic stem cells in a subject with leukemia is described. The method includes administering a therapeutically effective amount of a composition including a compound of formula I: I wherein R1 is independently selected from hydrogen and methyl; R2 is selected from the group consisting of 4,8-dimethyl-non-1-enyl, 4,8-dimethyl-nonyl, non-1-enyl, and nonanyl groups; X is a carboxyl, phosphonic, or sulfonic moiety, and n is an integer from 1 to 6, or a compound of Formula II: II wherein R1 is a C6-C12 alkyl or C6-C12 alkoxy group; R2 is independently selected from the group consisting of hydrogen, methoxy, and hydroxyl; and R3 is an alkyl or cycloalkyl group; or a pharmaceutically acceptable salt thereof.
Owner:OHIO STATE INNOVATION FOUND
Leukemic stem cell ablation
InactiveUS20090176759A1Reduce eliminateReduce the amount requiredBiocideAnimal repellantsTyrosine-kinase inhibitorHematology
Owner:TEVA PHARMACEUTICALS INTERNATIONAL GMBH
Detection of acute myeloid leukaemia (AML) leukaemic stem cells (LSC)
InactiveUS10191055B2Microbiological testing/measurementUnknown materialsLeukemic Stem CellGene expression profiling
Owner:OXFORD UNIV INNOVATION LTD
Anti-human CD45RA rat immune globulin variable region gene and application
ActiveCN101831434BImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsFhit geneLeukemia
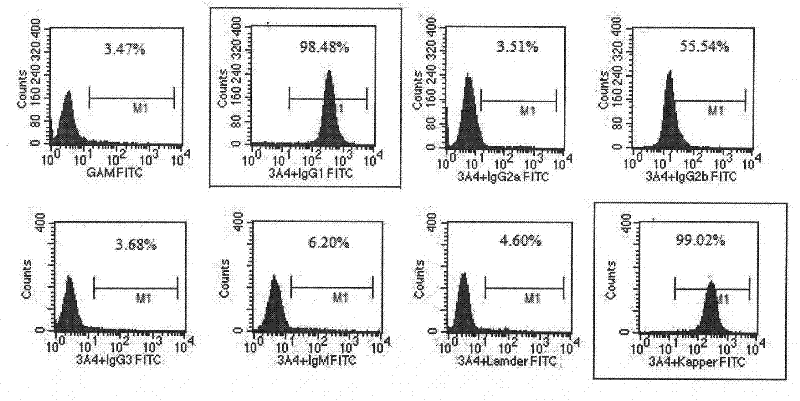
The invention provides an anti-human CD45RA rat immune globulin variable region gene, which comprises nucleotide sequences of SEQIDNO.1 and SEQIDNO.2 and encoded nucleotide sequences of SEQIDNO.3 and SEQIDNO.4. Research proves that anti-human CD45RA rat immune globulin variable region gene can effectively distinguish leukemia patients, leukemia cell strain CD34+CD38-CD123 stem cells and CD34+CD38+CD123 offspring leukemia cells, has the application prospects of leukemia targeted therapy, and can be used in preparing targeted therapy blood system malignant tumor medicines.
Owner:杭州永申生物科技有限公司
Leukemic Stem Cell Ablation
InactiveUS20150104416A1Reduce the amount requiredShorten the timeBiocidePeptide/protein ingredientsTyrosine-kinase inhibitorHematology
Owner:TEVA PHARMACEUTICALS INTERNATIONAL GMBH
Mouse having human leukemic stem cell and leukemic non-stem cell amplified therein, and method for production thereof
ActiveUS9125384B2EffectivenessRecurrenceMicrobiological testing/measurementLibrary screeningHuman leukemiaLeukemic Stem Cell
The invention relates to a method of selectively expanding human leukemic cells in a non-adult NOD / SCID / IL2rgnull mouse by transplanting a substance containing a leukemic stem cell derived from a human acute myelogenous leukemia patient to the mouse. In addition, the invention relates to screening for a medicament capable of eradicating leukemic stem cell (LSC), consideration of treatment methods suitable for individual patients, identification of a differentially expressed gene and the like, using a mouse with expanded human leukemic cells.
Owner:JACKSON LAB THE
Marine organism-derived extract, compound, and medical composition having niche formation suppressing activity of leukemic stem cells
ActiveUS20190022045A1Prevent relapseOrganic active ingredientsOrganic chemistryAbnormal tissue growthChemical composition
Using the cobblestone area (CA) formation inhibitory activity of human leukemic stem cell-like cells as an indicator, a fraction having the activity has been extracted from the fat-soluble fraction of Porifera, then, a compound having the activity has been isolated and purified from the aforementioned fraction, and then, the structure thereof has been determined, so that a Stelliferin compound comprising a novel compound has been identified. Moreover, it has been found that the isolated and purified Stelliferin compound significantly suppresses the niche formation of leukemic stem cell-like cells derived from human chronic myelogenous leukemia (CML) having resistance to existing antitumor agents and enhances the effects of antitumor agents on the cells. The present invention provides a pharmaceutical composition having inhibitory or suppressive activity against the niche formation of leukemic stem cells, a prophylactic agent against the recurrence of malignant tumor, which involves the combined use of other antitumor drugs, and the like.
Owner:WASEDA UNIV +1
Inhibition of leukemic stem cells by PP2A activating agents
A method of inhibiting the growth of leukemic hematopoietic stem cells in a subject with leukemia is described. The method includes administering a therapeutically effective amount of a composition including a compound of formula I: I wherein R1 is independently selected from hydrogen and methyl; R2 is selected from the group consisting of 4,8-dimethyl-non-1-enyl, 4,8-dimethyl-nonyl, non-1-enyl, and nonanyl groups; X is a carboxyl, phosphonic, or sulfonic moiety, and n is an integer from 1 to 6, or a compound of Formula II: II wherein R1 is a C6-C12 alkyl or C6-C12 alkoxy group; R2 is independently selected from the group consisting of hydrogen, methoxy, and hydroxyl; and R3 is an alkyl or cycloalkyl group; or a pharmaceutically acceptable salt thereof.
Owner:OHIO STATE INNOVATION FOUND
Methods and compositions for identifying leukemic stem cells
ActiveUS20190195878A1Microbiological testing/measurementBiological material analysisLeukemic Stem CellMolecular biology
Owner:CHARLOTTE MECKLENBURG HOSPITAL AUTHORITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com