Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
31 results about "Hypnotic agent" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Propofol is a popular sedative hypnotic agent which is commonly used for induction of general anesthesia in the operating room (OR) and sedation in the Intensive Care Unit (ICU).
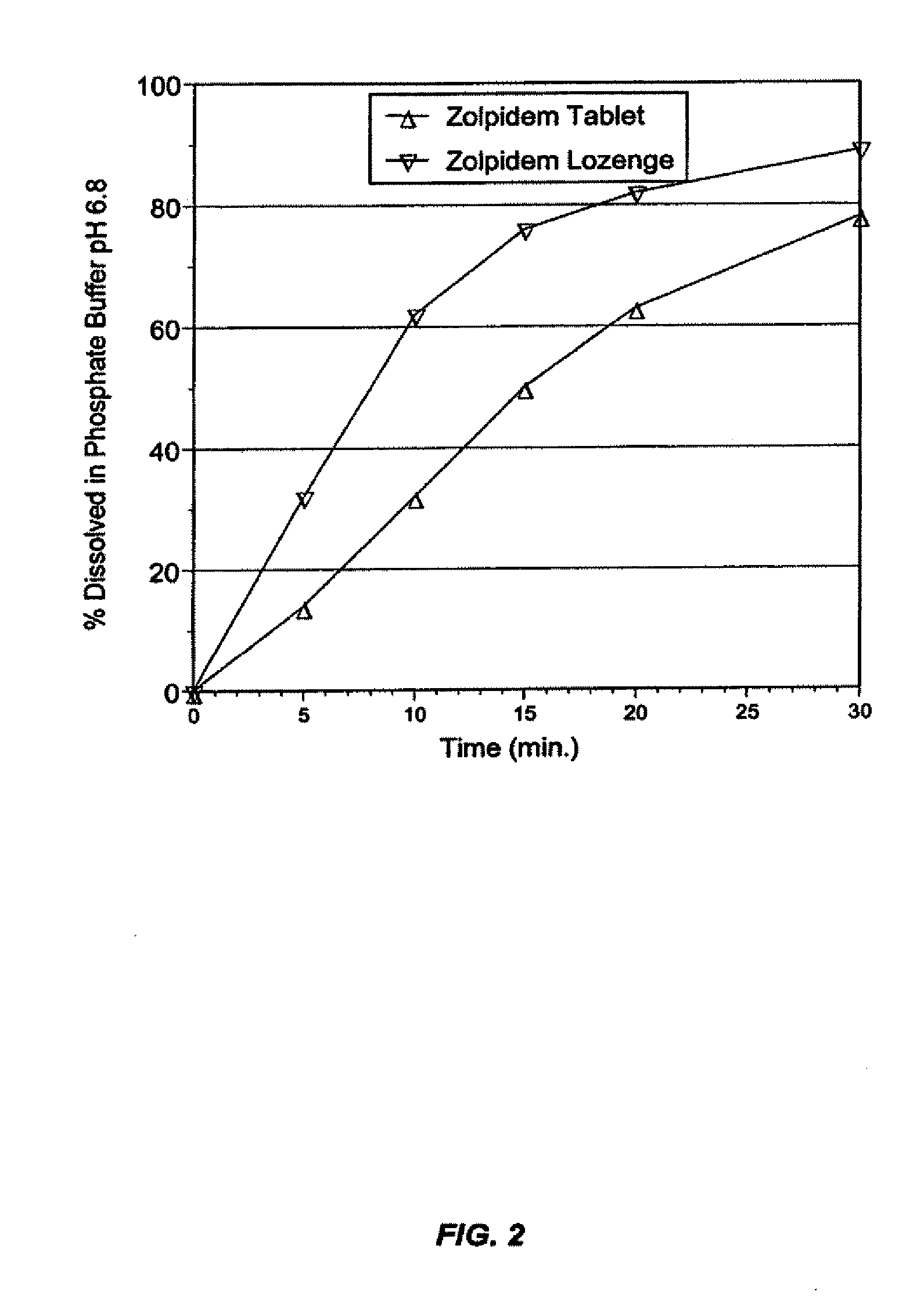
Compositions for delivering hypnotic agents across the oral mucosa and methods of use thereof
InactiveUS20080008753A1Rapidly and efficiently absorbedAvoid degradationBiocideNervous disorderHypnotic agentSleeping disorders
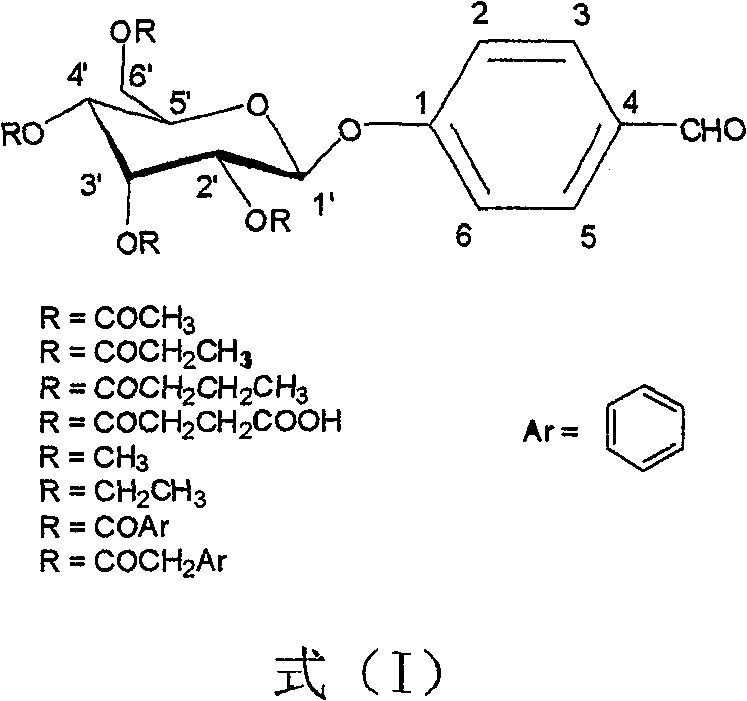
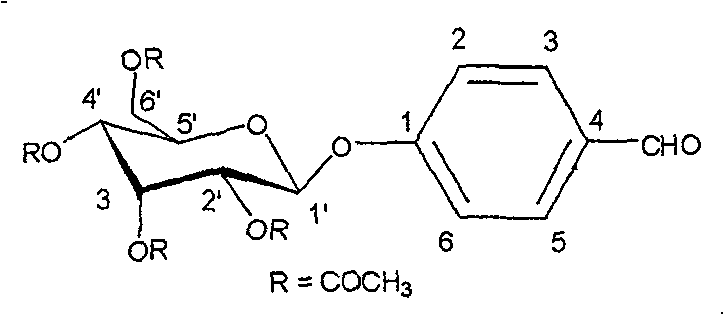
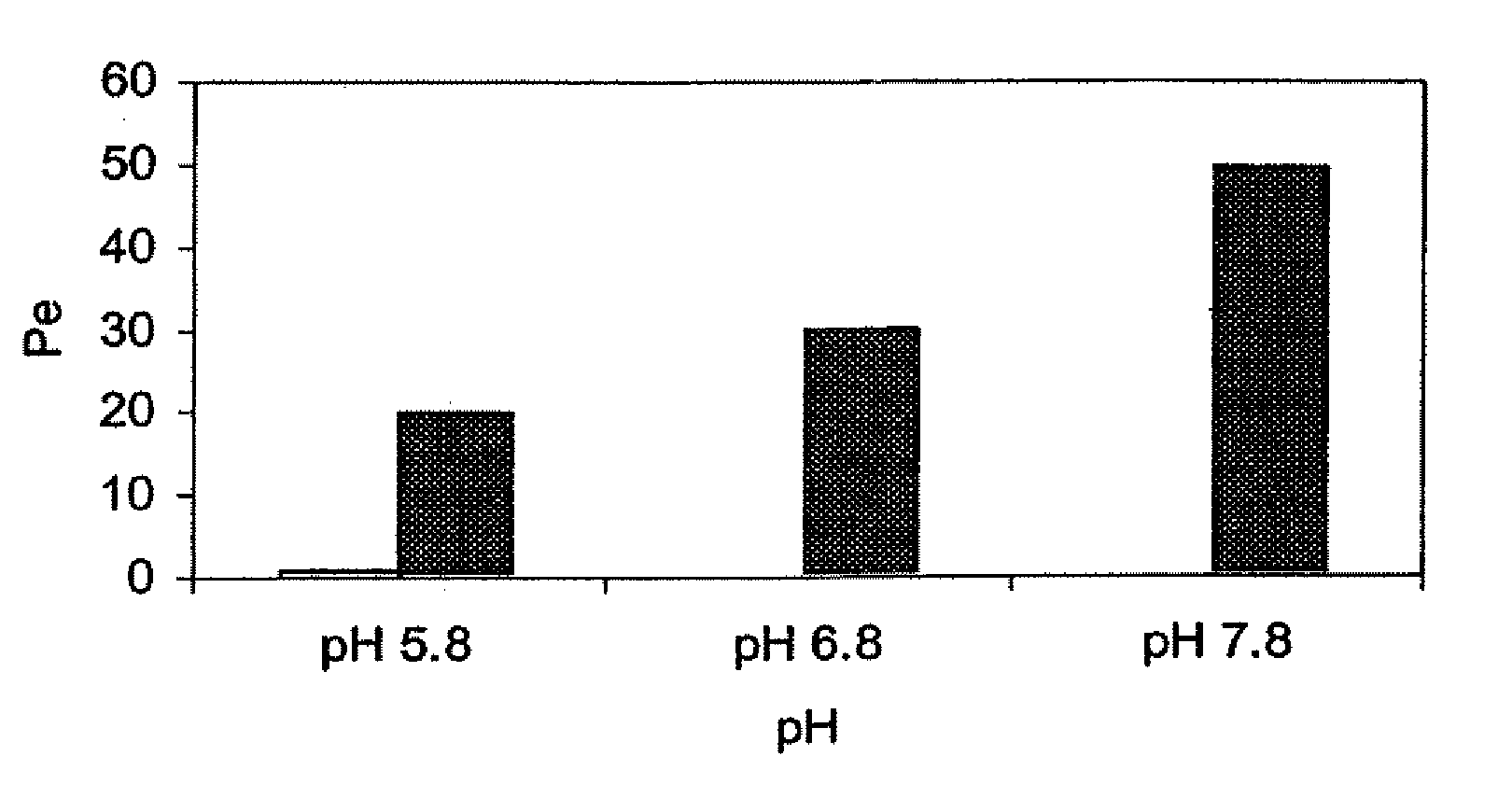
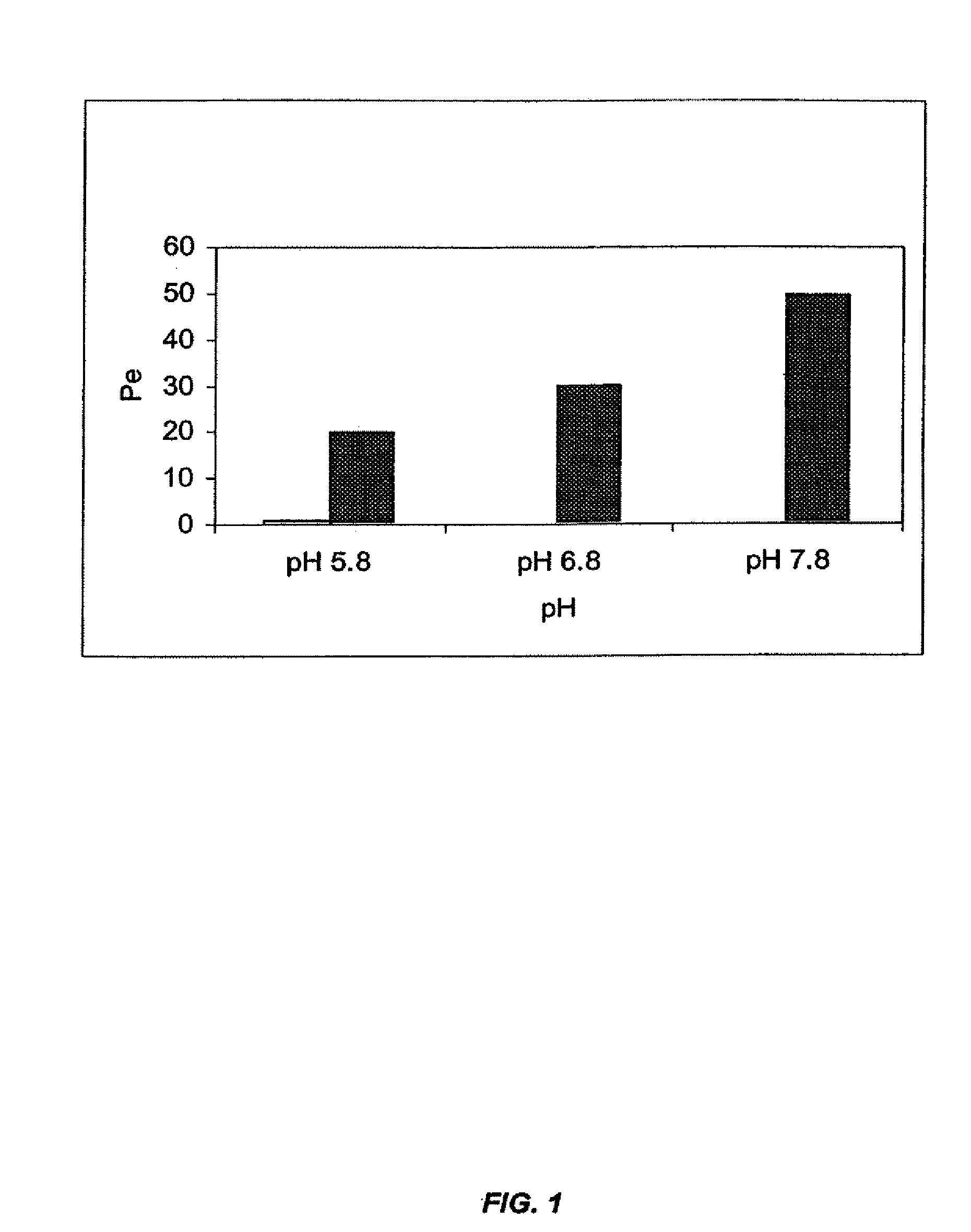
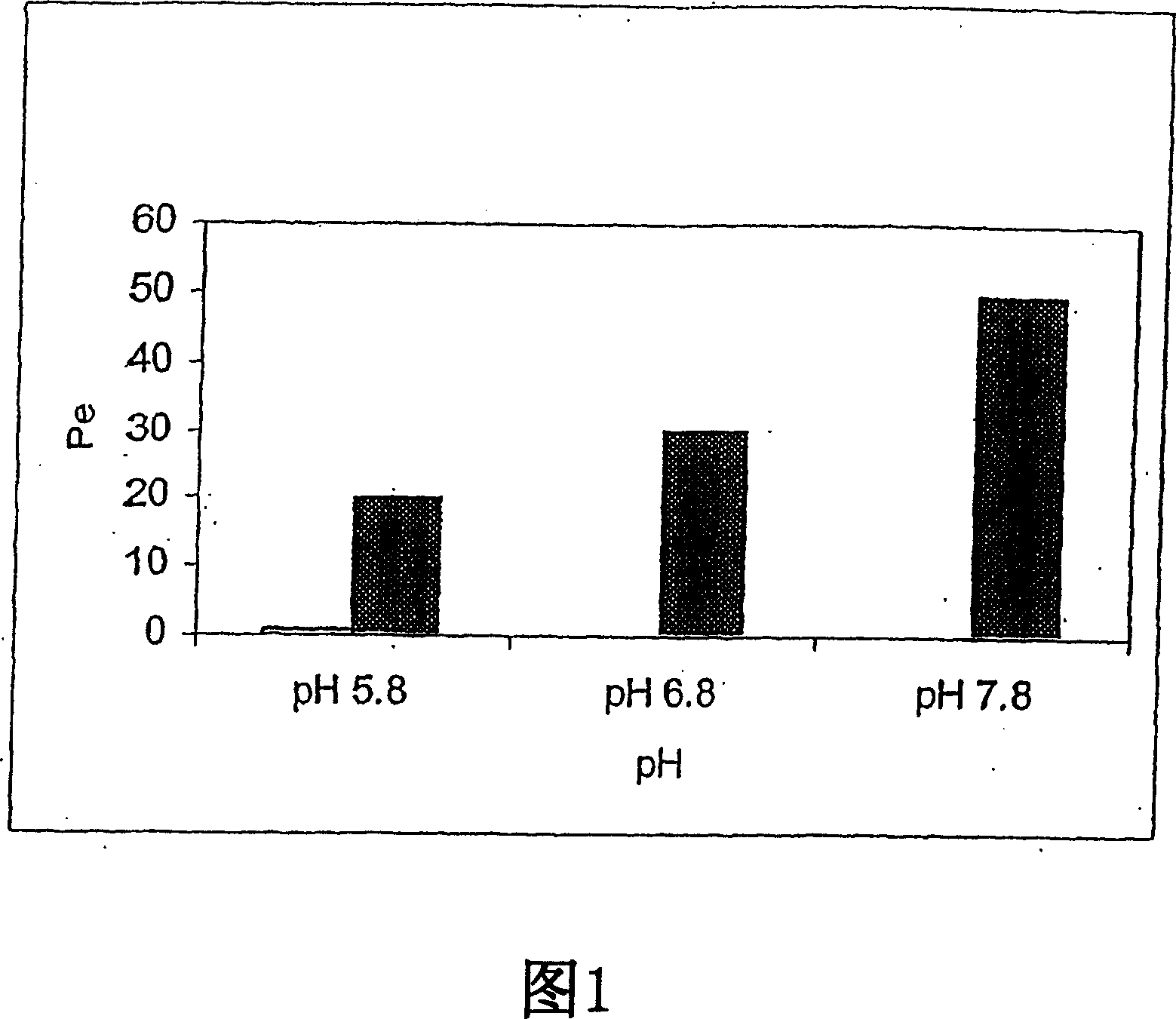
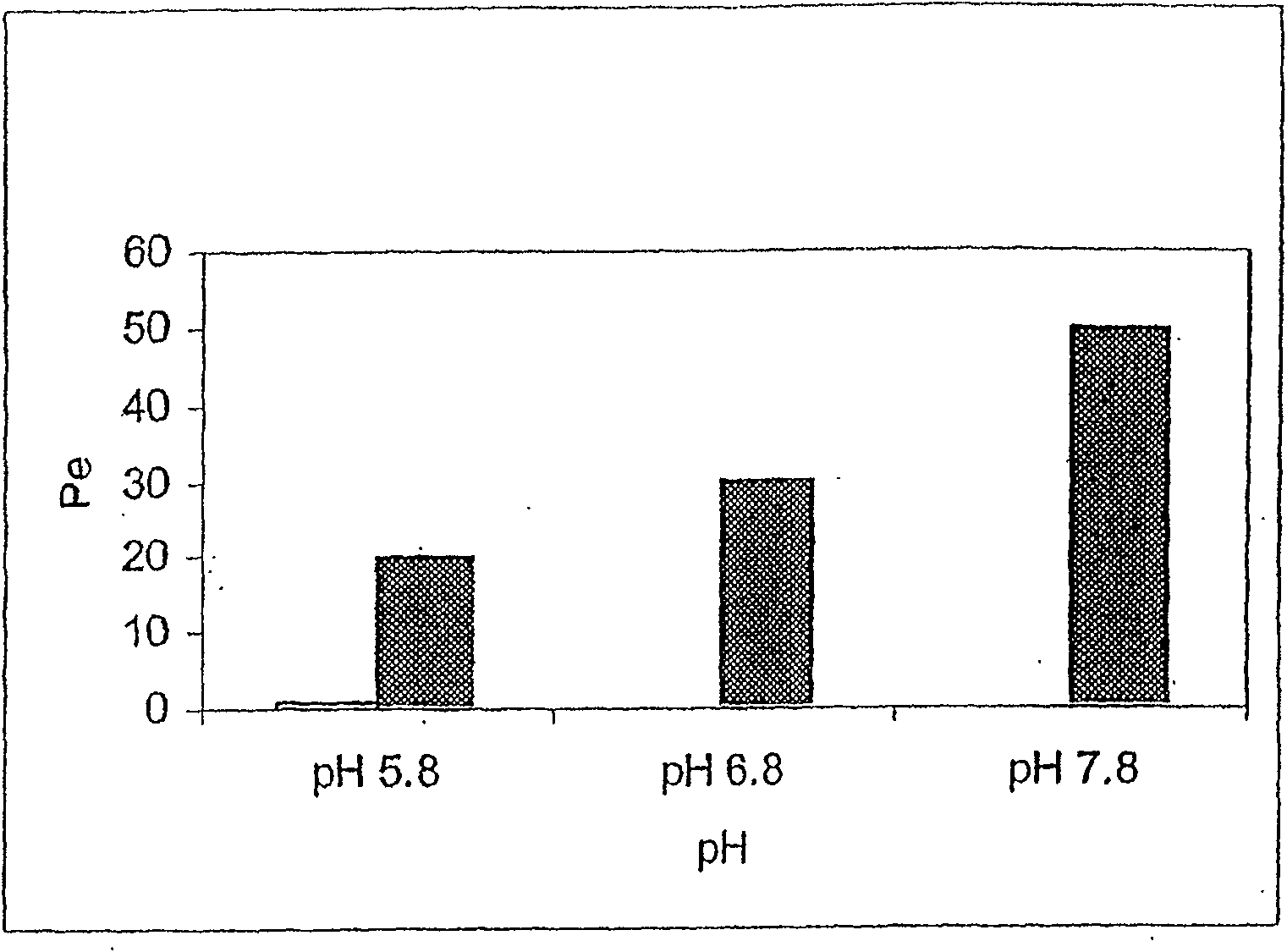
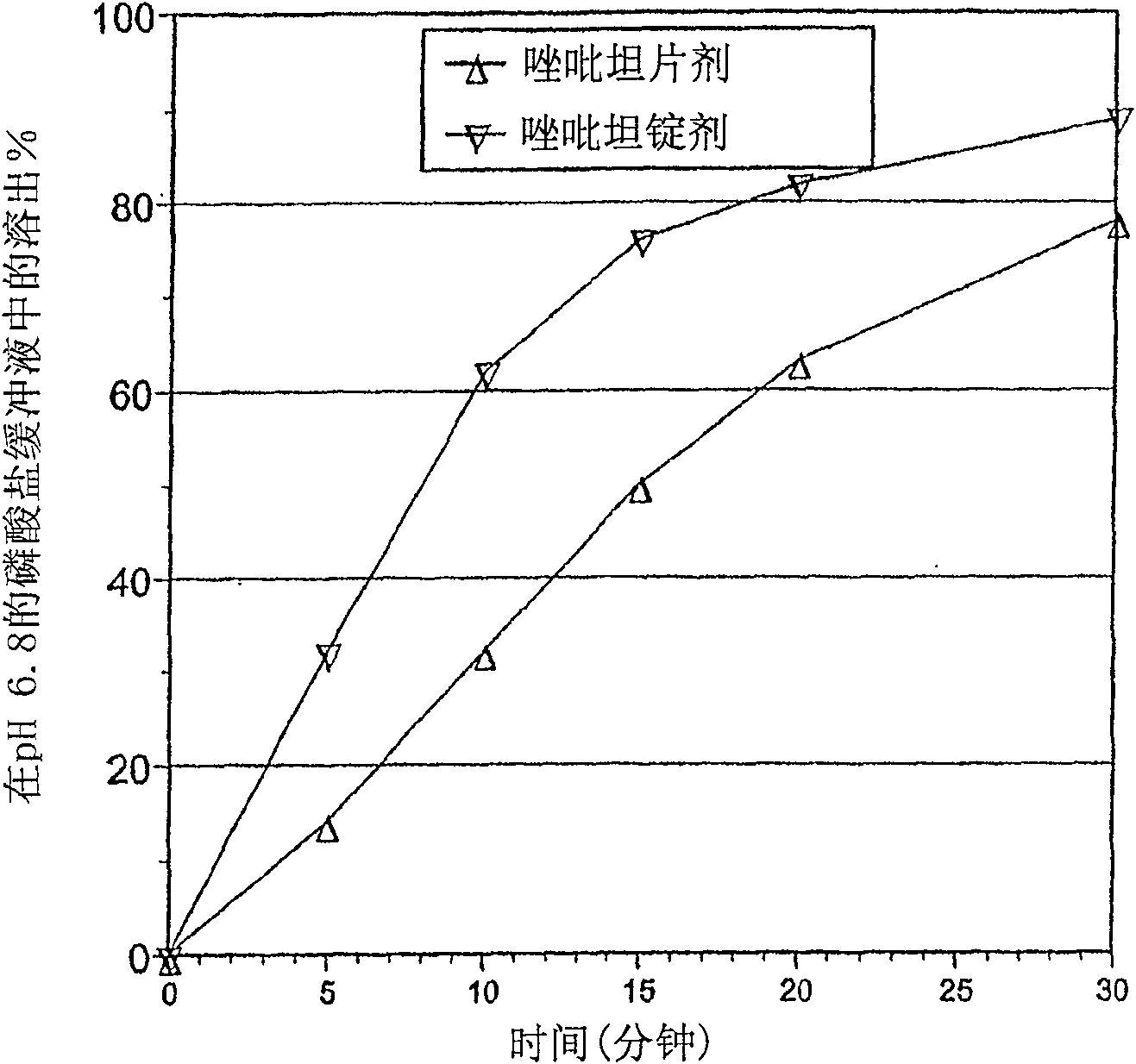
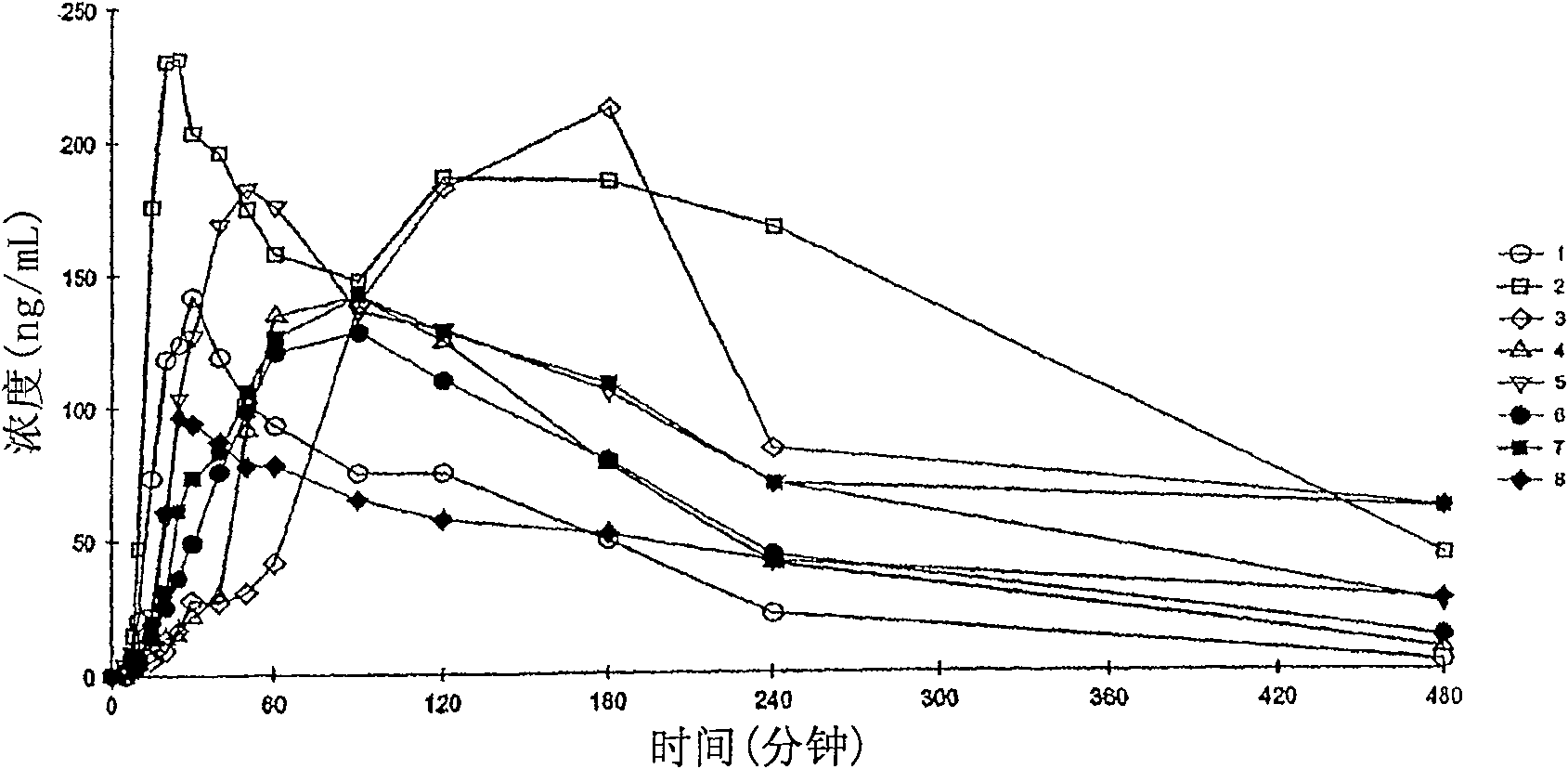
The present invention provides novel compositions for the delivery of a hypnotic agent across the oral mucosa. In particular, the buffer system in the compositions of the present invention raises the pH of saliva to a pH greater than about 7.8, thereby facilitating the substantially complete conversion of the hypnotic agent from its ionized to its un-ionized form. As a result, the dose of hypnotic agent is rapidly and efficiently absorbed by the oral mucosa with surprisingly low inter-subject variability. Furthermore, delivery of the hypnotic agent across the oral mucosa advantageously bypasses hepatic first pass metabolism of the drug and avoids enzymatic degradation of the drug within the gastrointestinal tract. Methods for using the compositions of the present invention for treating sleep disorders such as insomnia are also provided.
Owner:PARATEK PHARM INC
Lithium combinations, and uses related thereto
InactiveUS20080107756A1Prevent precipitating manic episodeLessening and preventing riskBiocideNervous disorderNorepinephrine reuptake inhibitorPsychoactive drug
The present invention relates to combinatorial therapies for treating anxiety, depression or psychotic conditions using a lithium salt and a psychoactive drug selected from the group consisting of serotonin reuptake inhibitor, a 5HT2 receptor antagonist, an anticonvulsant, a norepinephrine reuptake inhibitor, an α-adrenoreceptor antagonist, an NK-3 antagonist, an NK-1 receptor antagonist, a PDE4 inhibitor, an Neuropeptide Y5 Receptor Antagonists, a D4 receptor antagonist, a 5HT1A receptor antagonist, a 5HT1D receptor antagonist, a CRF antagonist, a monoamine oxidase inhibitor, a sedative-hypnotic drug, and an atypical antipsychotic.
Owner:NOVEN THERAPEUTICS
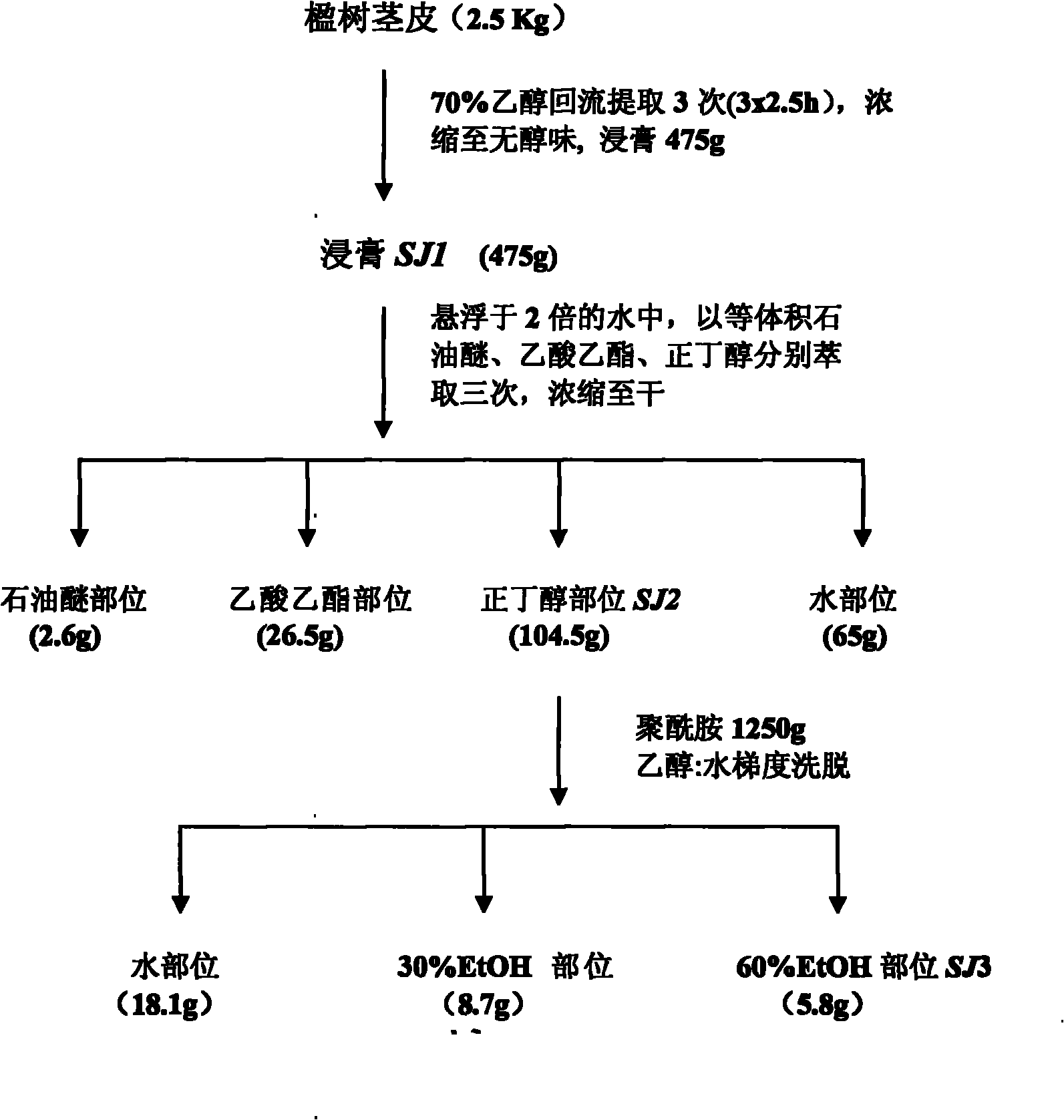
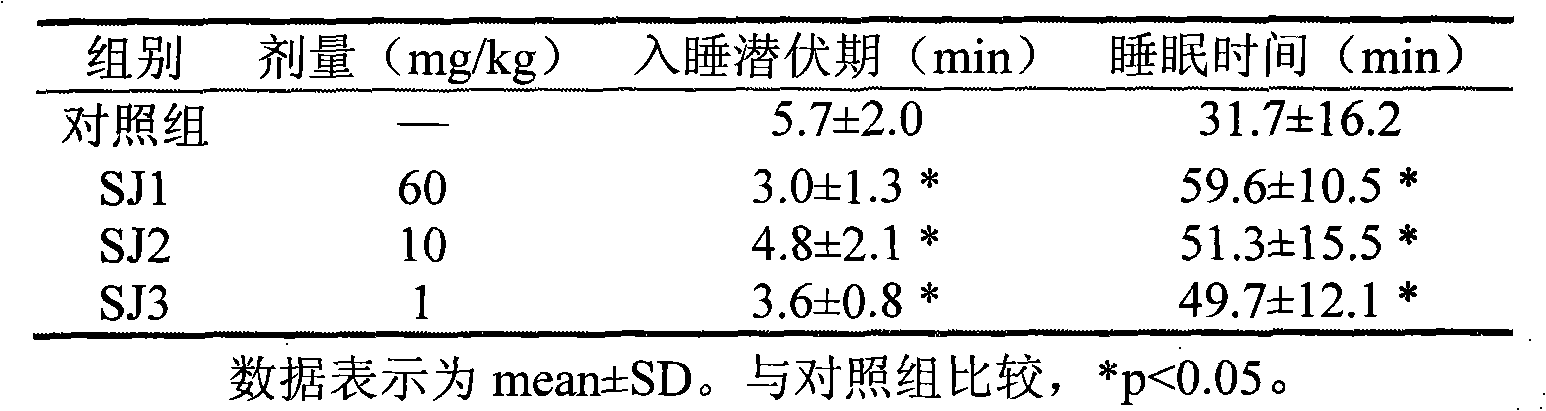
Extract of Albizia chinensis (Osbeck) Merr, preparation method, combination and purpose thereof
ActiveCN101766676ANo dependenciesAddiction freeNervous disorderPlant ingredientsMedicineCurative effect
The invention relates to the application of Albizia chinensis (Osbeck) Merr in the preparation of calming hypnotic medicine, an extract of the Albizia chinensis (Osbeck) Merr, a preparation method of the extract, and a medicine combination containing the extract. The stem skin of the Albizia chinensis (Osbeck) Merr is dried, crushed, extracted, concentrated and purified, so as to obtain the extract with the calming hypnotic function. Effective components include catechin compounds. Pharmacological tests show that the extract has the characteristics of low effective dose, quick medicinal effect exerting, obvious curative effect, good safety and high reliability.
Owner:INST OF MATERIA MEDICA CHINESE ACAD OF MEDICAL SCI
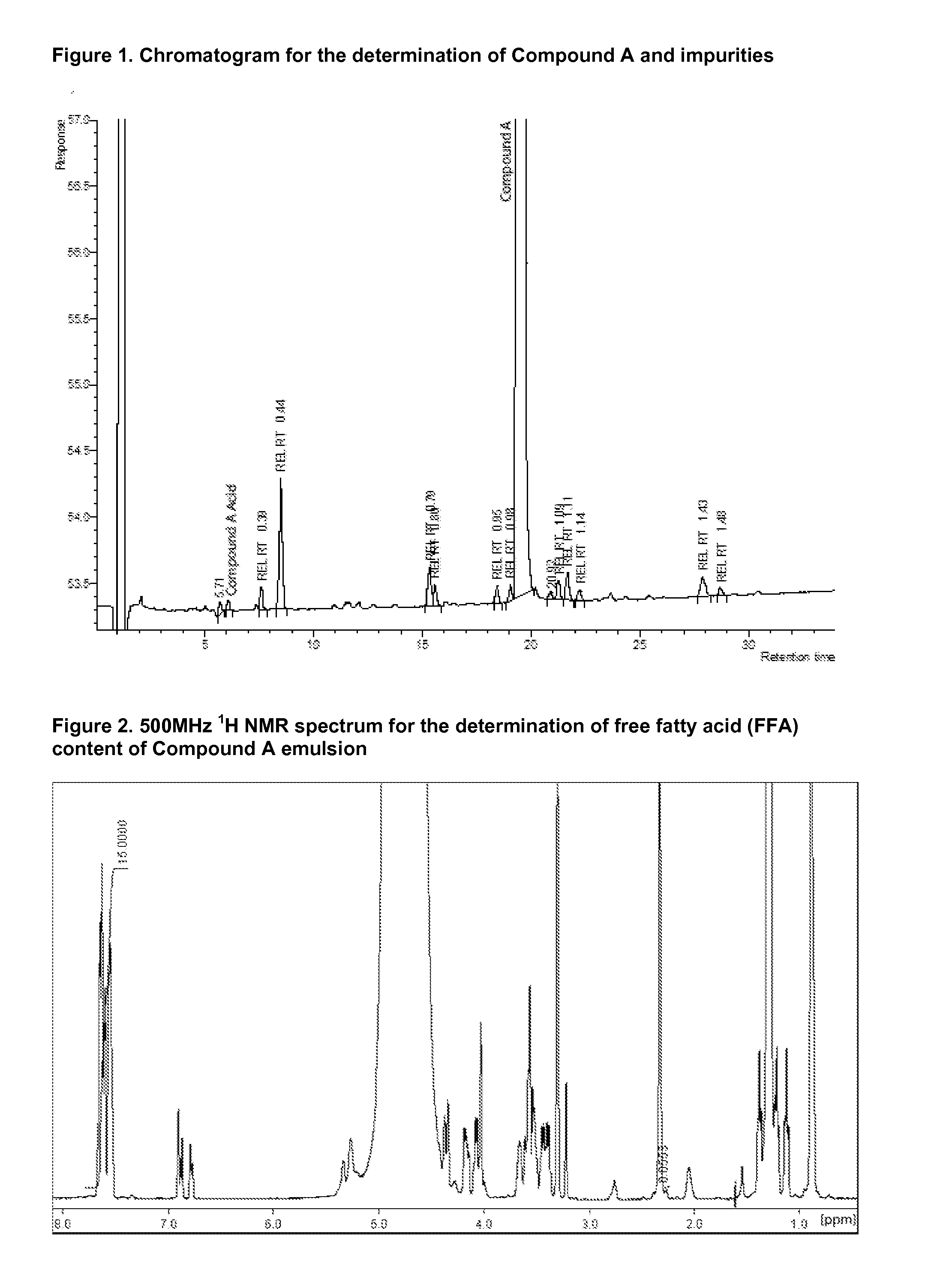
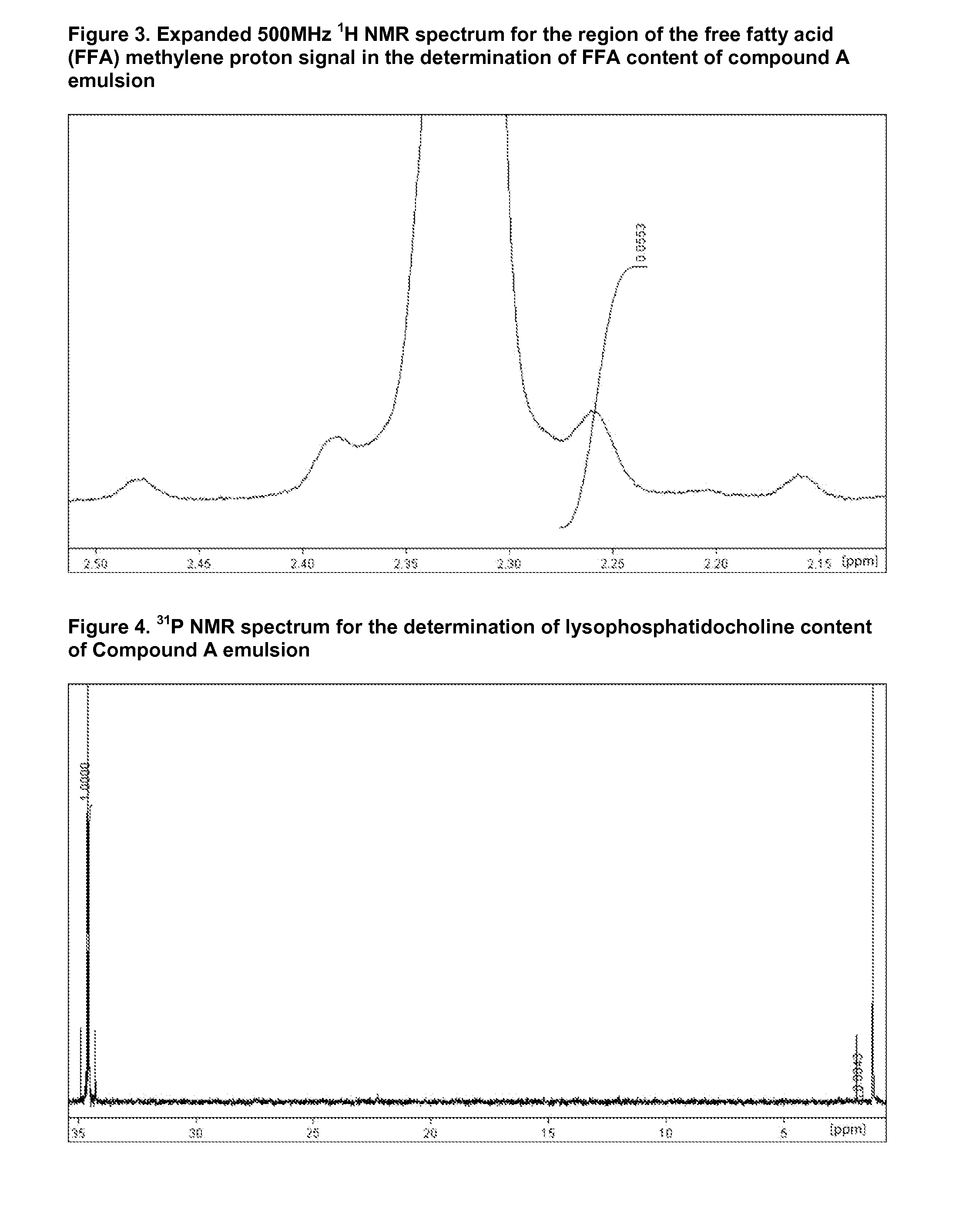
Injectable Emulsion of Sedative Hypnotic Agent
InactiveUS20130236501A1Improve physical stabilityGood storage stabilityBiocideNervous disorderHypnotic agentPhenylacetic acid
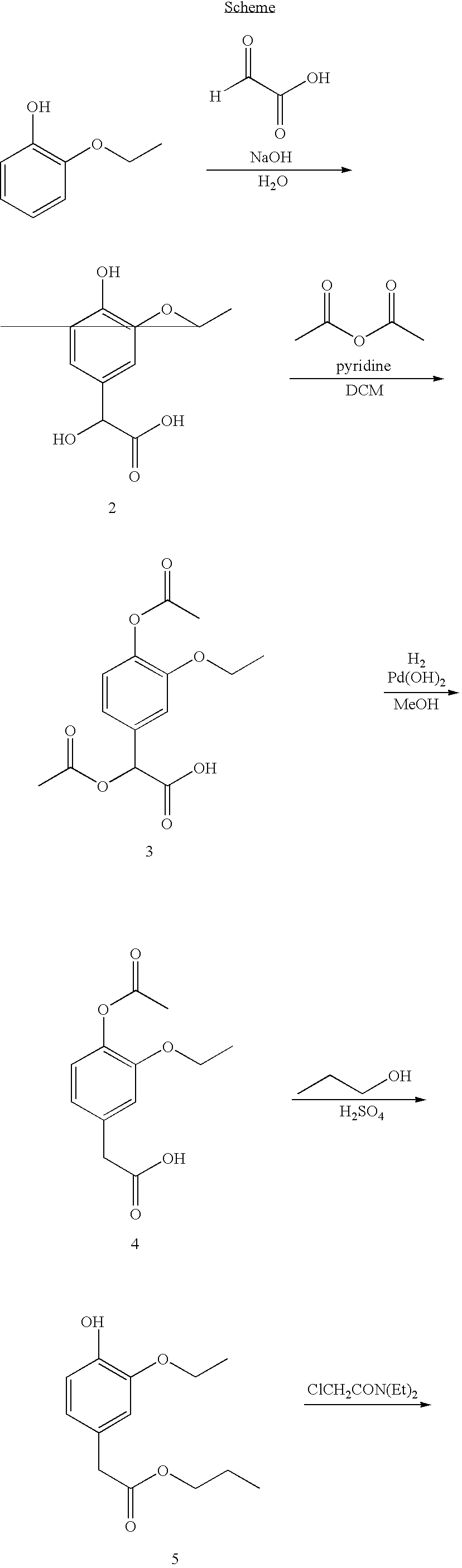
The present invention provides novel pharmaceutical formulations of a substituted phenylacetic acid ester compound, which is useful as a short-acting sedative hypnotic agent for anesthesia and sedation. The pharmaceutical formulations are oil-in-water emulsions suitable for administration by injection. The invention further provides processes for the preparation of the formulation and the use of the formulation in medical treatment of a mammal.
Owner:ASTRAZENECA AB
Pharmaceutical compositions of short-acting hypnotic agents in modified-release forms and the procedures to prepare the mentioned formulation
InactiveUS20090155358A1Minimizes unwanted gastrointestinal effectAvoid stimulationBiocideNervous disorderHypnotic agentActive agent
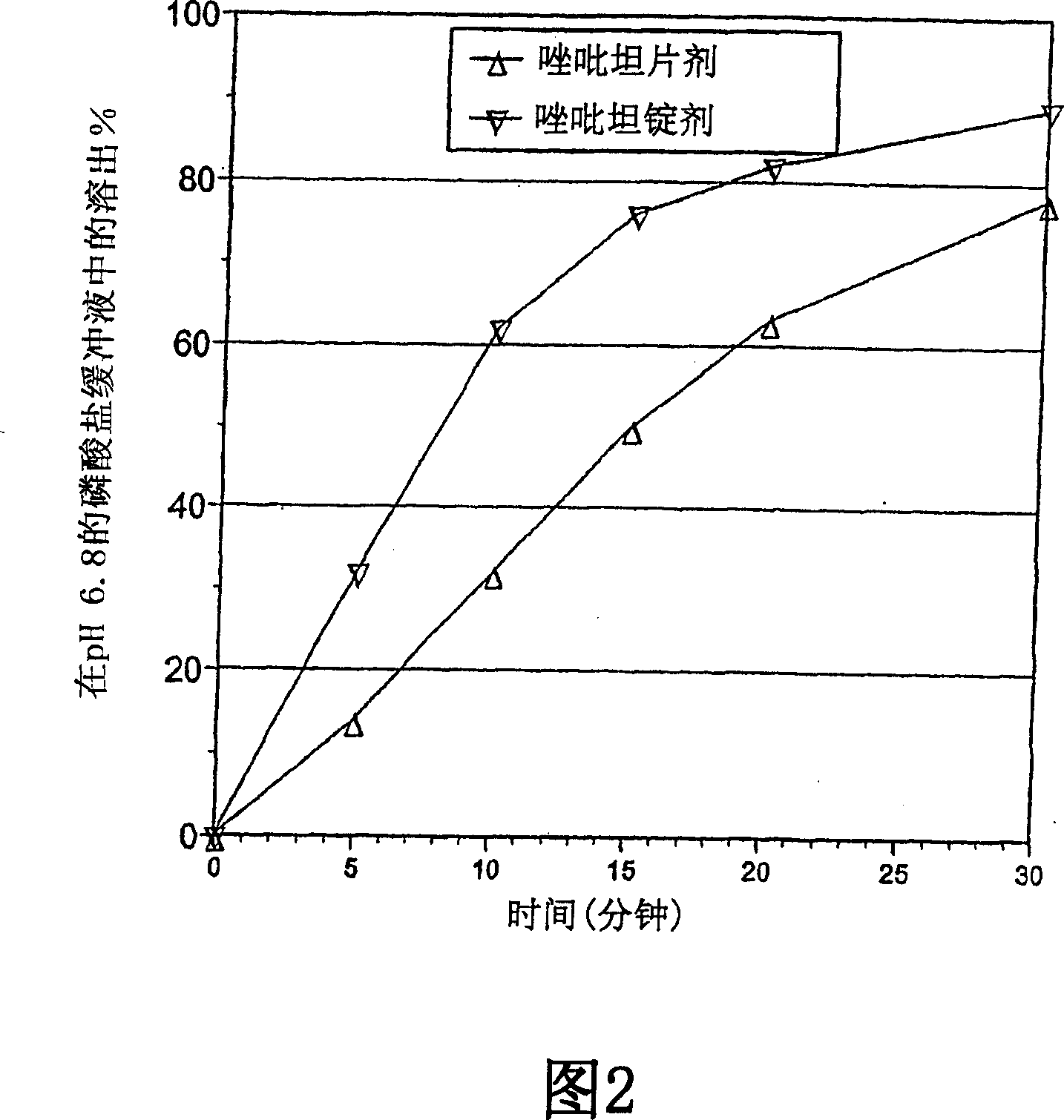
This application refers to a modified-release pharmaceutical composition containing, as the active agent, a short-acting hypnotic agent or a pharmaceutically acceptable salt thereof, comprising two sustained-release pharmaceutical entities, differentiated from each other by a different release rate of the active agent wherein the release of the active agent from one of the entities starts before the release of the active agent from the second entity.
Owner:GADOR
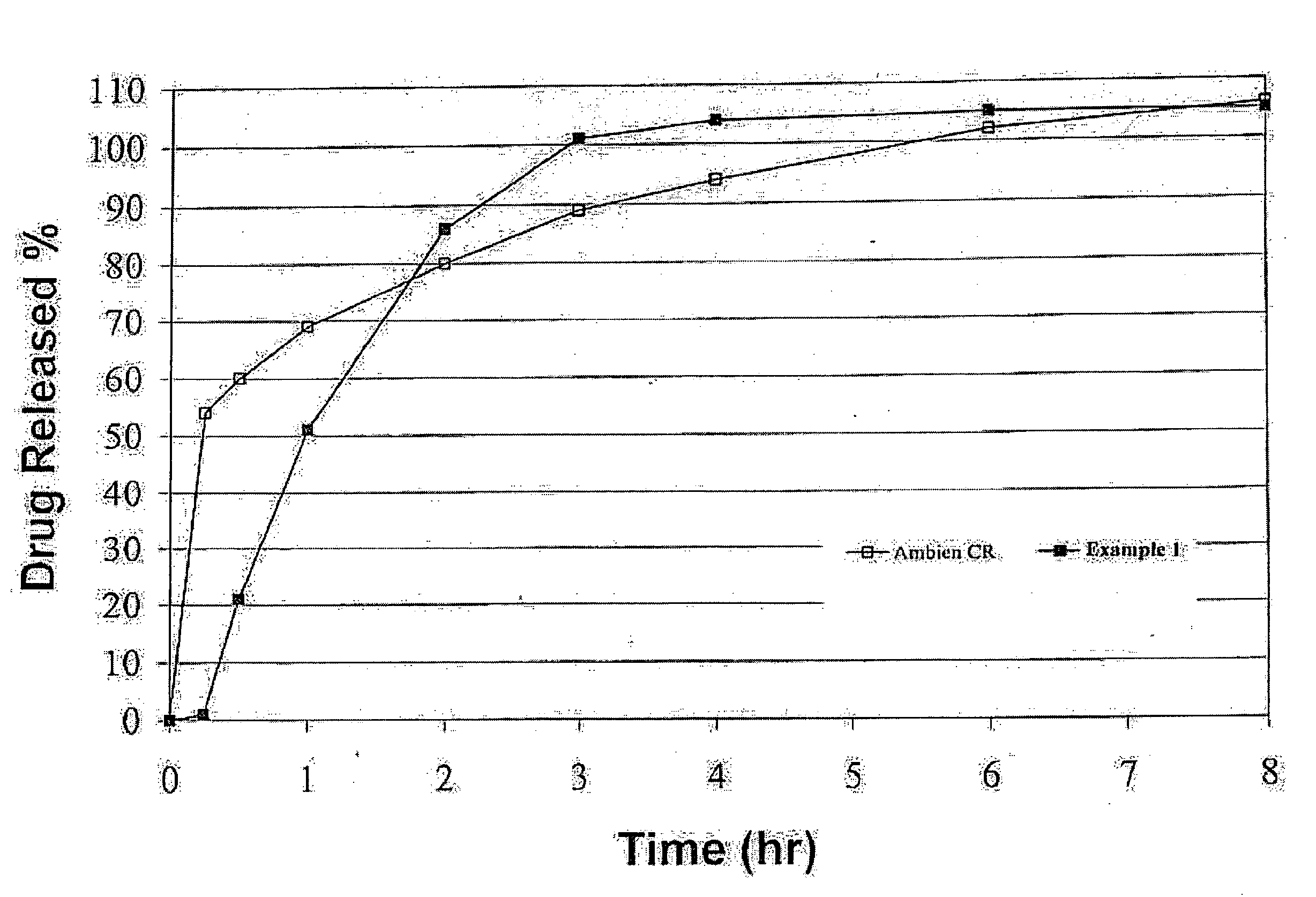
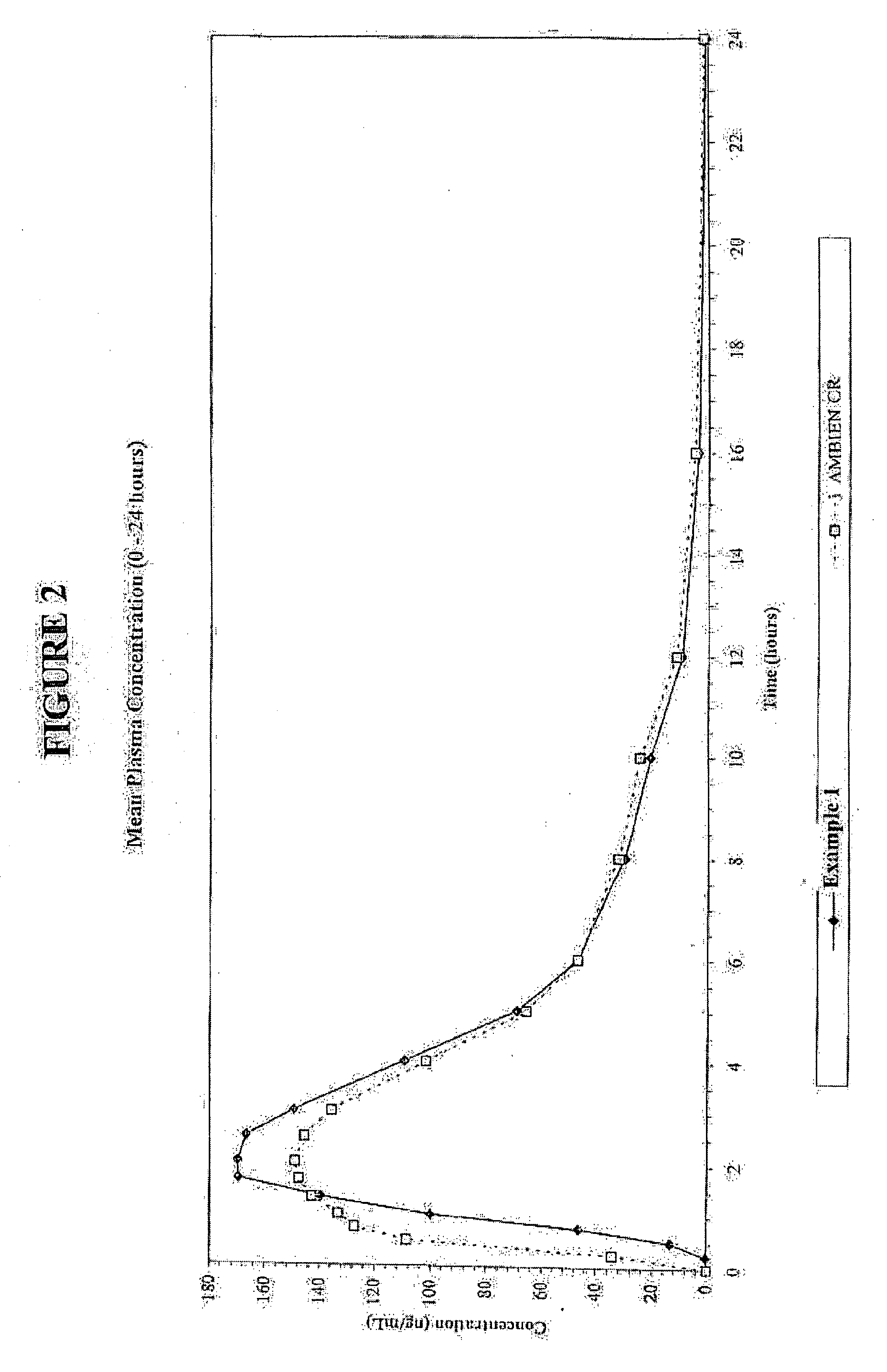
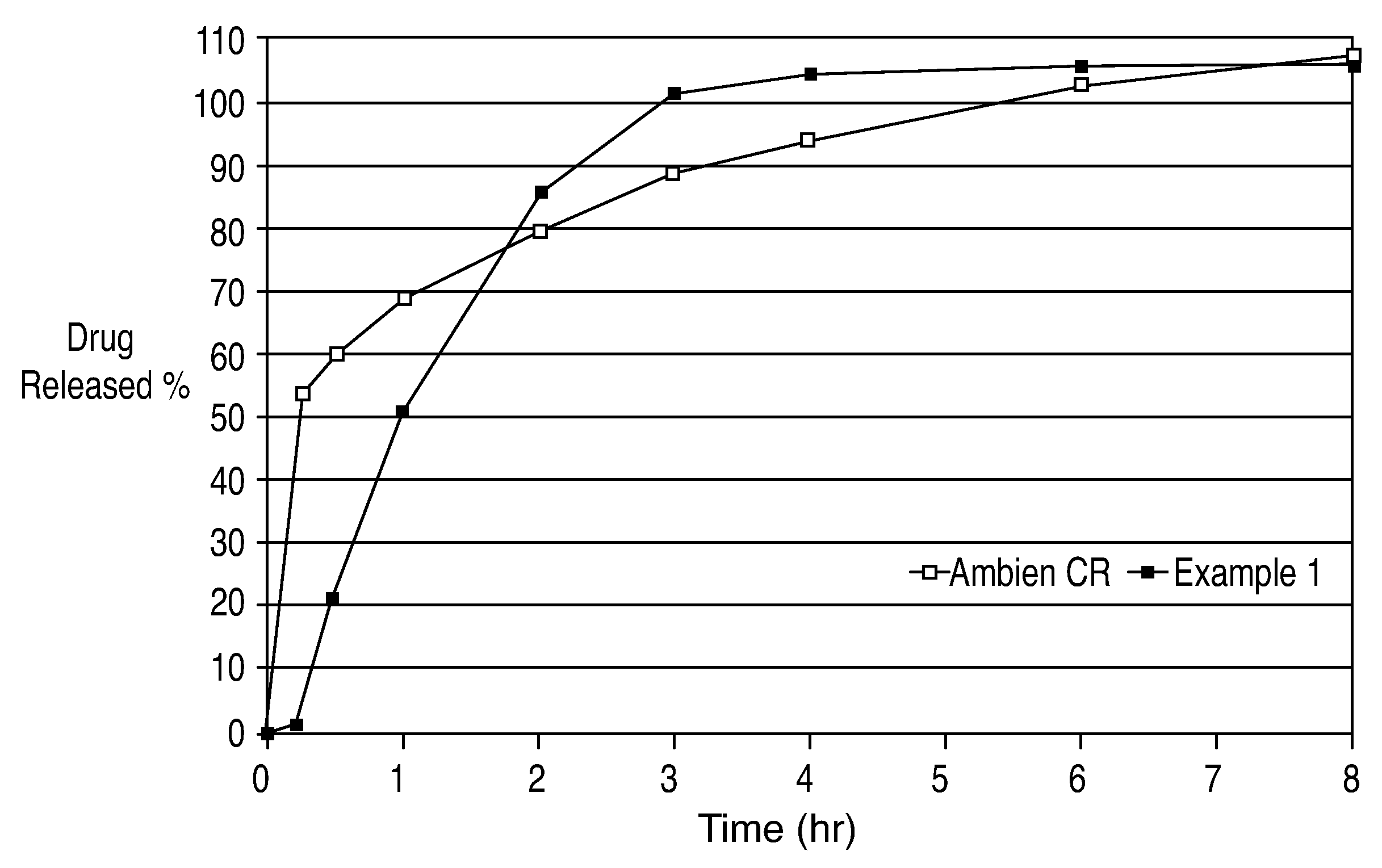
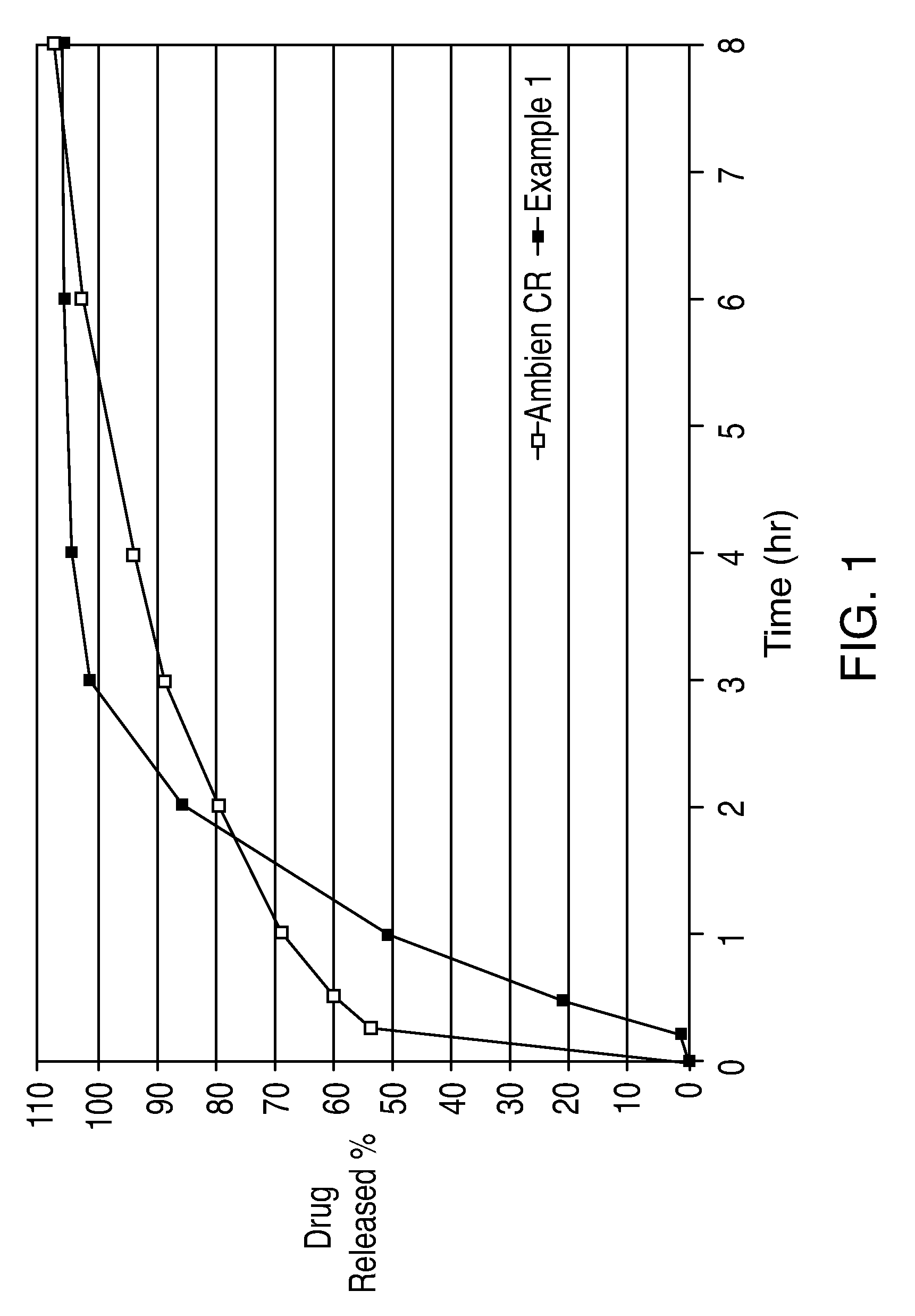
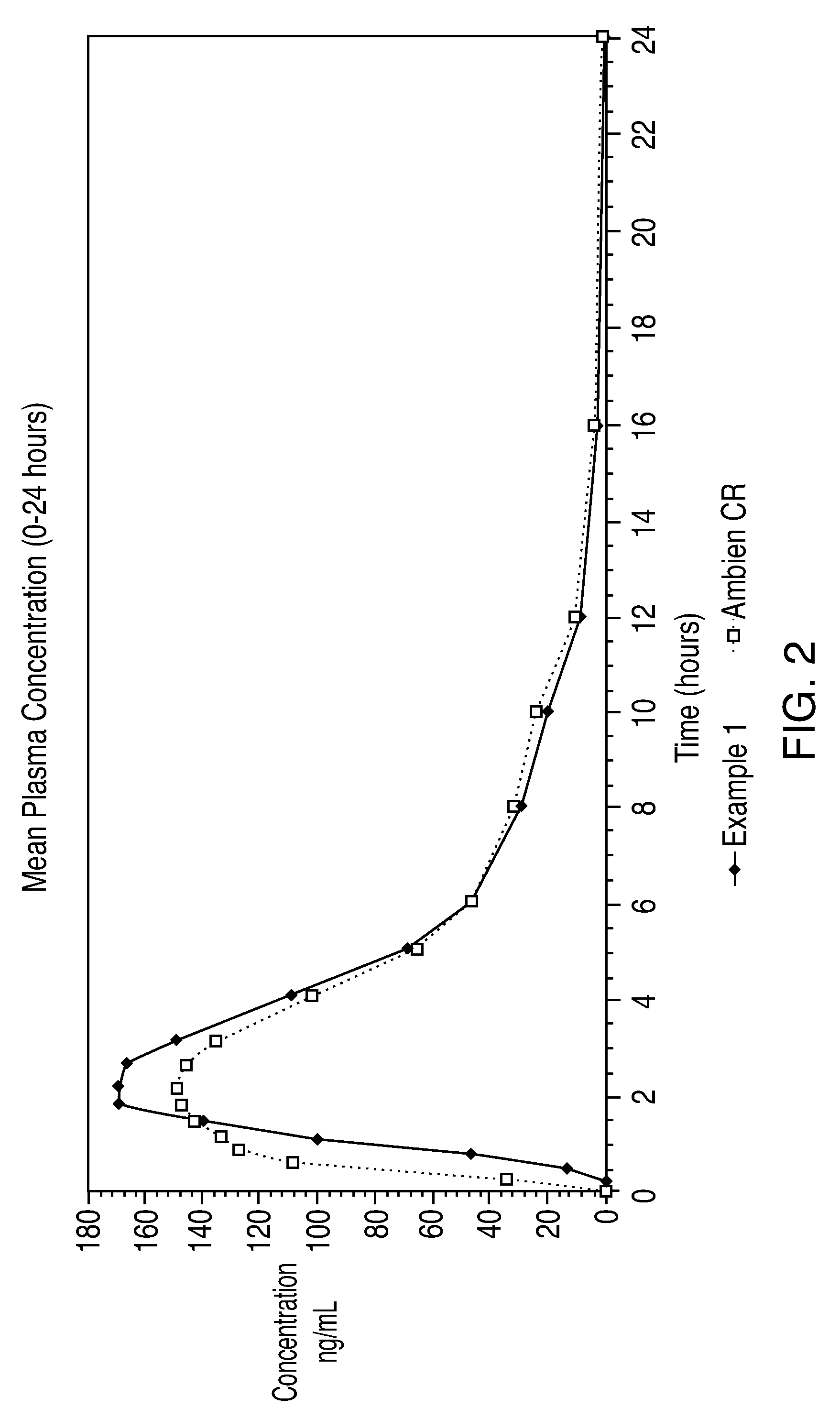
Oral controlled release formulation for sedative and hypotnic agents
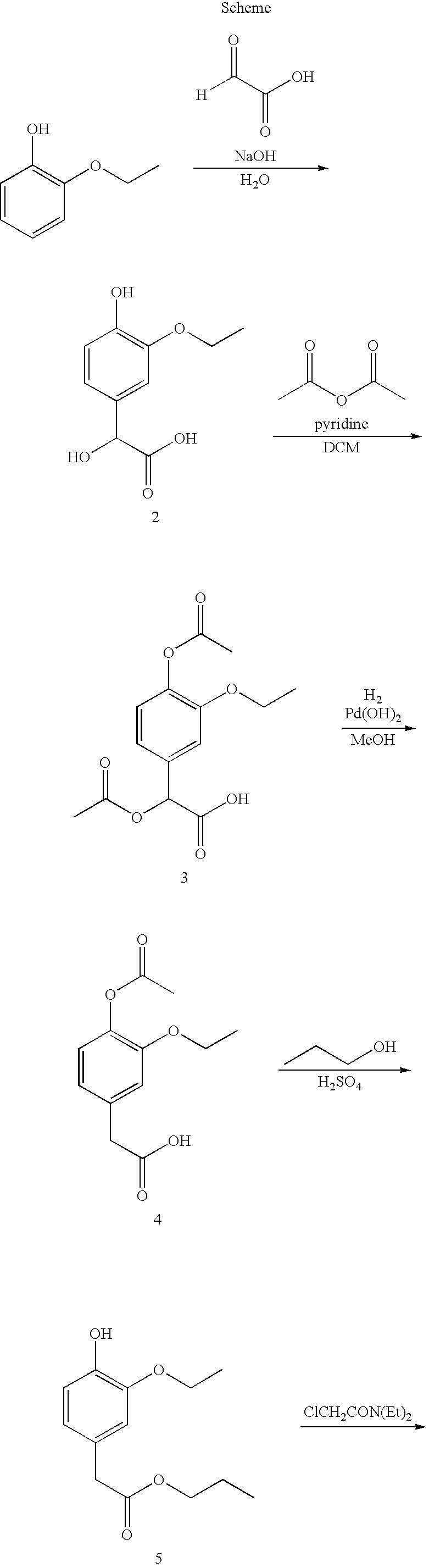
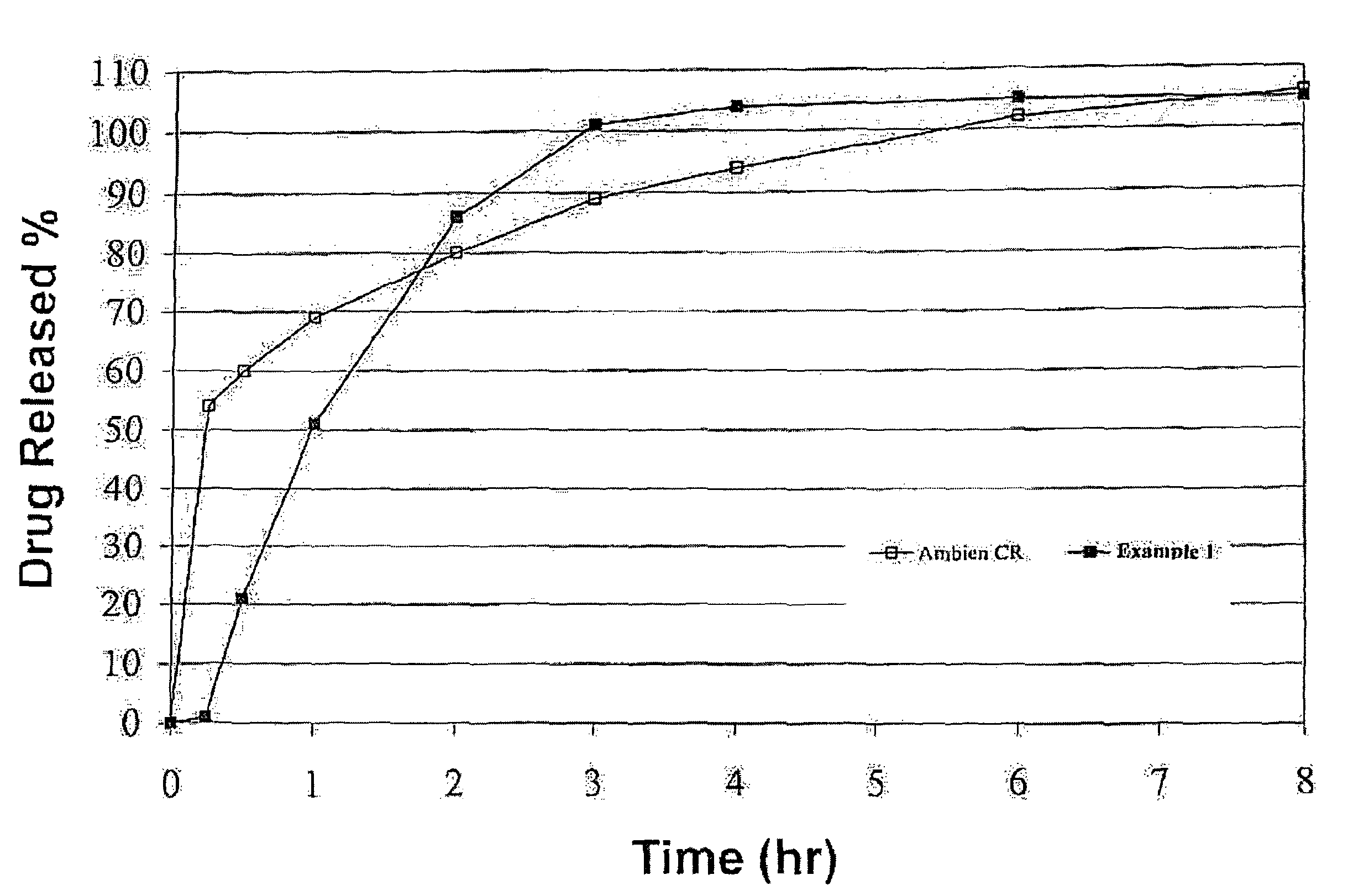
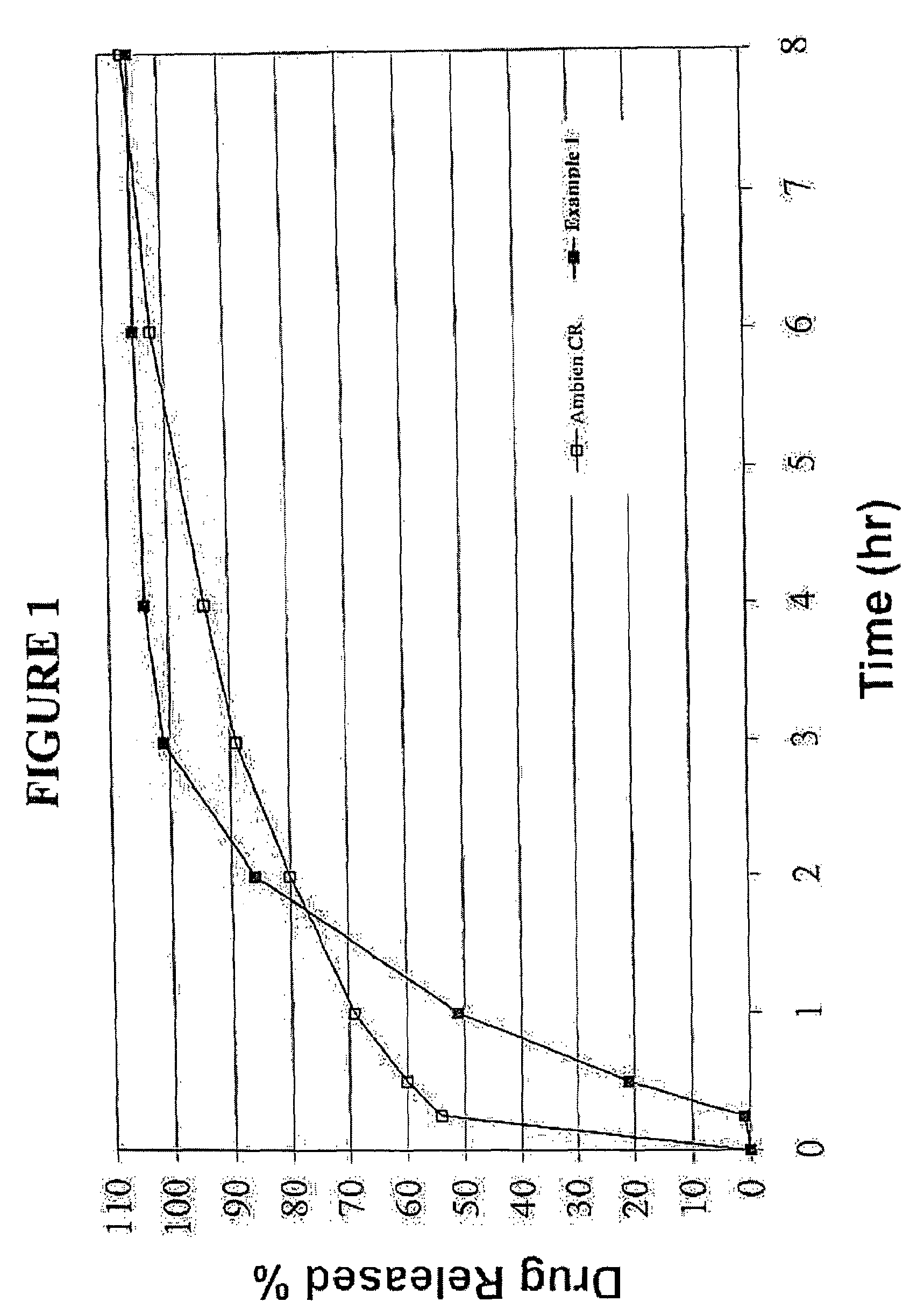
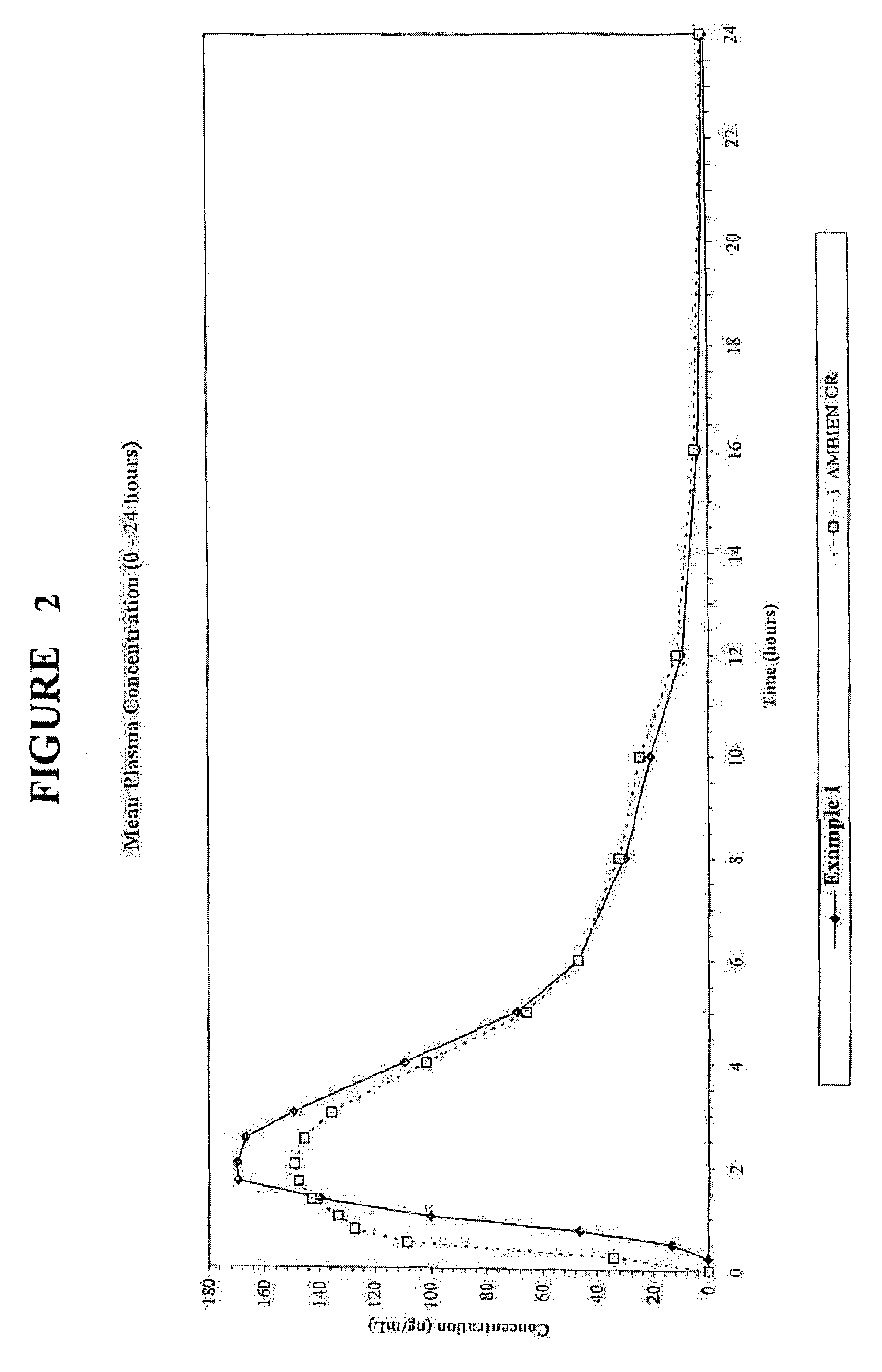
InactiveUS20070207203A1Simple manufacturing processDecrease in AUC and CmaxNervous disorderCoatingsHypnotic agentTreatment level
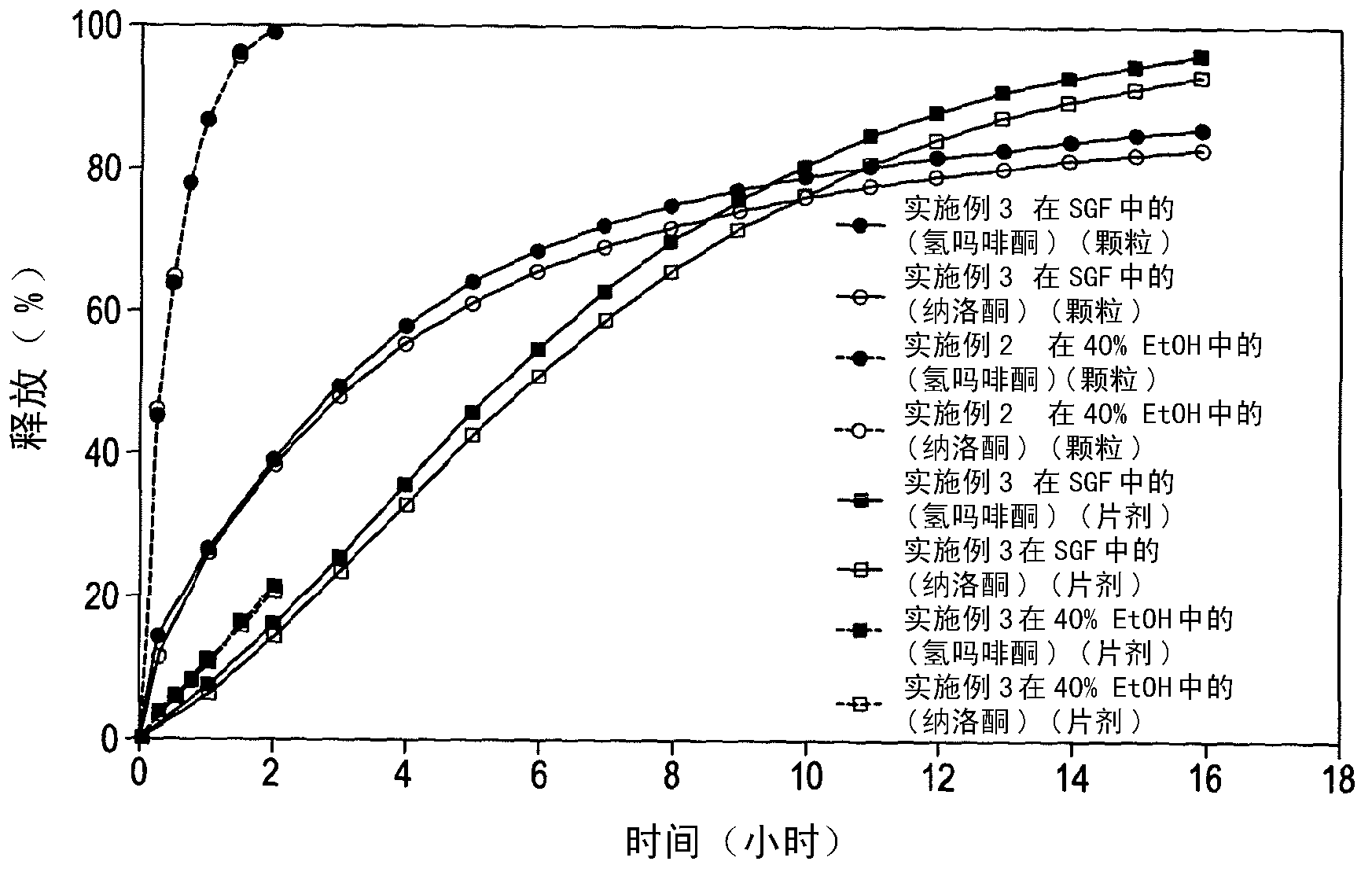
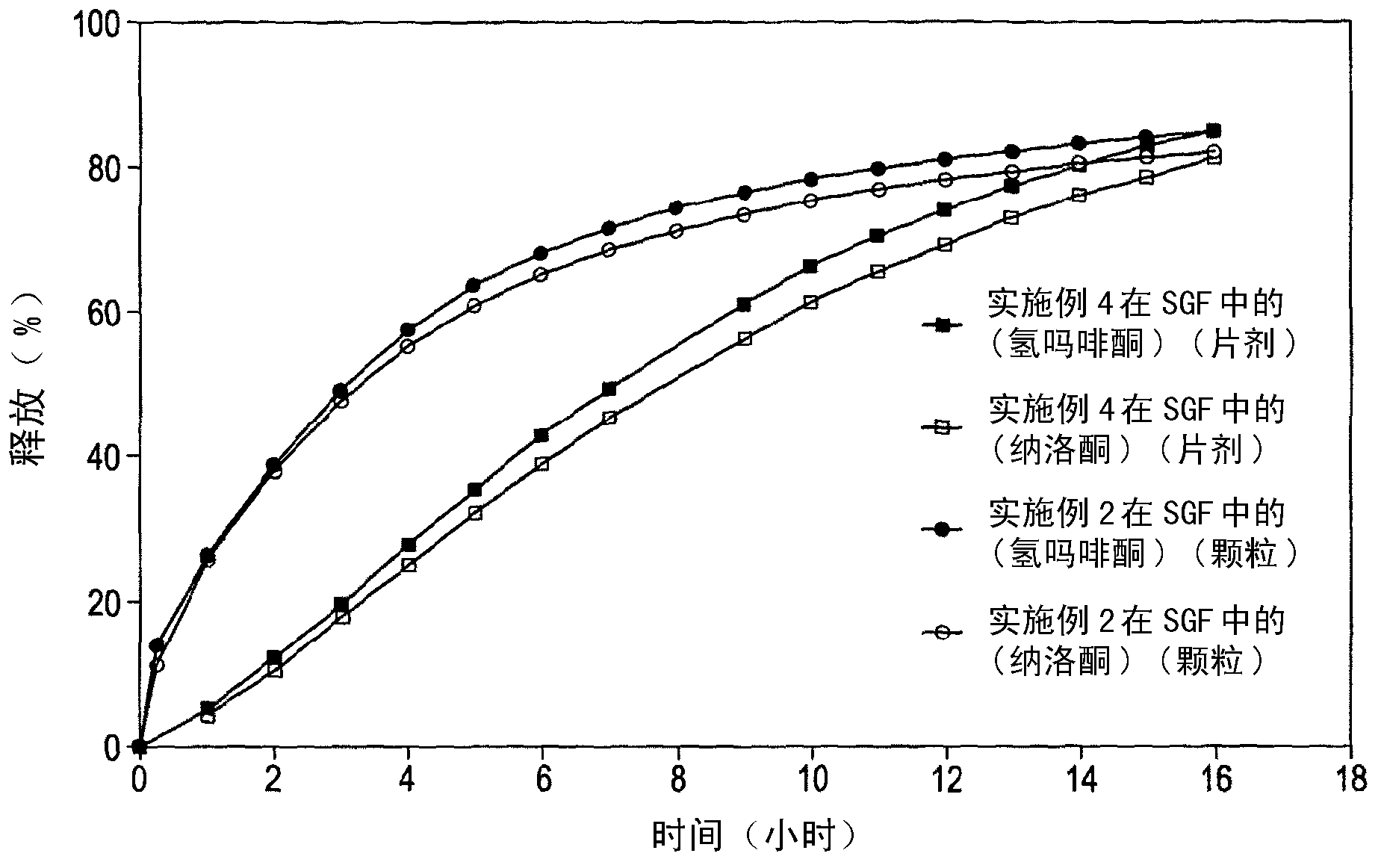
The present invention relates to a novel controlled release dosage form that releases therapeutic amounts of a sedative or hypnotic agent rapidly after administration and maintains therapeutic levels for about eight hours after administration.
Owner:ACTAVIS HOLDCO US INC
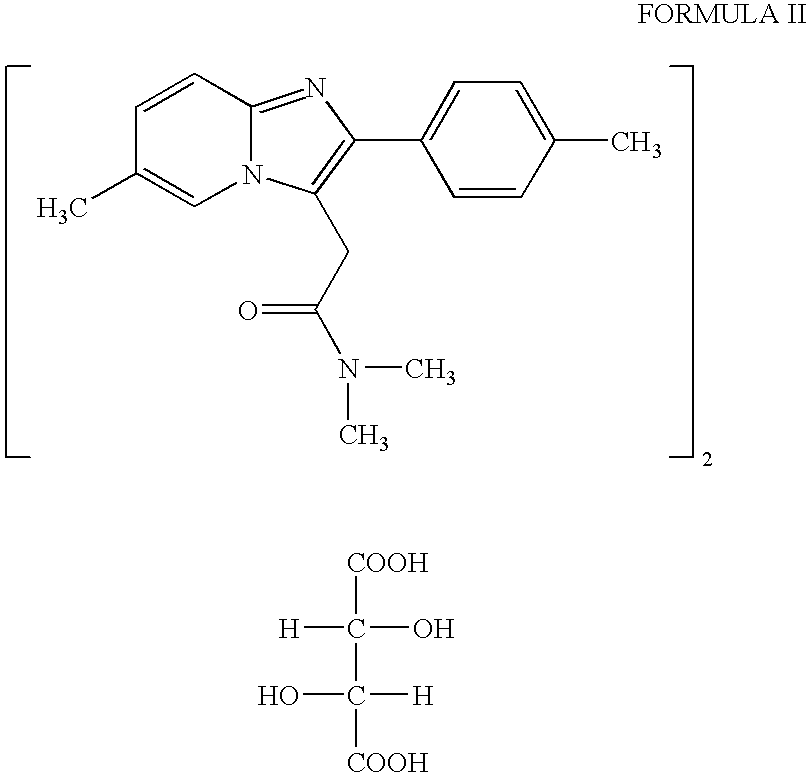
Polymorph of N-methyl-N-(3-{3-[2-thienylcarbonyl]-pyrazol-[1,5-alpha]-pyrimidin-7-yl}phenyl)acetamide and compositions and methods related thereto
Polymorph Form III of N-methyl-N-(3-{3-[2-thienylcarbonyl]-pyrazol-[1,5-α]-pyrimidin-7-yl}phenyl)acetamide, and use thereof as a sedative-hypnotic, anxiolytic, anticonvulsant, and / or skeletal muscle relaxant agent. Related compositions and methods are also disclosed, particularly with regard to treatment of insomnia.
Owner:NEUROCRINE BIOSCI INC
Polymorphs of N-methyl-N-(3-{3-[2-thienylcarbonyl]-pyrazol-[1,5-alpha]-pyrimidin-7-yl}phenyl)acetamide and compositions and methods related thereto
The invention discloses N-methyl-N-(3-{3-[2-thienylcarbonyl]-pyrazole-[1,5-α]-pyrimidin-7-yl}phenyl)acetamide (compound ( 1)), and their use as sedative-hypnotics, anxiolytics, anticonvulsants and skeletal muscle relaxants. At the same time, methods for preparing the substance and related compositions are also disclosed, especially for the treatment of insomnia. And provides the exceptional physical and thermal stability possessed by the Form I polymorph. Type II polymorphs are also contemplated.
Owner:NEUROCRINE BIOSCI INC
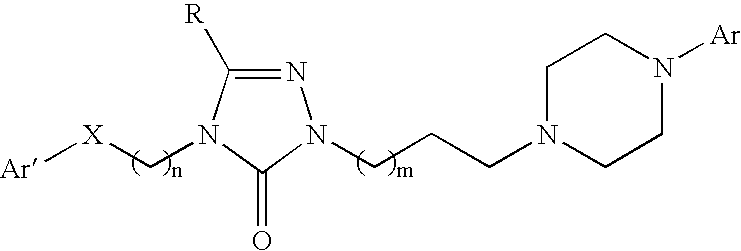
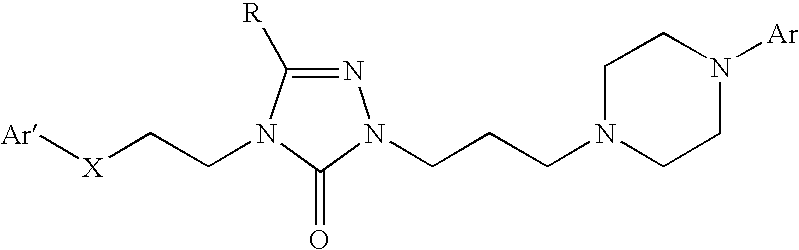
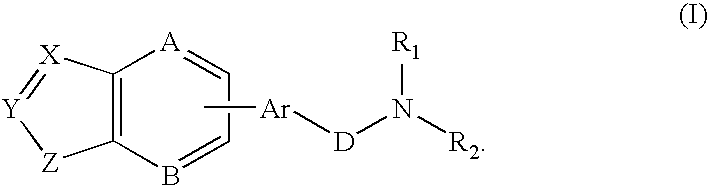
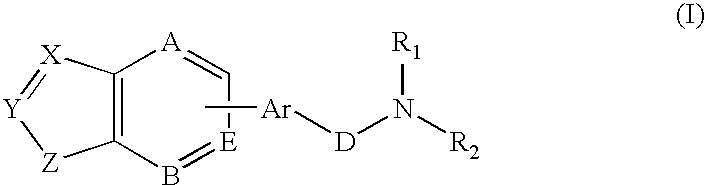
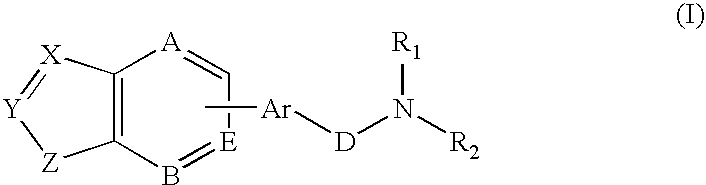
Combination of a hypnotic agent and substituted bis aryl and heteroaryl compound and therapeutic application thereof
The invention concerns the combination of a short-acting hypnotic agent and a compound of formula (I):Wherein X, Y, Z, A, B, D, Ar, R1 and R2 are as defined herein. The combination of this invention is useful in treating a variety of sleep disorders.
Owner:AVENTISUB LLC
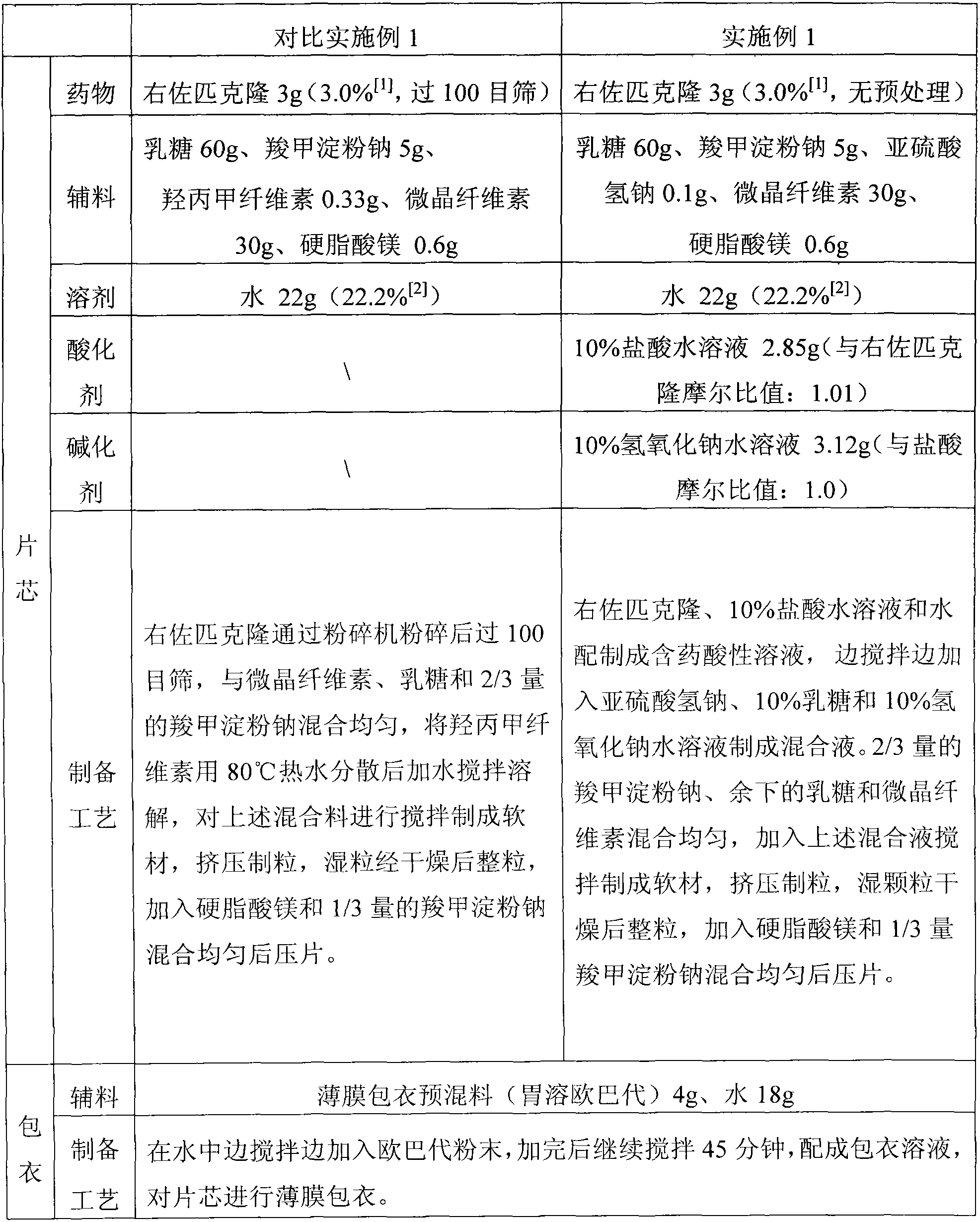
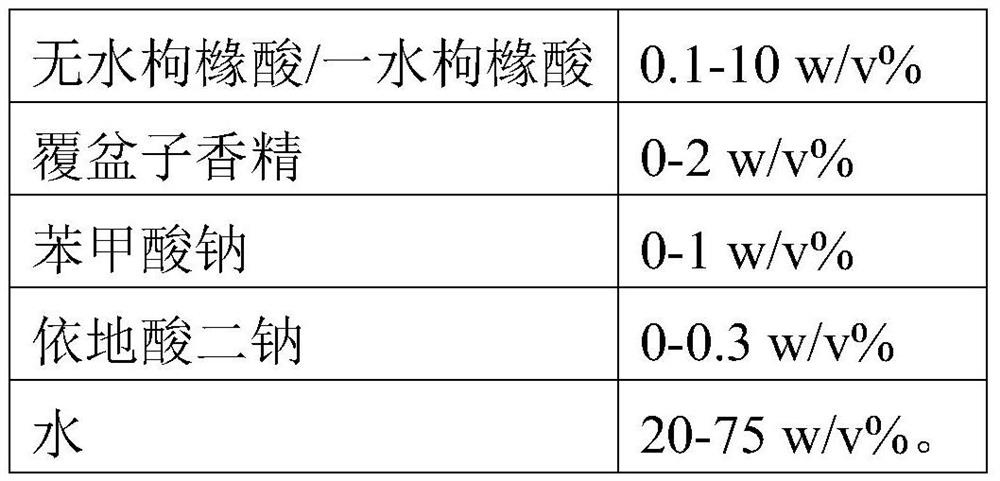
Sedative hypnotic pharmaceutical preparation and its preparation method
ActiveCN102846538AChange surface propertiesImprove wettabilityOrganic active ingredientsNervous disorderOrganic acidSubstance content
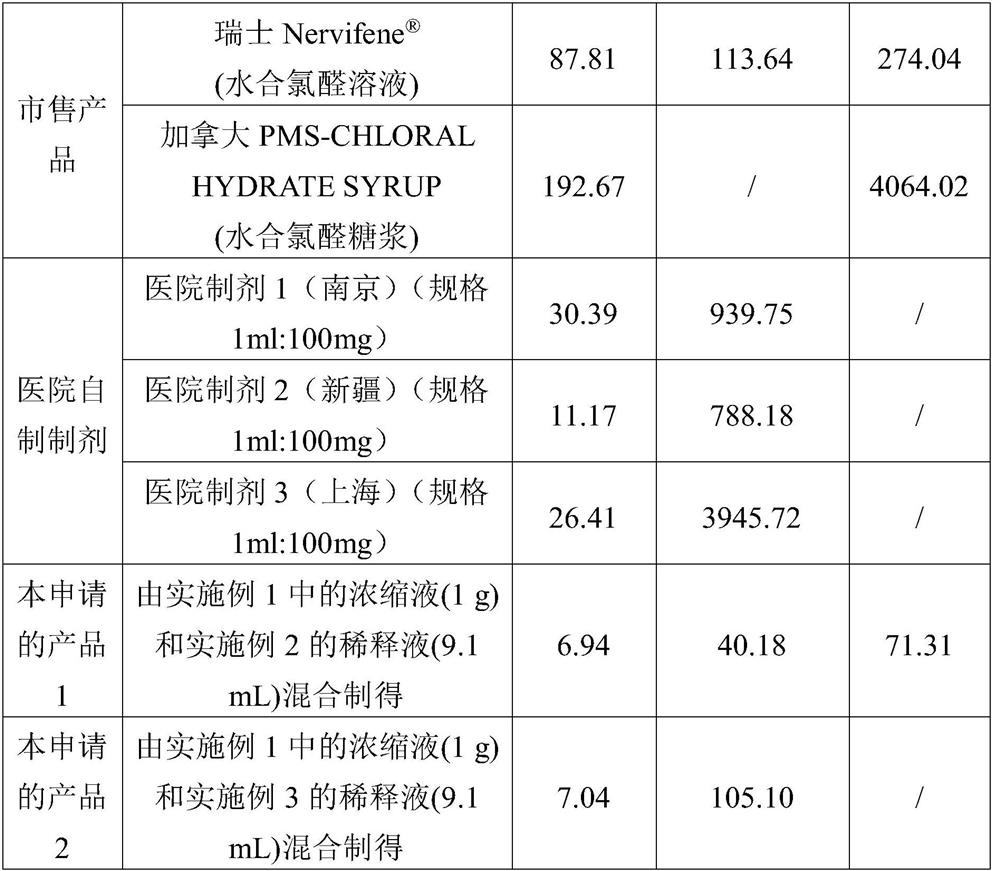
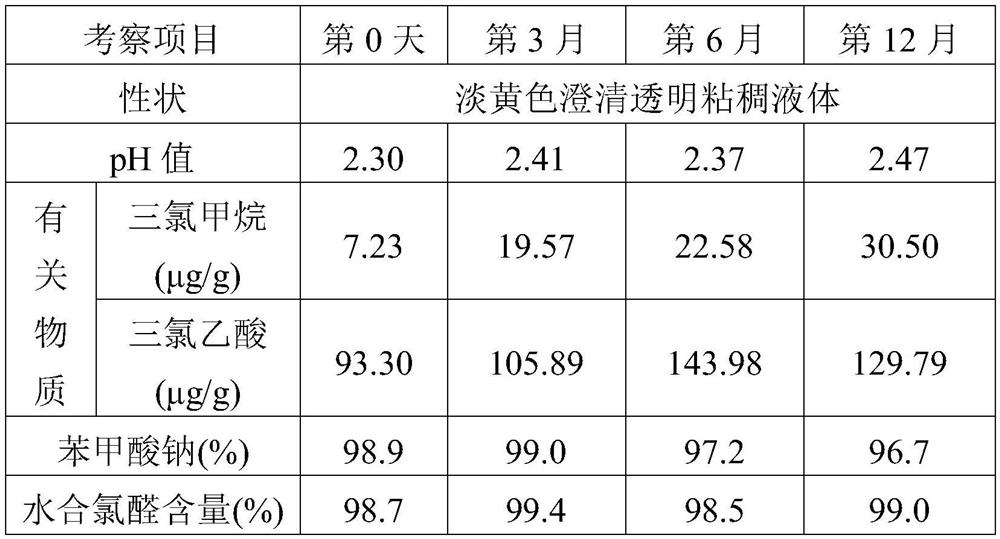
The invention discloses a preparation method of a sedative hypnotic pharmaceutical preparation. The preparation method includes the steps of dissolving active ingredient dexzopiclone or zopiclone in acidifier-containing acidic solution to give drug-containing acidic solution, and then performing wet granulation with the obtained drug-containing acidic solution, basifier and adjuvant. The acidifier is hydrochloric acid, and the basifier is sodium hydroxide. One or more of organic weak acid, acid salt and conjugate base of organic weak acid are added before or during the addition of the basifier. The invention also discloses the sedative hypnotic pharmaceutical preparation prepared by the method. The inventive preparation method has no potential safety hazard, simple and convenient operation, low pollution and loss, low cost, and good process controllability. The obtained pharmaceutical preparation has excellent dissolution and stability, and lower related substance content.
Owner:SHANGHAI ZHONGXI PHARMA
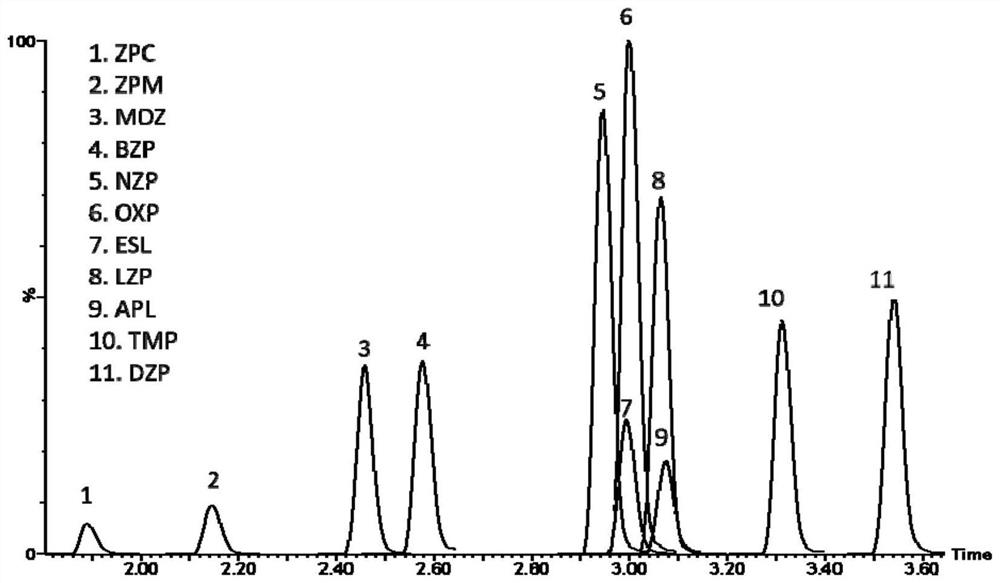
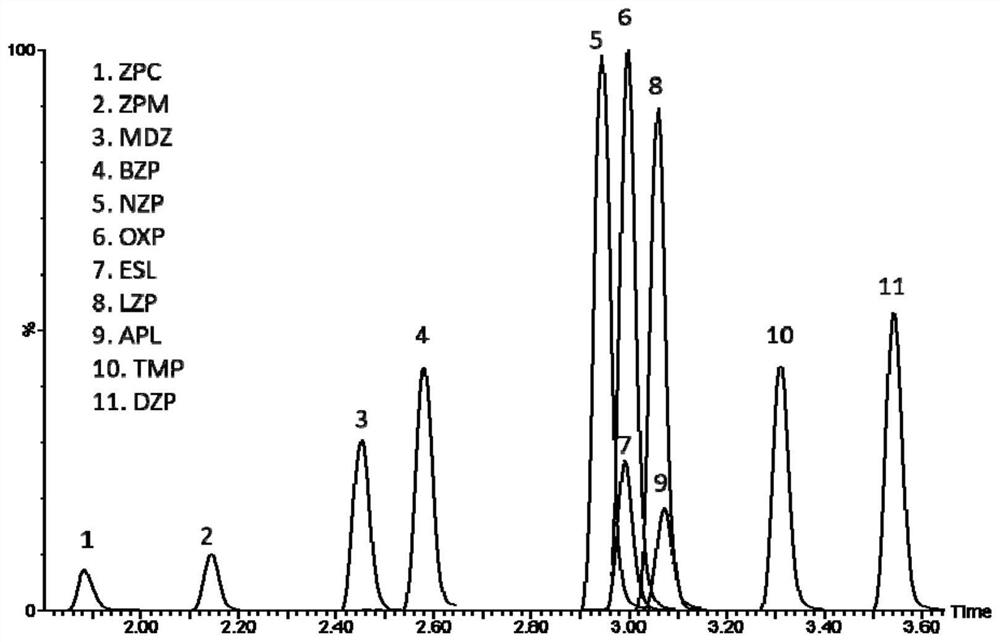
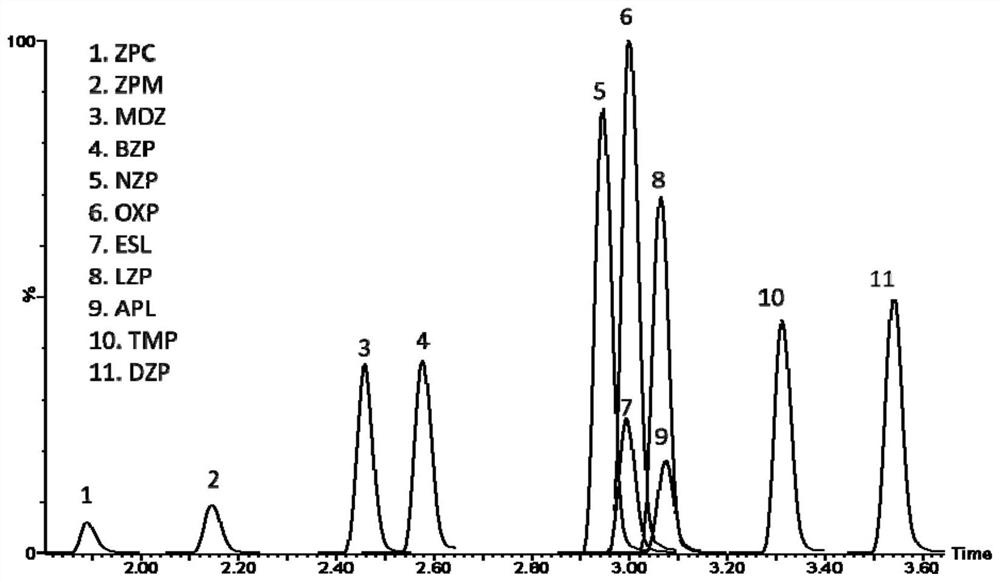
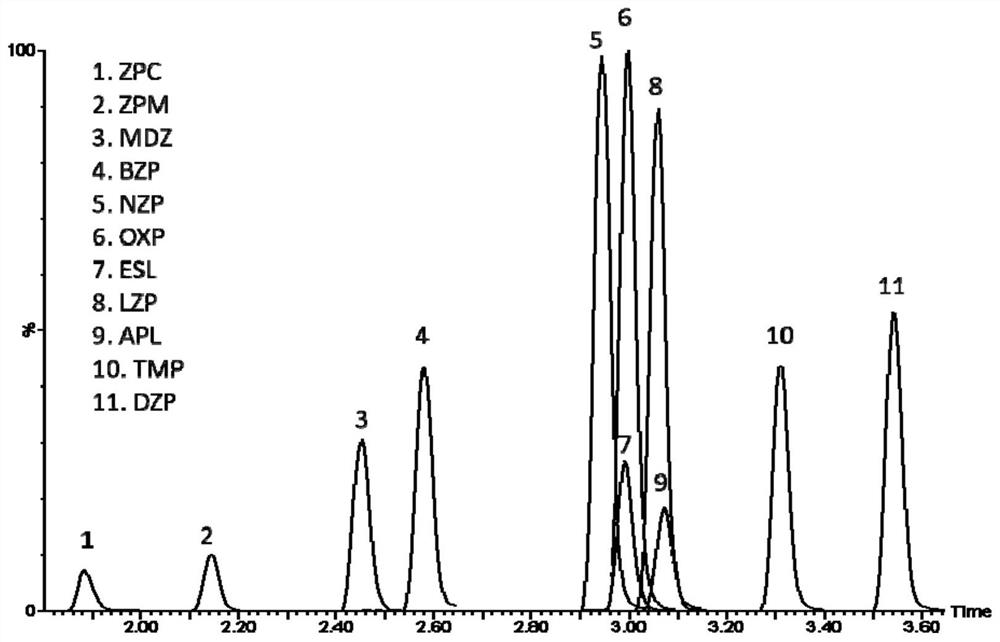
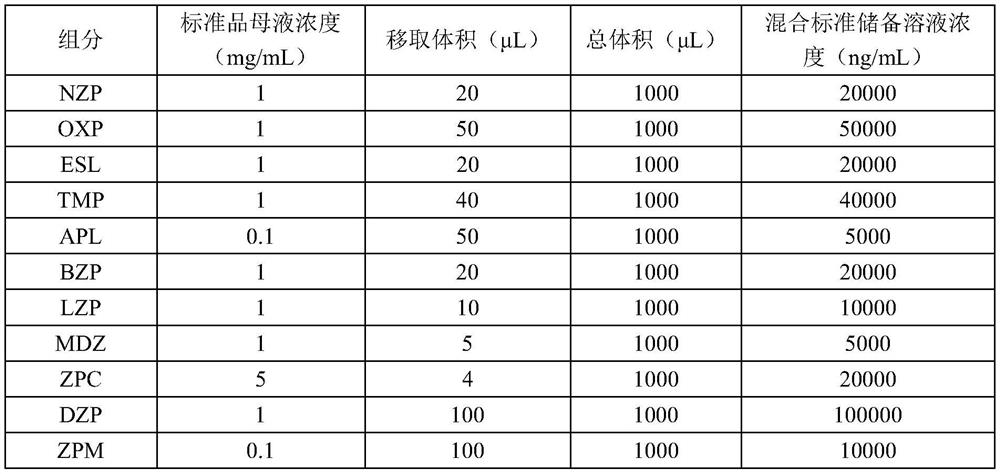
Method for detecting concentrations of anxiolytic and hypnotic drugs in serum by using ultra-high performance liquid chromatography-tandem mass spectrometry technology
ActiveCN111812225AHigh sensitivityThe pre-processing process is simpleComponent separationMetaboliteHypnotic agent
The invention relates to a method for detecting anxiolytic and hypnotic drugs in serum by using an ultra-high performance liquid chromatography-tandem mass spectrometry technology. The method can be used for detecting 11 kinds of anxiolytic and hypnotic drugs at one time, monitors target drugs and metabolites at the same time, is simple in pretreatment process, low in cost, high in sensitivity andhigh in specificity, can complete separation and detection of the anxiolytic and hypnotic drugs within 5 minutes, basically meets the requirements for accuracy degree and precision, can be used for quantitative analysis of the anxiolytic and hypnotic drugs clinically, and provides a simple and rapid detection method for monitoring the treatment concentrations of the anxiolytic and hypnotic drugsin serum clinically.
Owner:南京品生医学检验实验室有限公司 +2
Pharmaceutical compositions of short-acting sedative hypnotic agent
The invention provides pharmaceutical compositions comprising a phenylacetic acid ester compound useful for inducing or maintaining general anesthesia or sedation in mammals, methods for preparing such compositions, and methods for inducing or maintaining anesthesia or sedation using such compositions.
Owner:THERAVANCE BIOPHARMA R&D IP LLC
Compositions for delivering hypnotic agents across the oral mucosa and methods of use thereof
The present invention provides novel compositions for the delivery of a hypnotic agent across the oral mucosa. In particular, the buffer system in the compositions of the present invention raises the pH of saliva to a pH greater than about 7.8, thereby facilitating the substantially complete conversion of the hypnotic agent from its ionized to its un-ionized form. As a result, the dose of hypnotic agent is rapidly and efficiently absorbed by the oral mucosa with surprisingly low inter-subject variability. Furthermore, delivery of the hypnotic agent across the oral mucosa advantageously bypasses hepatic first pass metabolism of the drug and avoids enzymatic degradation of the drug within the gastrointestinal tract. Methods for using the compositions of the present invention for treating sleep disorders such as insomnia are also provided.
Owner:创塞伯特制药公司
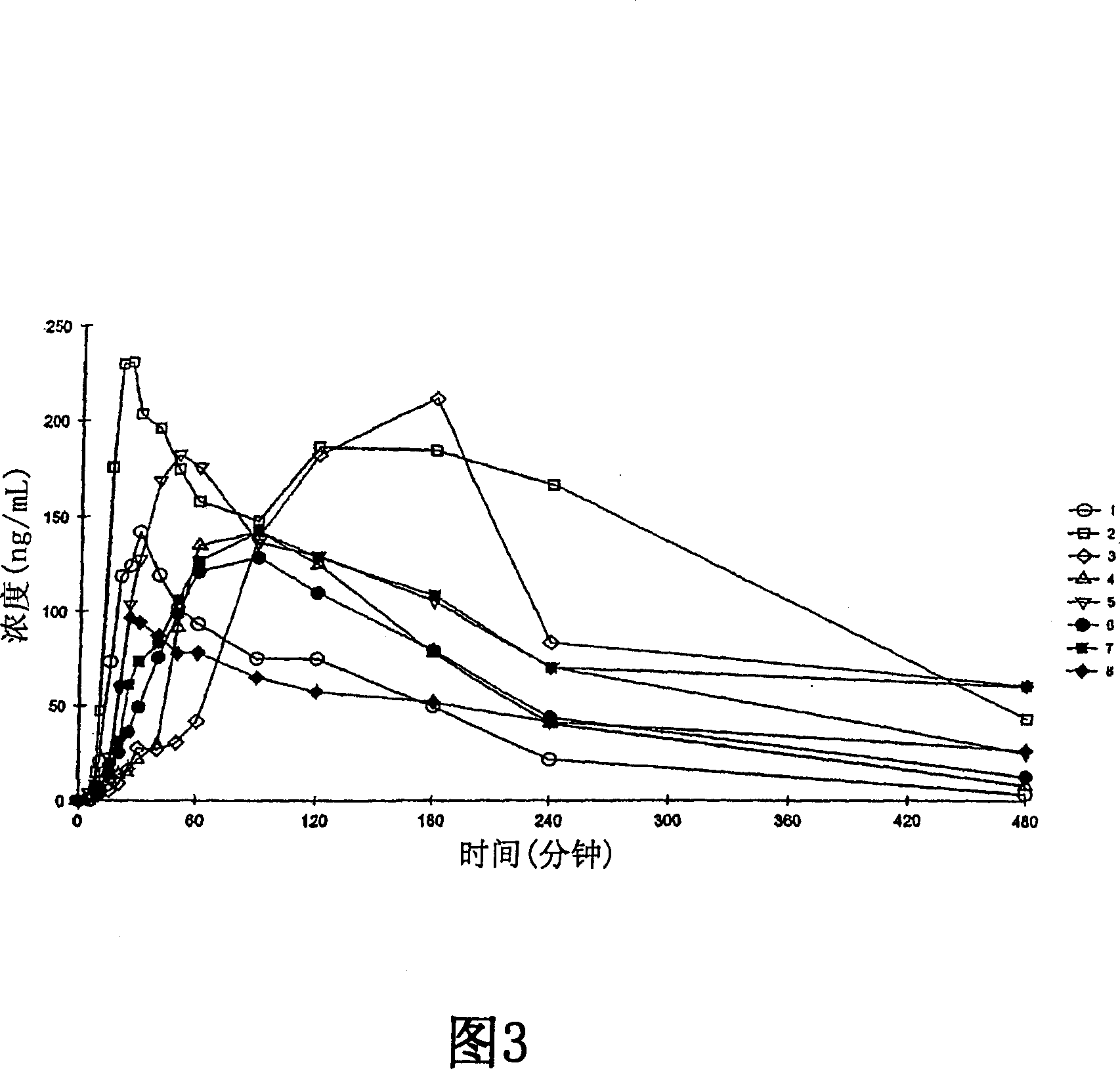
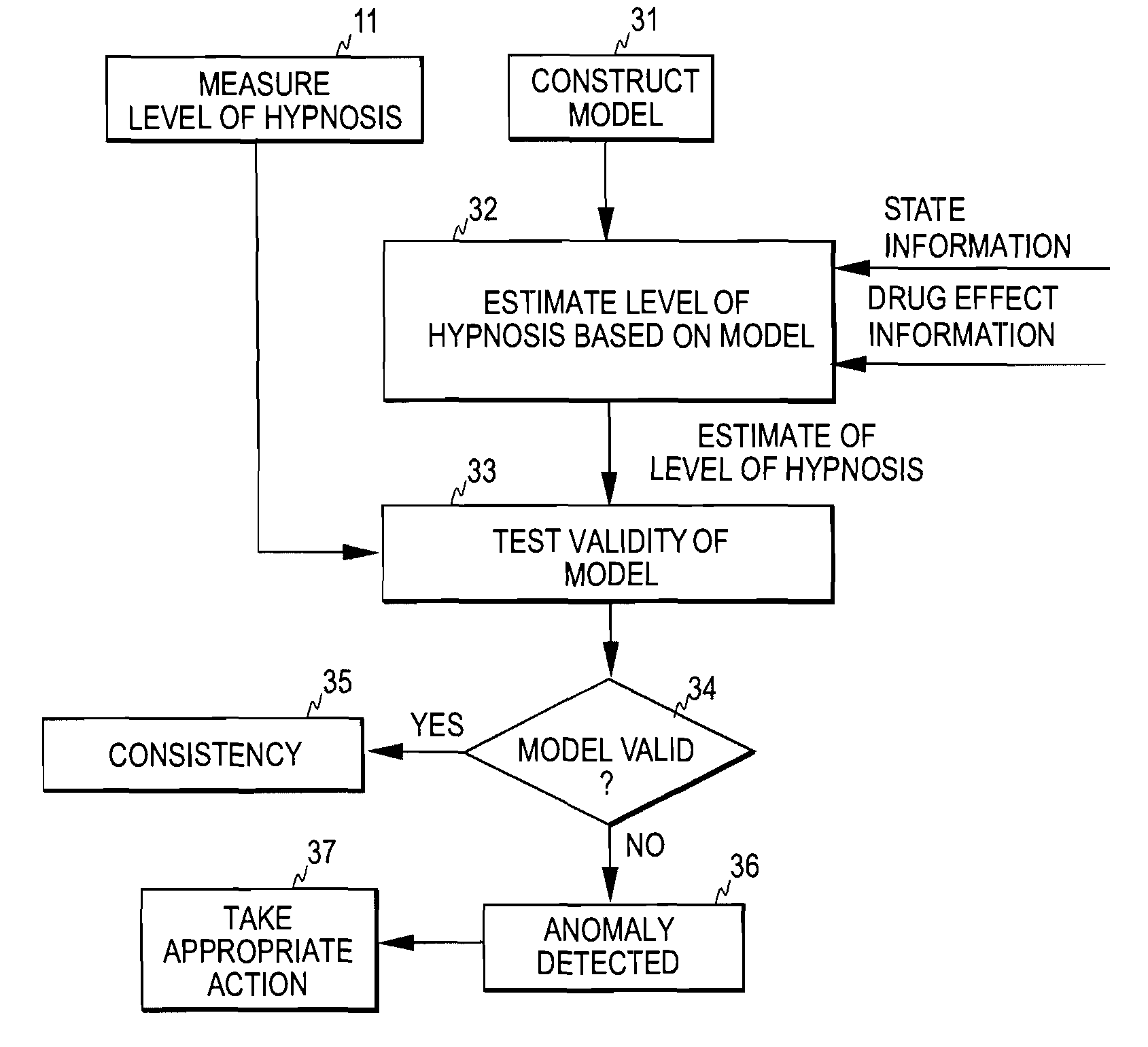
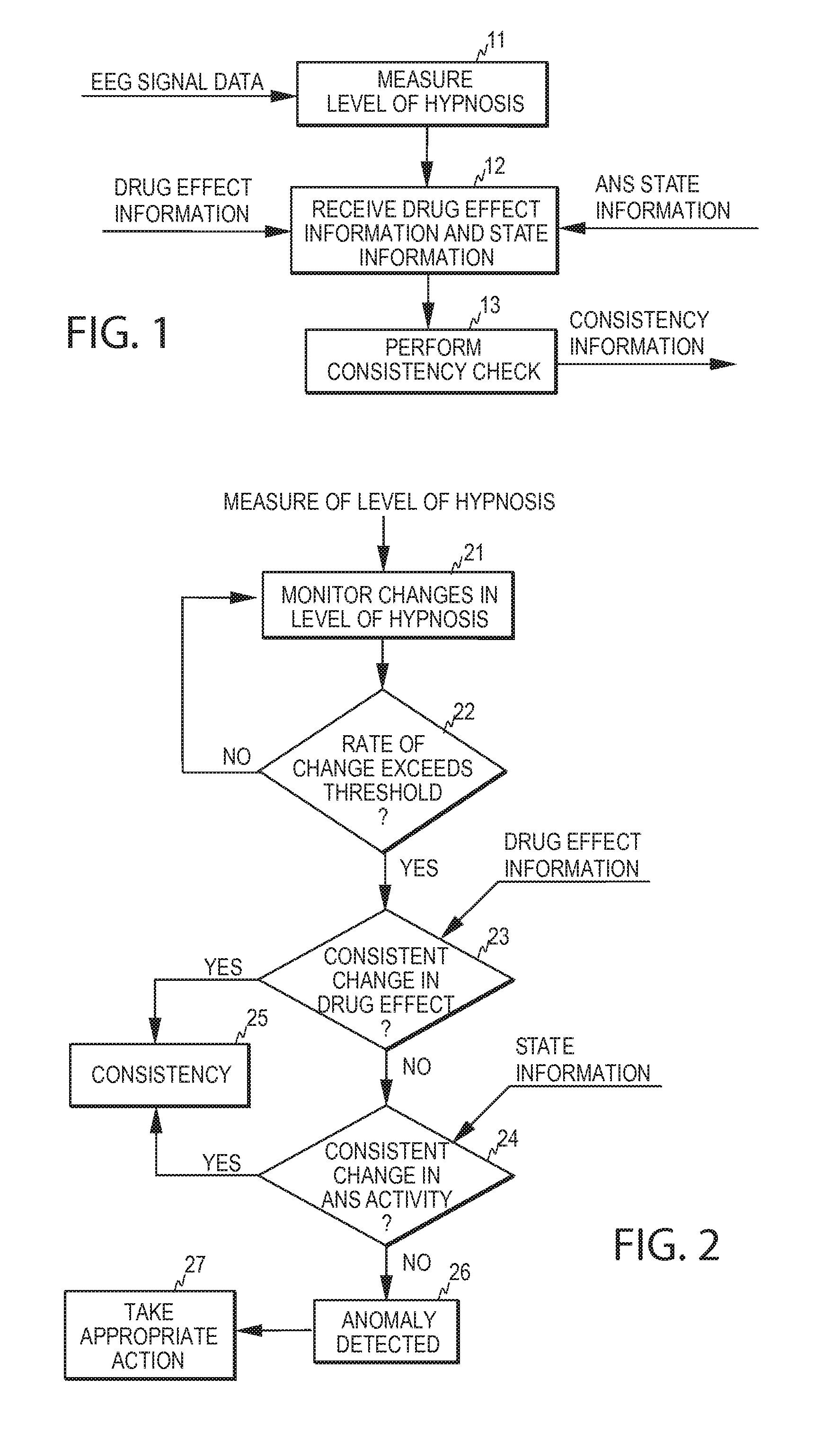
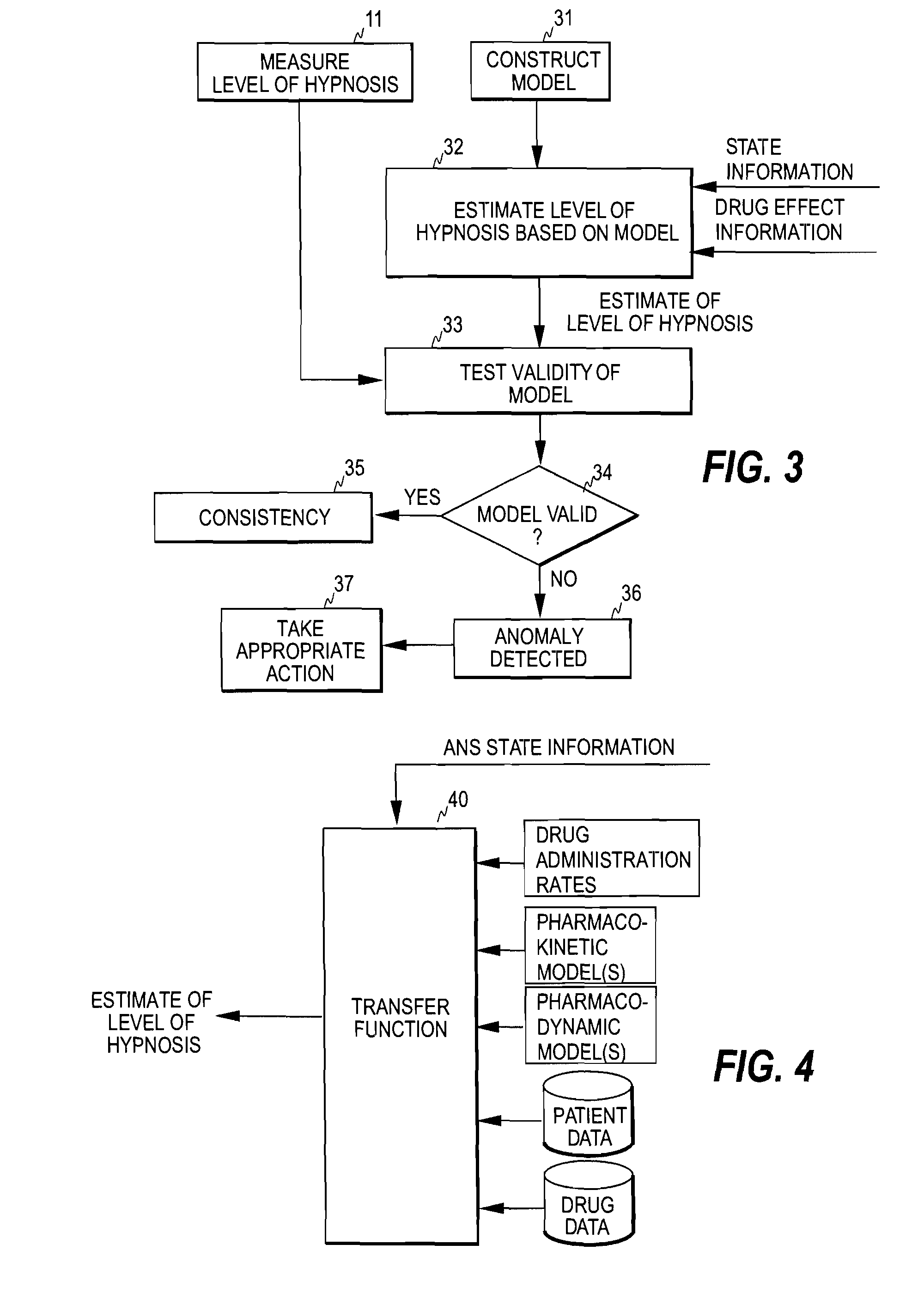
Detection of anomalies in measurement of level of hypnosis
The invention relates to detection of anomalies related to a measurement of the hypnotic level of a subject. In order to detect when a measure indicative of the hypnotic level of a subject is anomalous either due to a medical reason or due to an interference, the measure is monitored and state information indicative of the activity of the autonomous nervous system of the subject and drug effect information indicative of the hypnotic drug effect in the subject are employed to check whether the measure fulfills a predetermined consistency condition requiring that the measure changes consistently with at least one of the drug effect information and the state information. An anomaly, indicative of an abnormal change in the measure, is detected when the measure fails to fulfill the predetermined consistency condition.
Owner:GENERAL ELECTRIC CO
Kit for anxiolytic and hypnotic drug in serum by ultra-high performance liquid chromatography-tandem mass spectrometry technology
The invention relates to a kit for detecting an anxiolytic and hypnotic drug in serum by an ultra-high performance liquid chromatography-tandem mass spectrometry technology. 11 kinds of anxiolytic andhypnotic drugs can be detected at one time; target drugs and metabolites are monitored at the same time; the pretreatment process is simple, low in cost, high in sensitivity and high in specificity,separation and detection of the anxiolytic and hypnotic drugs are completed within 5 minutes, the accuracy and precision basically meet the requirements, the kit can be used for quantitative analysisof the anxiolytic and hypnotic drugs clinically, and a simple and rapid detection method is provided for monitoring the treatment concentration of the anxiolytic and hypnotic drugs in serum clinically.
Owner:NANJING PINSHENG MEDICAL TECH CO LTD +3
Compositions for delivering hypnotic agents across the oral mucosa and methods of use thereof
Owner:创塞伯特制药公司
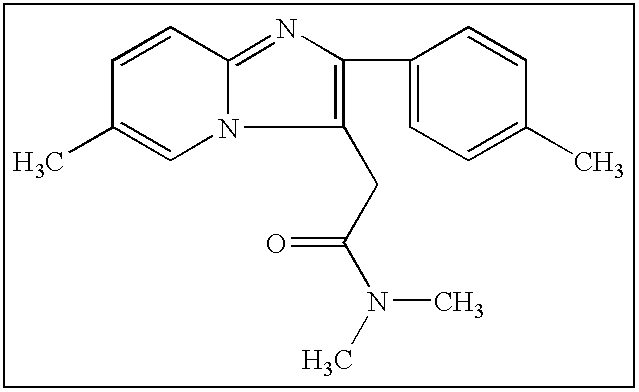
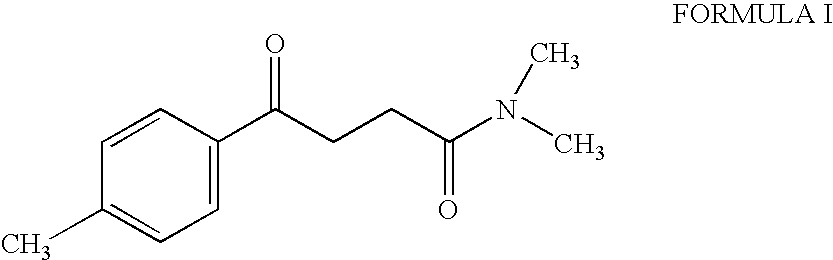
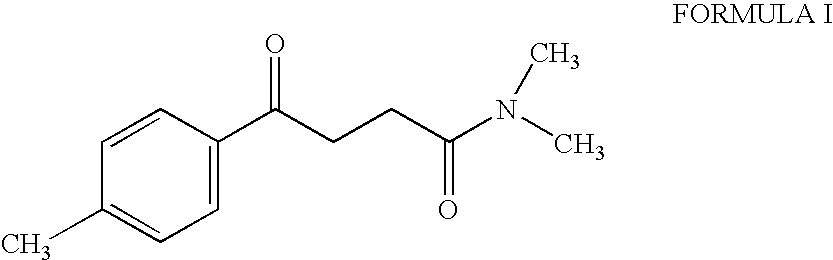
Synthesis of N,N-dimethyl-3-(4-methyl) benzoyl propionamide a key intermediate of zolpidem
InactiveUS6921839B2Organic compound preparationCarboxylic acid amides preparationPropanoic acidHypnotic agent
The present invention relates to an improved and industrially advantageous process for the preparation of N,N-dimethyl-3-(4-methyl)benzoyl propionamide of Formula I, which is a key intermediate in the synthesis of zolpidem hemitartrate, a non-benzodiazepine hypnotic agent. The process includes reacting 3-(4-methyl)-benzoyl propionic acid of Formula IV, with alkyl chloroformate or pivaloyl chloride to get a mixed anhydride of Formula V; and reacting the mixed anhydride of Formula V with dimethylamine of Formula VI to get the N,N-dimethyl-3-(4-methyl)benzoyl propionamide of Formula I.
Owner:RANBAXY LAB LTD
Polymorphs of N-methyl-N-(3-{3-[2-thienylcarbonyl]-pyrazol-[1,5-alpha]-pyrimidin-7-yl}phenyl)acetamide and compositions and methods related thereto
Polymorphs of N-methyl-N-(3-{3-[2-thienylcarbonyl]-pyrazol-[1,5-α]-pyrimidin-7-yl}phenyl)acetamide (Compound 1), and use of the same as a sedative-hypnotic, anxiolytic, anticonvulsant, and skeletal muscle relaxant agent. Processes for making the same, as well as related compositions and methods are also disclosed, particularly with regard to treatment of insomnia. A polymorph Form I possessing exception physical and heat stability is provided. A polymorph Form II:
Owner:NEUROCRINE BIOSCI INC
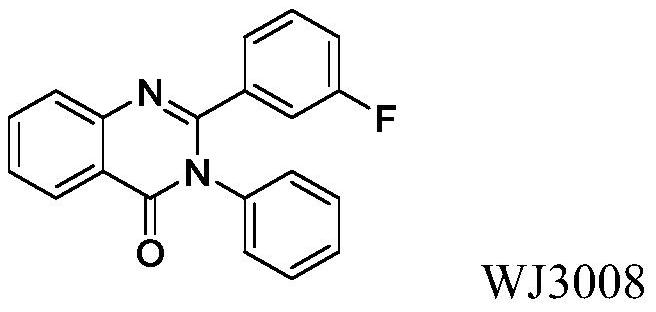
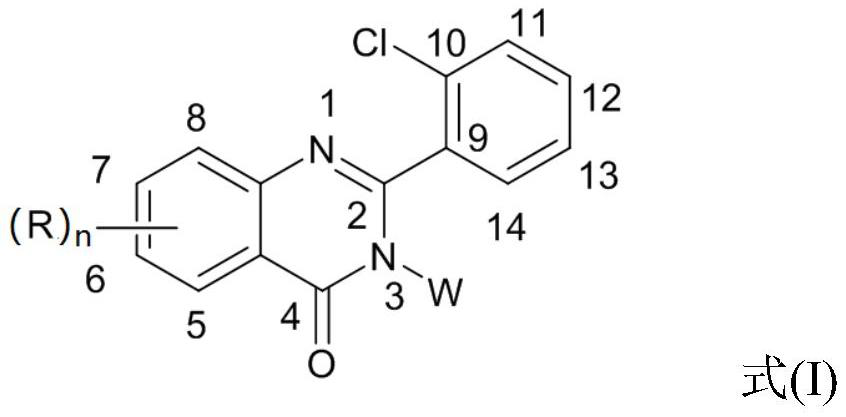
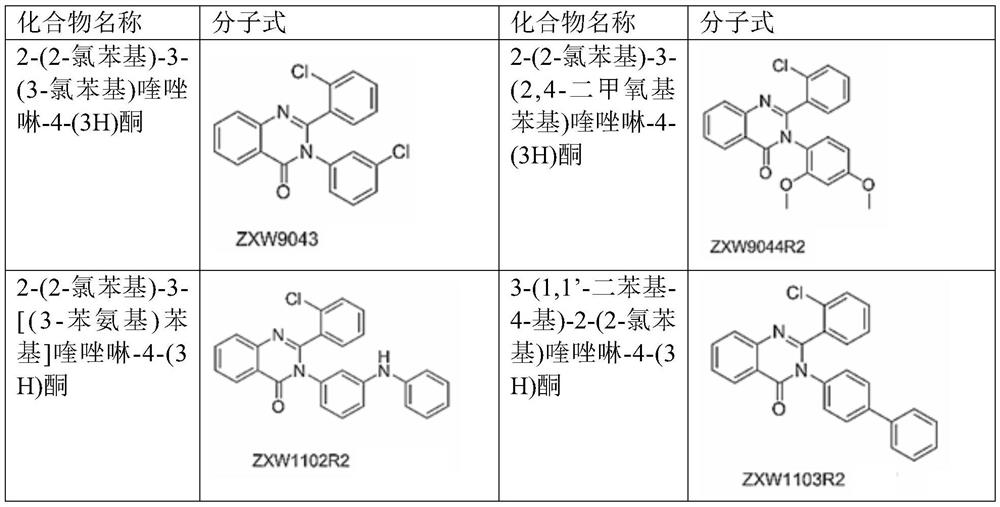
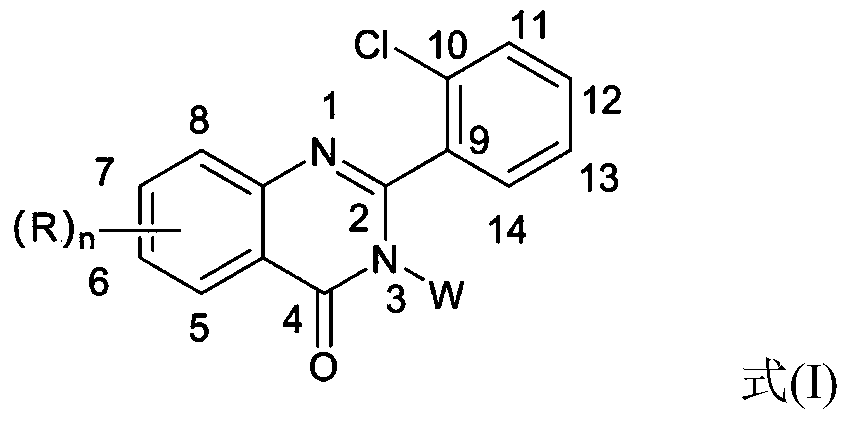
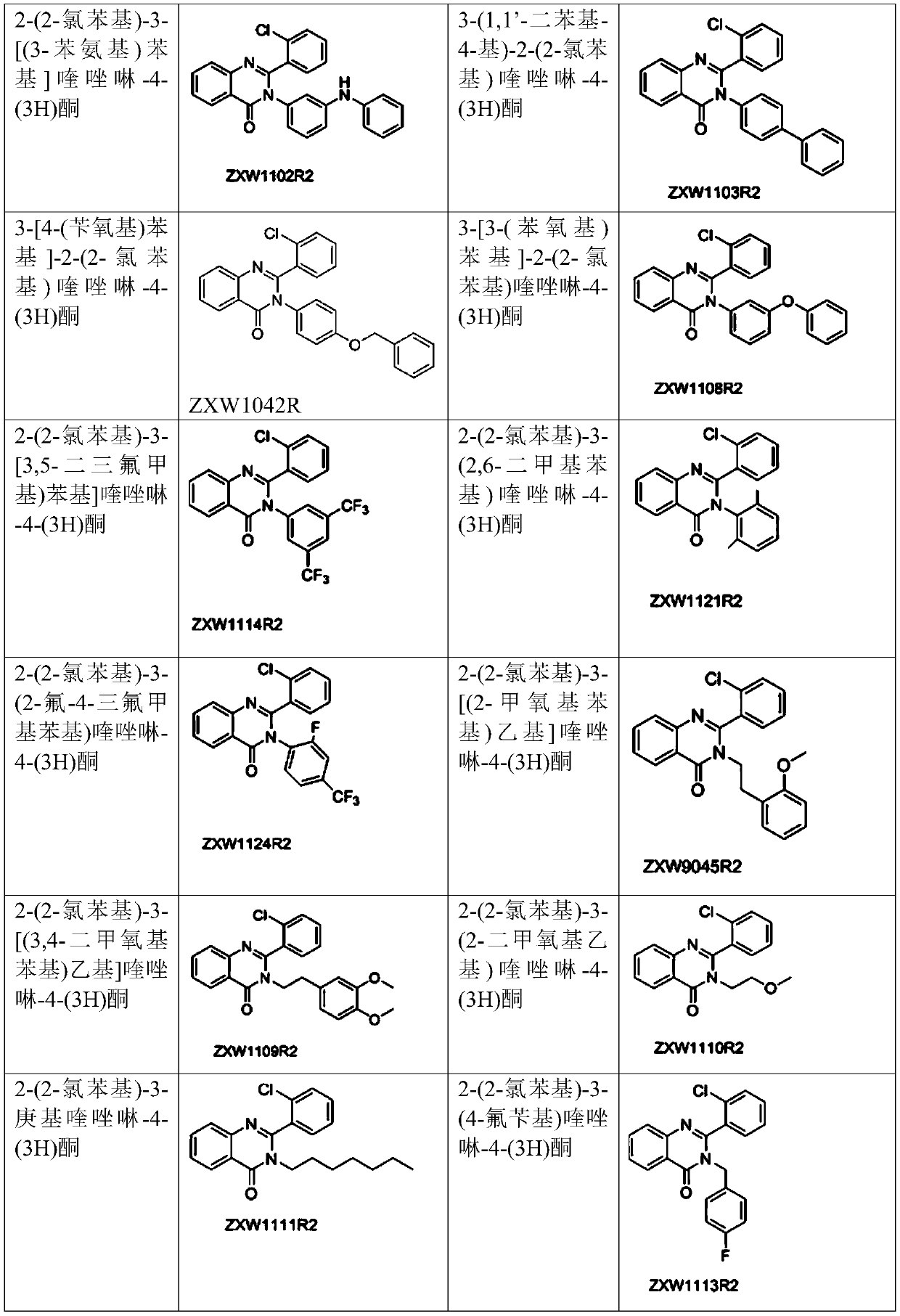
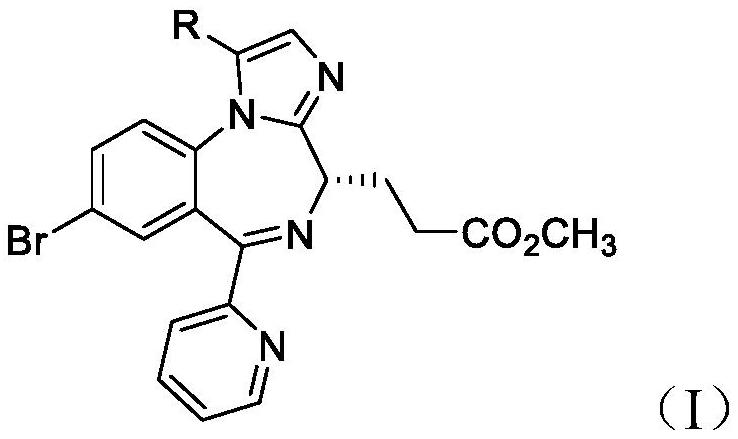
2-(2-chlorophenyl) quinazoline-4 (3H)-one derivative and preparation method and application thereof
ActiveCN110041272AStrong medicineFast metabolismAntibacterial agentsOrganic active ingredientsDiazepamHypnotic drug
The invention discloses a 2-(2-chlorophenyl) quinazoline-4 (3H)-one derivative shown in formula (I) or pharmaceutically acceptable salts thereof. Compared with the existing first-line sedative and hypnotic drugs diazepam and midazolam, the compound has stronger drug effect and faster metabolism speed, can reduce the next-day residual effect, and is expected to be developed into a novel high-efficiency low-toxicity sedative and hypnotic drug.
Owner:ACADEMY OF MILITARY MEDICAL SCI
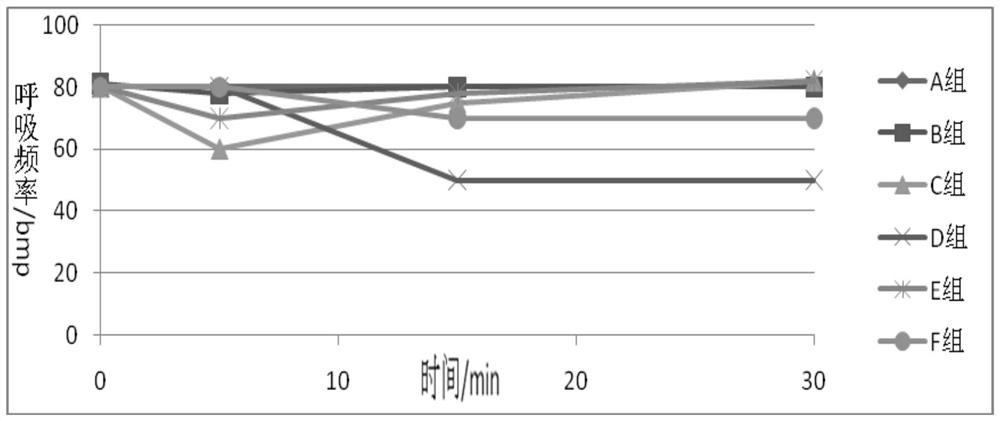
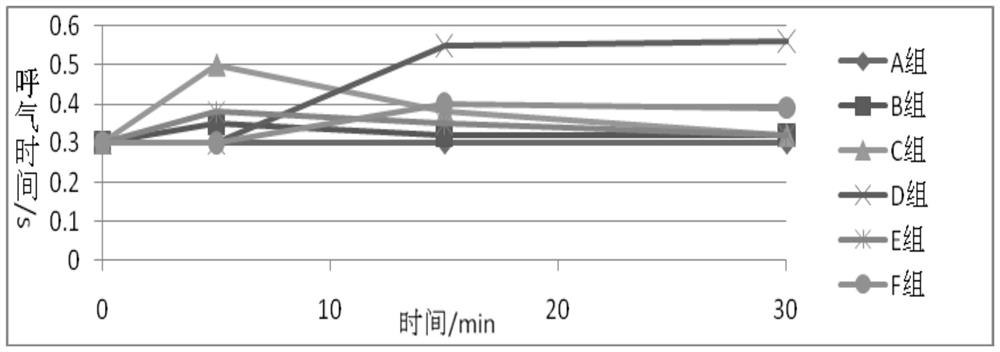
A kind of narcotic and analgesic pharmaceutical composition and preparation method thereof
ActiveCN108143733BFast onsetLong-lasting analgesic and sedative effectsPowder deliveryNervous disorderCompounding drugsHypnotic agent
Owner:YICHANG HUMANWELL PHARMA
Polymorphs of N-methyl-N-(3-3-{2-thienylcarbonyl]-pyrazol -[1,5-alpha]-pyrimidin-7-yl}phenyl)a cetamide and compositions and methods related thereton
Owner:NEUROCRINE BIOSCI INC
Oral Controlled Release Formulation for Sedatives and Hypnotic Agents
InactiveUS20090123535A1Simple manufacturing processBiocideNervous disorderHypnotic agentTreatment level
The present invention relates to a novel controlled release dosage form that releases therapeutic amounts of a sedative or hypnotic agent rapidly after administration and maintains therapeutic levels for about eight hours after administration.
Owner:CHENG XIU XIU +1
Dosage form
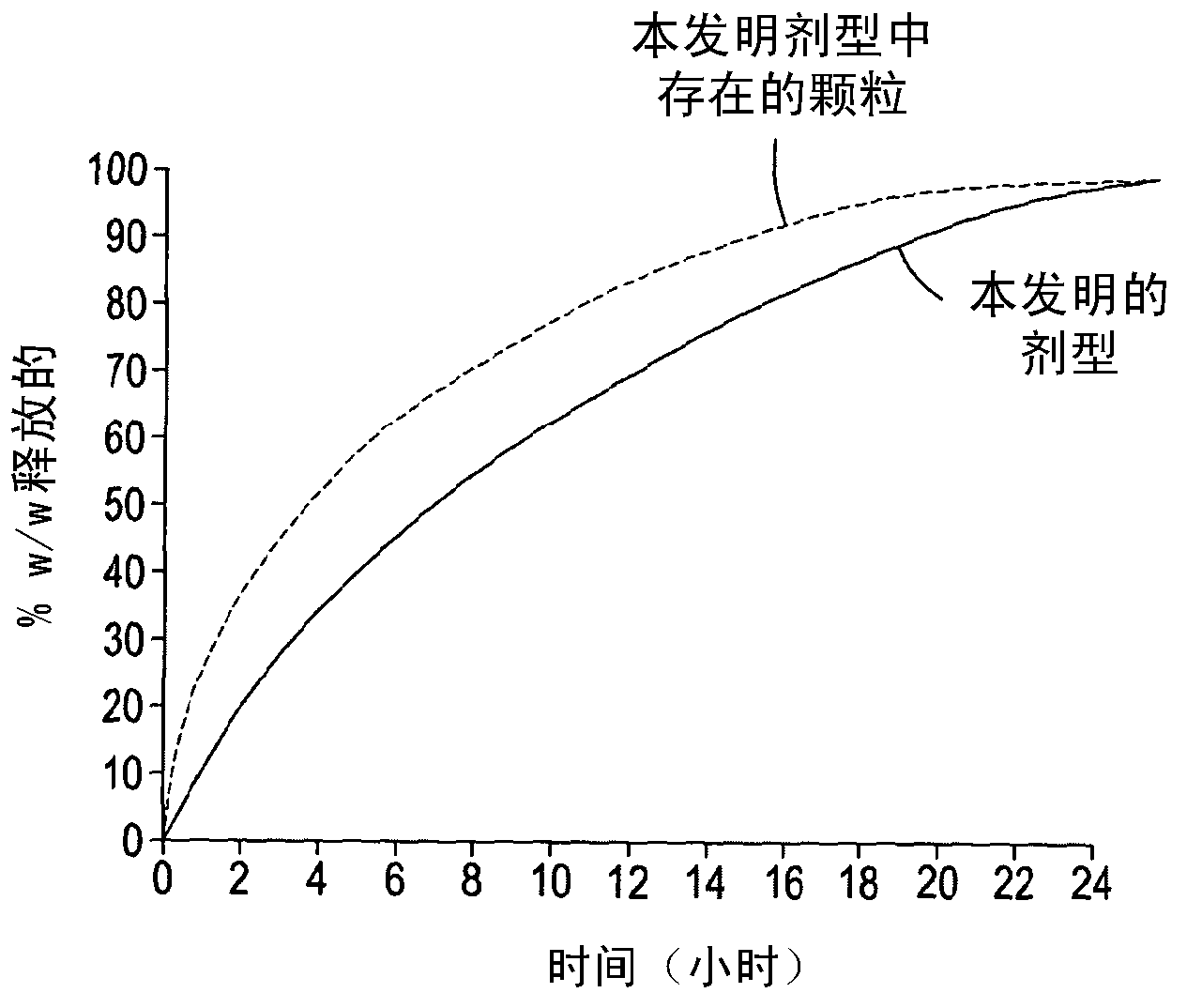
InactiveCN103282026AReduce lossesReduce degradationOrganic active ingredientsNervous disorderParticulatesSedative/hypnotic
The present invention provides a dosage form, particularly a tamper resistant dosage form, comprising; non-stretched melt extruded particulates comprising a drug selected from an opioid agonist, a tranquilizer, a CNS depressant, a CNS stimulant or a sedative hypnotic; and a matrix; wherein said melt extruded particulates are present as a discontinuous phase in said matrix.
Owner:EURO-CELTIQUE SA
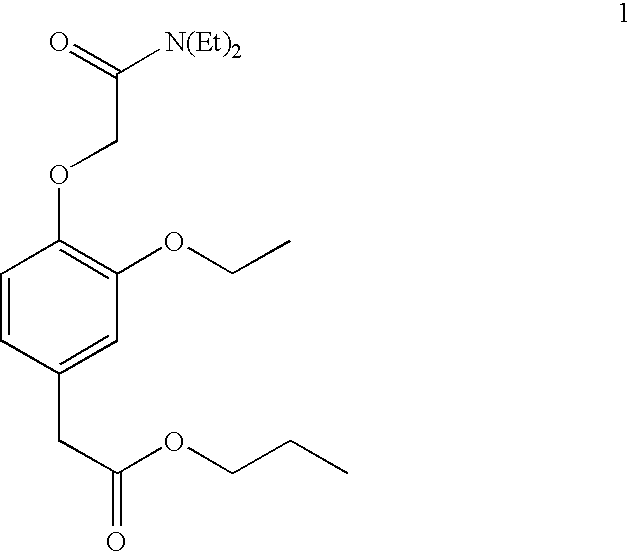
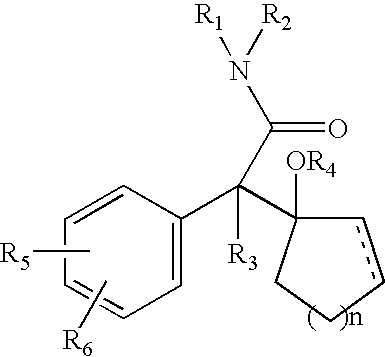
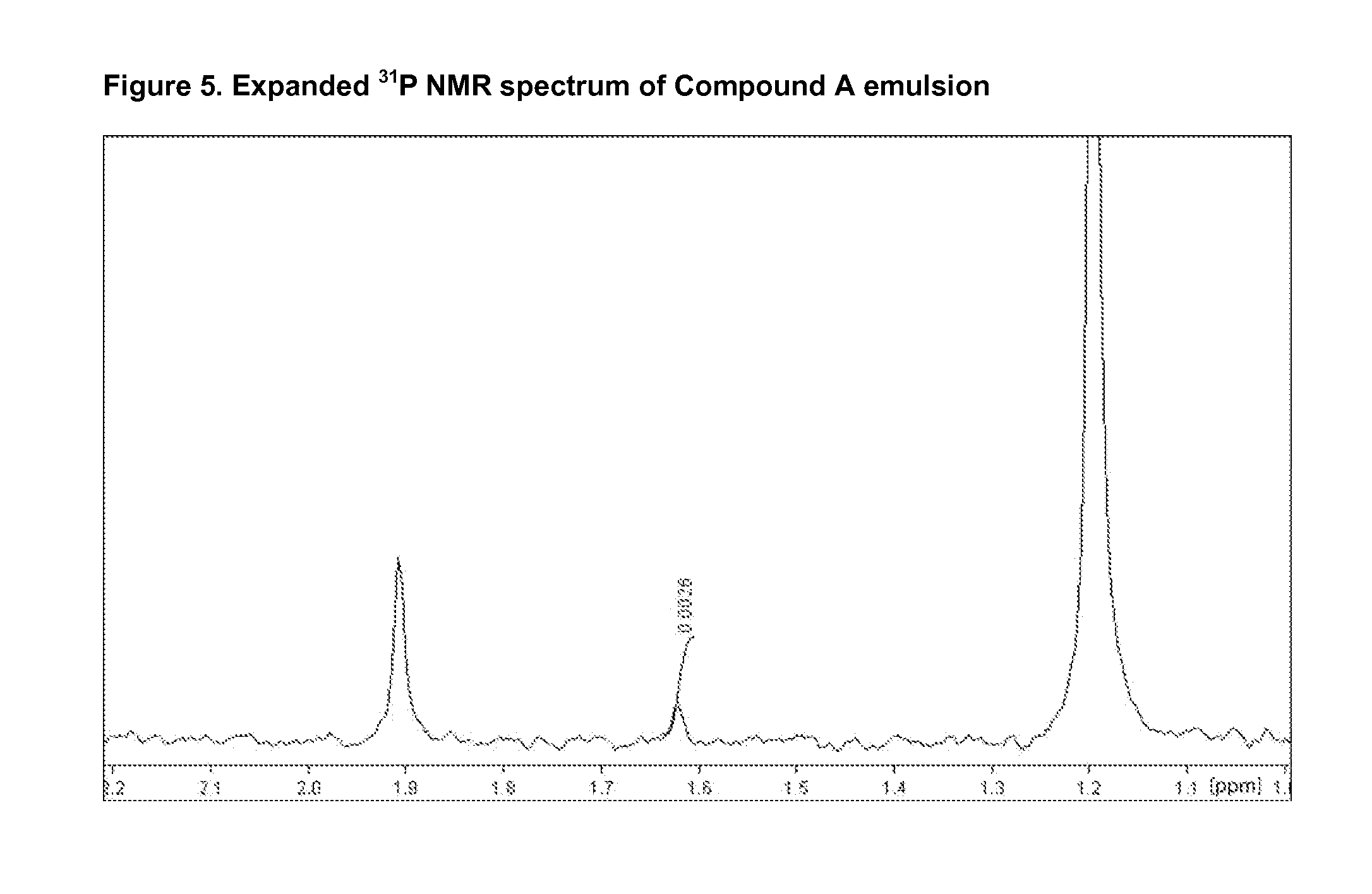
Combination of a hypnotic agent and r (+)-alpha-(2,3-dimethoxy-phenyl)-1-[2-(4-fluorophenyl)ethyl]-4-piperidinemethanol and therapeutic application thereof
The invention concerns the combination of a short-acting hypnotic agent and R-(+)-α-(2,3-dimethoxyphenyl)-1-[2-(4-fluorophenyl)ethyl]-4-piperidinemethanol (Compound A) or its prodrug having the Formula II:wherein R is C1-C20 alkyl or a pharmaceutically acceptable salt thereof. The combination of this invention is useful in treating a variety of sleep disorders.
Owner:AVENTIS PHARMA INC
2-(2-Chlorophenyl)quinazolin-4(3h)-one derivatives and their preparation methods and uses
ActiveCN110041272BStrong medicineFast metabolismAntibacterial agentsOrganic active ingredientsChlorobenzeneSedative/hypnotic
The invention discloses a 2-(2-chlorophenyl) quinazoline-4 (3H)-one derivative shown in formula (I) or pharmaceutically acceptable salts thereof. Compared with the existing first-line sedative and hypnotic drugs diazepam and midazolam, the compound has stronger drug effect and faster metabolism speed, can reduce the next-day residual effect, and is expected to be developed into a novel high-efficiency low-toxicity sedative and hypnotic drug.
Owner:ACADEMY OF MILITARY MEDICAL SCI
Pharmaceutical Compositions Of Short-Acting Sedative Hypnotic Agent
The invention provides pharmaceutical compositions comprising a phenylacetic acid ester compound useful for inducing or maintaining general anesthesia or sedation in mammals, methods for preparing such compositions, and methods for inducing or maintaining anesthesia or sedation using such compositions.
Owner:THERAVANCE BIOPHARMA R&D IP LLC
Oral controlled release formulation for sedative and hypnotic agents
InactiveUS8309104B2Simple manufacturing processNervous disorderCoatingsHypnotic agentTranquilizing Agents
The present invention relates to a novel controlled release dosage form that releases therapeutic amounts of a sedative or hypnotic agent rapidly after administration and maintains therapeutic levels for about eight hours after administration.
Owner:ACTAVIS HOLDCO US INC
Helicid modifier and its preparation method and uses
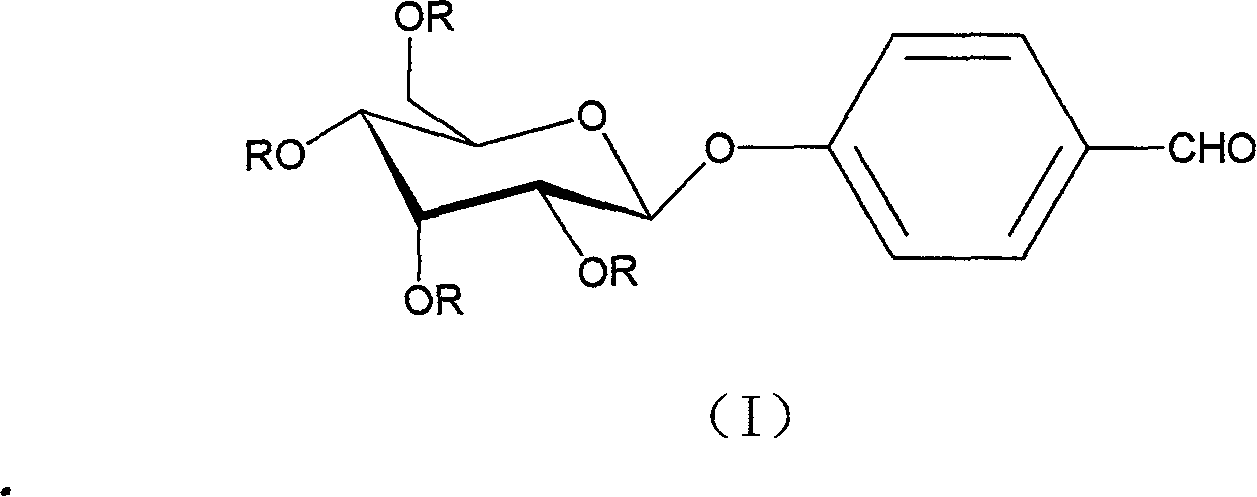
The invention discloses a new decorative of bean curd with structure formula (I), which is characterized by the following: adopting the decorative as active component of drug component; fitting for preparing sedative-hypnotic and antiphlogistic anodyne.
Owner:KUNMING INST OF BOTANY - CHINESE ACAD OF SCI +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com


![Polymorph of N-methyl-N-(3-{3-[2-thienylcarbonyl]-pyrazol-[1,5-alpha]-pyrimidin-7-yl}phenyl)acetamide and compositions and methods related thereto Polymorph of N-methyl-N-(3-{3-[2-thienylcarbonyl]-pyrazol-[1,5-alpha]-pyrimidin-7-yl}phenyl)acetamide and compositions and methods related thereto](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/e89c9398-ca99-4dab-9d3b-309edeb3602f/US06903106-20050607-D00001.png)
![Polymorph of N-methyl-N-(3-{3-[2-thienylcarbonyl]-pyrazol-[1,5-alpha]-pyrimidin-7-yl}phenyl)acetamide and compositions and methods related thereto Polymorph of N-methyl-N-(3-{3-[2-thienylcarbonyl]-pyrazol-[1,5-alpha]-pyrimidin-7-yl}phenyl)acetamide and compositions and methods related thereto](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/e89c9398-ca99-4dab-9d3b-309edeb3602f/US06903106-20050607-D00002.png)
![Polymorph of N-methyl-N-(3-{3-[2-thienylcarbonyl]-pyrazol-[1,5-alpha]-pyrimidin-7-yl}phenyl)acetamide and compositions and methods related thereto Polymorph of N-methyl-N-(3-{3-[2-thienylcarbonyl]-pyrazol-[1,5-alpha]-pyrimidin-7-yl}phenyl)acetamide and compositions and methods related thereto](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/e89c9398-ca99-4dab-9d3b-309edeb3602f/US06903106-20050607-D00003.png)
![Polymorphs of N-methyl-N-(3-{3-[2-thienylcarbonyl]-pyrazol-[1,5-alpha]-pyrimidin-7-yl}phenyl)acetamide and compositions and methods related thereto Polymorphs of N-methyl-N-(3-{3-[2-thienylcarbonyl]-pyrazol-[1,5-alpha]-pyrimidin-7-yl}phenyl)acetamide and compositions and methods related thereto](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/fe5cdd4d-2af8-4f4e-8238-58deb8d51f2f/A0081315800441.PNG)
![Polymorphs of N-methyl-N-(3-{3-[2-thienylcarbonyl]-pyrazol-[1,5-alpha]-pyrimidin-7-yl}phenyl)acetamide and compositions and methods related thereto Polymorphs of N-methyl-N-(3-{3-[2-thienylcarbonyl]-pyrazol-[1,5-alpha]-pyrimidin-7-yl}phenyl)acetamide and compositions and methods related thereto](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/fe5cdd4d-2af8-4f4e-8238-58deb8d51f2f/A0081315800442.PNG)
![Polymorphs of N-methyl-N-(3-{3-[2-thienylcarbonyl]-pyrazol-[1,5-alpha]-pyrimidin-7-yl}phenyl)acetamide and compositions and methods related thereto Polymorphs of N-methyl-N-(3-{3-[2-thienylcarbonyl]-pyrazol-[1,5-alpha]-pyrimidin-7-yl}phenyl)acetamide and compositions and methods related thereto](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/fe5cdd4d-2af8-4f4e-8238-58deb8d51f2f/A0081315800451.PNG)


![Polymorphs of N-methyl-N-(3-{3-[2-thienylcarbonyl]-pyrazol-[1,5-alpha]-pyrimidin-7-yl}phenyl)acetamide and compositions and methods related thereto Polymorphs of N-methyl-N-(3-{3-[2-thienylcarbonyl]-pyrazol-[1,5-alpha]-pyrimidin-7-yl}phenyl)acetamide and compositions and methods related thereto](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/204fe2ef-ff7f-4e7c-b30e-2121b4f11353/US06958342-20051025-D00001.png)
![Polymorphs of N-methyl-N-(3-{3-[2-thienylcarbonyl]-pyrazol-[1,5-alpha]-pyrimidin-7-yl}phenyl)acetamide and compositions and methods related thereto Polymorphs of N-methyl-N-(3-{3-[2-thienylcarbonyl]-pyrazol-[1,5-alpha]-pyrimidin-7-yl}phenyl)acetamide and compositions and methods related thereto](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/204fe2ef-ff7f-4e7c-b30e-2121b4f11353/US06958342-20051025-D00002.png)
![Polymorphs of N-methyl-N-(3-{3-[2-thienylcarbonyl]-pyrazol-[1,5-alpha]-pyrimidin-7-yl}phenyl)acetamide and compositions and methods related thereto Polymorphs of N-methyl-N-(3-{3-[2-thienylcarbonyl]-pyrazol-[1,5-alpha]-pyrimidin-7-yl}phenyl)acetamide and compositions and methods related thereto](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/204fe2ef-ff7f-4e7c-b30e-2121b4f11353/US06958342-20051025-D00003.png)


![Polymorphs of N-methyl-N-(3-3-{2-thienylcarbonyl]-pyrazol -[1,5-alpha]-pyrimidin-7-yl}phenyl)a cetamide and compositions and methods related thereton Polymorphs of N-methyl-N-(3-3-{2-thienylcarbonyl]-pyrazol -[1,5-alpha]-pyrimidin-7-yl}phenyl)a cetamide and compositions and methods related thereton](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/02b93387-406b-4643-a44b-2e42094775ae/C0081315800441.PNG)
![Polymorphs of N-methyl-N-(3-3-{2-thienylcarbonyl]-pyrazol -[1,5-alpha]-pyrimidin-7-yl}phenyl)a cetamide and compositions and methods related thereton Polymorphs of N-methyl-N-(3-3-{2-thienylcarbonyl]-pyrazol -[1,5-alpha]-pyrimidin-7-yl}phenyl)a cetamide and compositions and methods related thereton](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/02b93387-406b-4643-a44b-2e42094775ae/C0081315800442.PNG)
![Polymorphs of N-methyl-N-(3-3-{2-thienylcarbonyl]-pyrazol -[1,5-alpha]-pyrimidin-7-yl}phenyl)a cetamide and compositions and methods related thereton Polymorphs of N-methyl-N-(3-3-{2-thienylcarbonyl]-pyrazol -[1,5-alpha]-pyrimidin-7-yl}phenyl)a cetamide and compositions and methods related thereton](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/02b93387-406b-4643-a44b-2e42094775ae/C0081315800451.PNG)
![Combination of a hypnotic agent and r (+)-alpha-(2,3-dimethoxy-phenyl)-1-[2-(4-fluorophenyl)ethyl]-4-piperidinemethanol and therapeutic application thereof Combination of a hypnotic agent and r (+)-alpha-(2,3-dimethoxy-phenyl)-1-[2-(4-fluorophenyl)ethyl]-4-piperidinemethanol and therapeutic application thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/4bd3de28-4549-44bd-987d-ae96a3d5b362/US20080139615A1-20080612-C00001.png)
![Combination of a hypnotic agent and r (+)-alpha-(2,3-dimethoxy-phenyl)-1-[2-(4-fluorophenyl)ethyl]-4-piperidinemethanol and therapeutic application thereof Combination of a hypnotic agent and r (+)-alpha-(2,3-dimethoxy-phenyl)-1-[2-(4-fluorophenyl)ethyl]-4-piperidinemethanol and therapeutic application thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/4bd3de28-4549-44bd-987d-ae96a3d5b362/US20080139615A1-20080612-C00002.png)
![Combination of a hypnotic agent and r (+)-alpha-(2,3-dimethoxy-phenyl)-1-[2-(4-fluorophenyl)ethyl]-4-piperidinemethanol and therapeutic application thereof Combination of a hypnotic agent and r (+)-alpha-(2,3-dimethoxy-phenyl)-1-[2-(4-fluorophenyl)ethyl]-4-piperidinemethanol and therapeutic application thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/4bd3de28-4549-44bd-987d-ae96a3d5b362/US20080139615A1-20080612-C00003.png)