Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
5430 results about "Elastic component" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Tendons provide what is called a series-elastic component because they are somewhat elastic and in line (in series) with the force of muscle contraction. The series-elastic component absorbs some of the tension as a muscle contracts, and it must be pulled tight before muscle contraction can result in muscle shortening.
Stretchable outer cover for an absorbent article and process for making the same
A stretchable outer cover for use with an absorbent article including an elastomeric film. The elastomeric film includes at least one skin layer that is less tacky than at least one core layer. The outer cover can include a nonwoven layer different structural combinations of spunbond fibers, meltblown fibers, and / or nanofibers. The combination of plastic and elastic components results in an outer cover that has favorable mechanical, physical, and aesthetic properties. The outer cover can be rendered either uniaxially or biaxially stretchable via a mechanical activation process.
Owner:THE PROCTER & GAMBLE COMPANY
Biaxially stretchable outer cover for an absorbent article
ActiveUS20070287348A1Improve versatilityImprove fitPersonal careSynthetic resin layered productsFiberElastic component
An outer cover for use with an absorbent article having a layer of nonwoven fibrous material and optionally including a polymeric layer laminated or printed onto the layer of nonwoven fibrous material. The outer cover includes at least one plastic component and at least one elastic component in the nonwoven fibrous material and / or optional polymeric layer. The outer cover can have different structural combinations of spunbond fibers, meltblown fibers, and / or nanofibers. The combination of plastic and elastic components results in an outer cover that has favorable mechanical, physical, and aesthetic properties. The outer cover can be rendered either uniaxially or biaxially stretchable via a mechanical activation process.
Owner:THE PROCTER & GAMBLE COMPANY
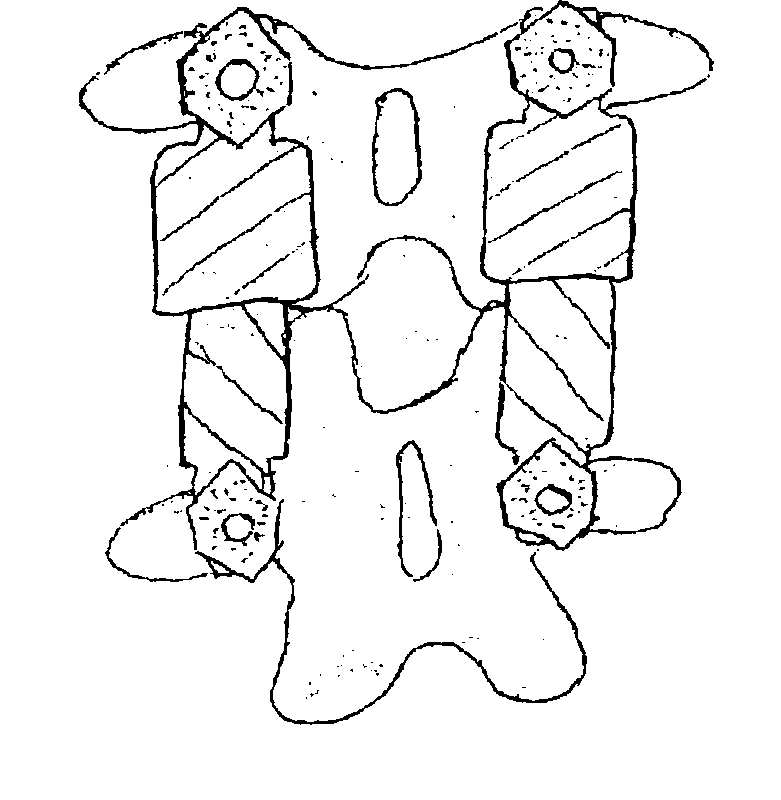
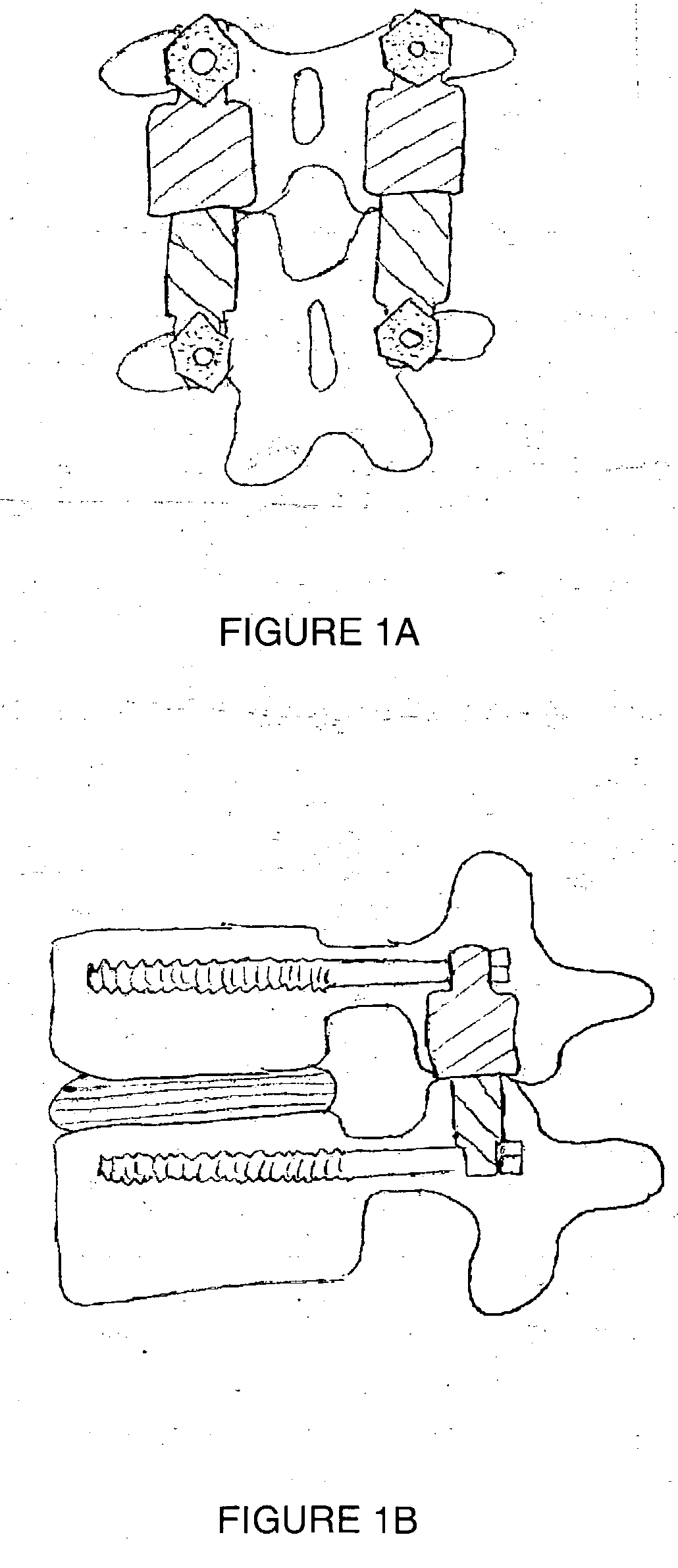
Vertebral shock absorbers
InactiveUS20050261682A1Extended range of motionFacilitate lateral applicationInternal osteosythesisJoint implantsElastic componentLamina terminalis
A vertebral shock absorber in the form of an elongated compressible member having two ends, one fastened to an upper vertebra, and the other fastened to a lower vertebra. The elongated member may be fastened using pedicle screws or by way of ball-and-socket joints for enhanced range of motion. In the preferred embodiment, the elongated compressible member is constructed using telescoping sleeves to create a cavity wherein there is disposed a compressible, resilient component such as a spring, elastomeric material, liquid, gel, hydrogel, or other suitable substance. The shock absorber may be combined with an intradiscal component and / or one or more plates that extends at least partially onto a vertebral endplate to facilitate lateral application.
Owner:FERREE BRET A
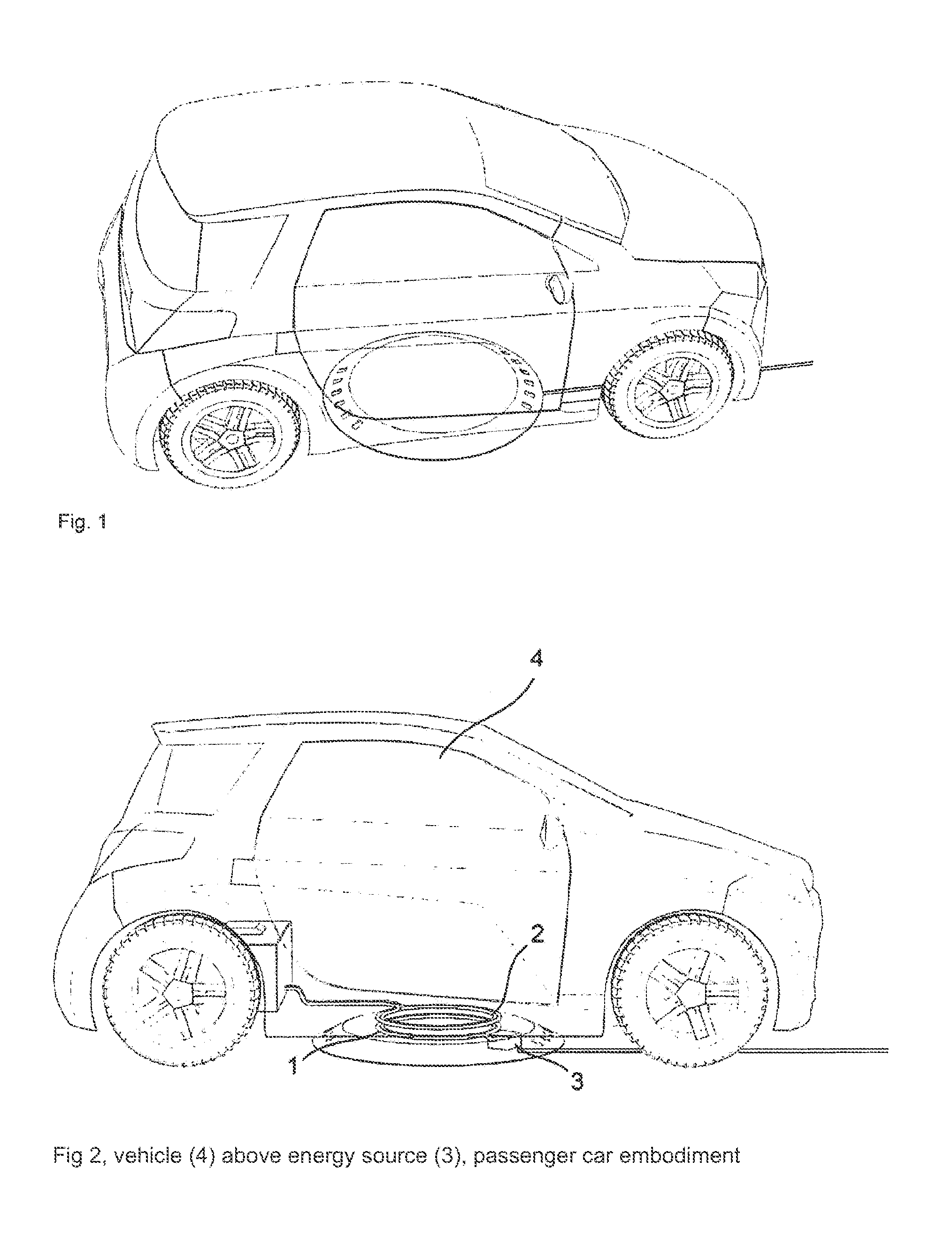
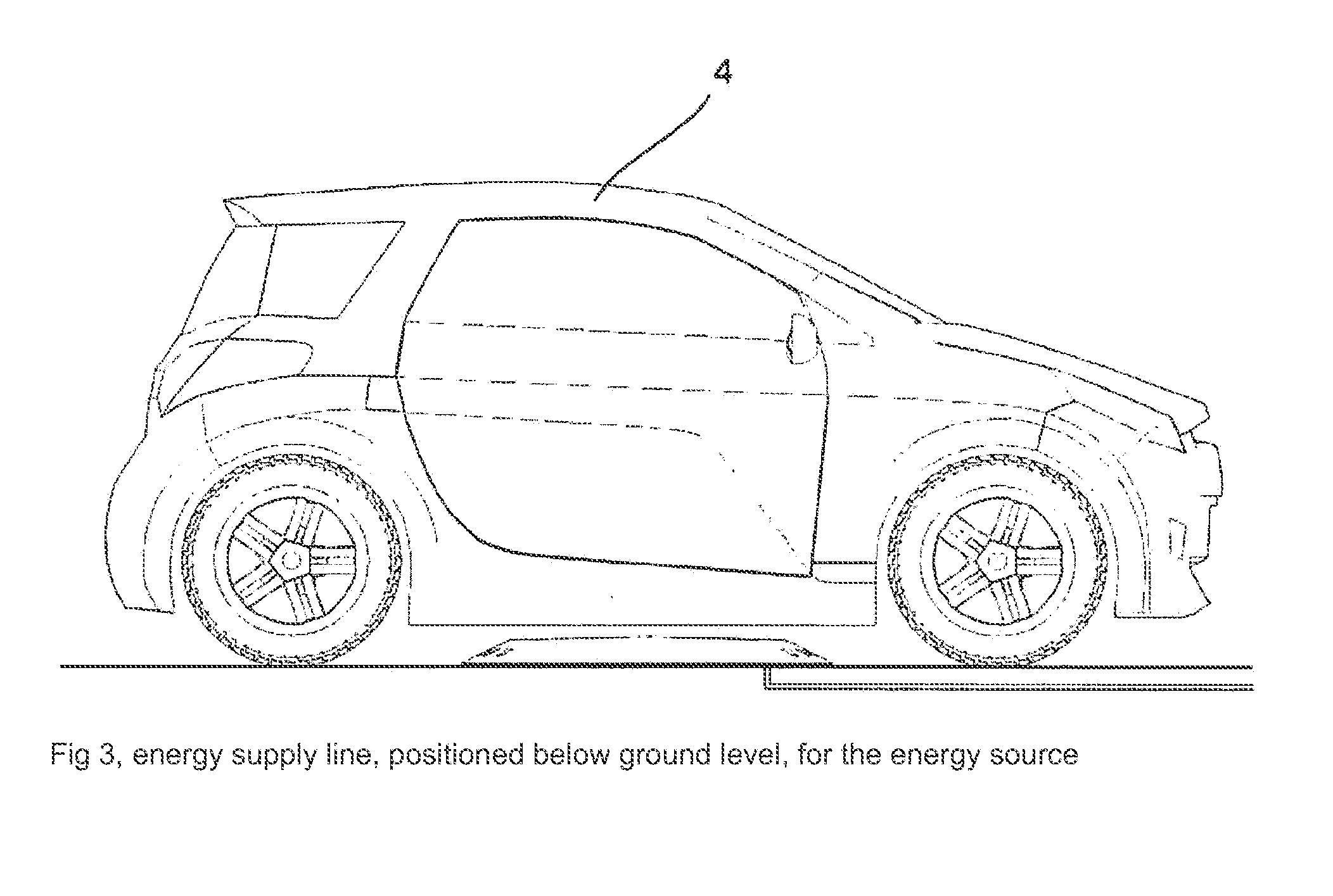
System for inductively charging vehicles, comprising an electronic positioning aid
InactiveUS20120203410A1High standardization effortMaximum efficiencyDigital data processing detailsVehicular energy storageElastic componentDriver/operator
The main claim involves a system that ensures a self-guiding, electronic positioning of a secondary coil in a vehicle, without the aid of indicators or kinematic or mechanical aids, in relation to a primary coil that is fixed in a structure, in order to guarantee a transfer of energy with over 90% efficiency without the disadvantages of moving, frictional and elastic components in terms of energy consumption, functional safety and wear. To achieve this aim, the coil housing in the structure fulfills the role of an electronics housing, reflective element and cooling element thanks to the choice of material used, the surface and the inner supports and can thus be retrofitted, as a single installation on the structure in the form of an operation-ready complete package, to any flat base with an electric connection. The vehicle can be used both for transporting passengers and loads and can be steered by a vehicle driver or can be operated without a driver, for example for cleaning areas, for the protection of the countryside or for intralogistics
Owner:CONDUCTIX WAMPFLER
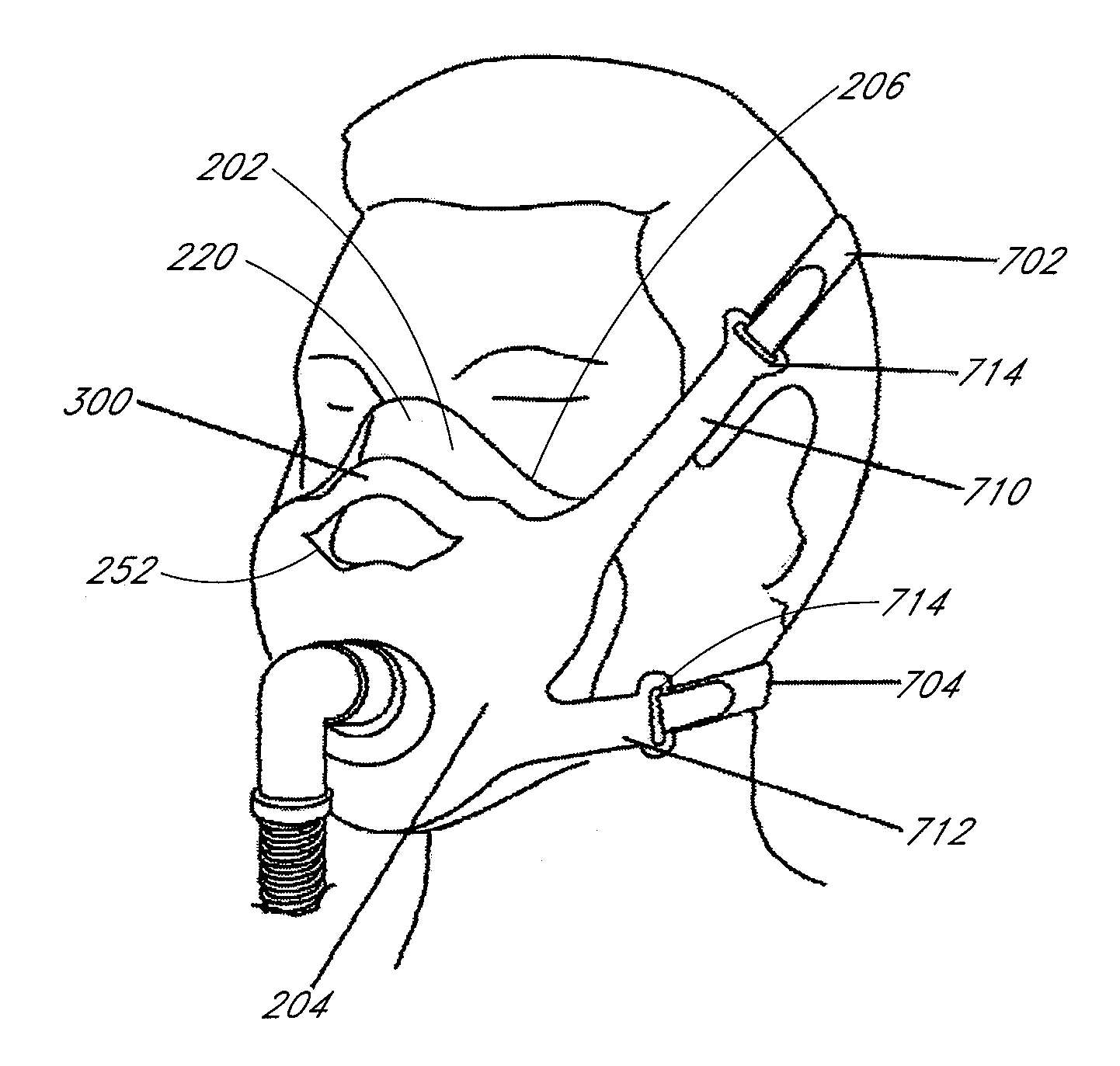
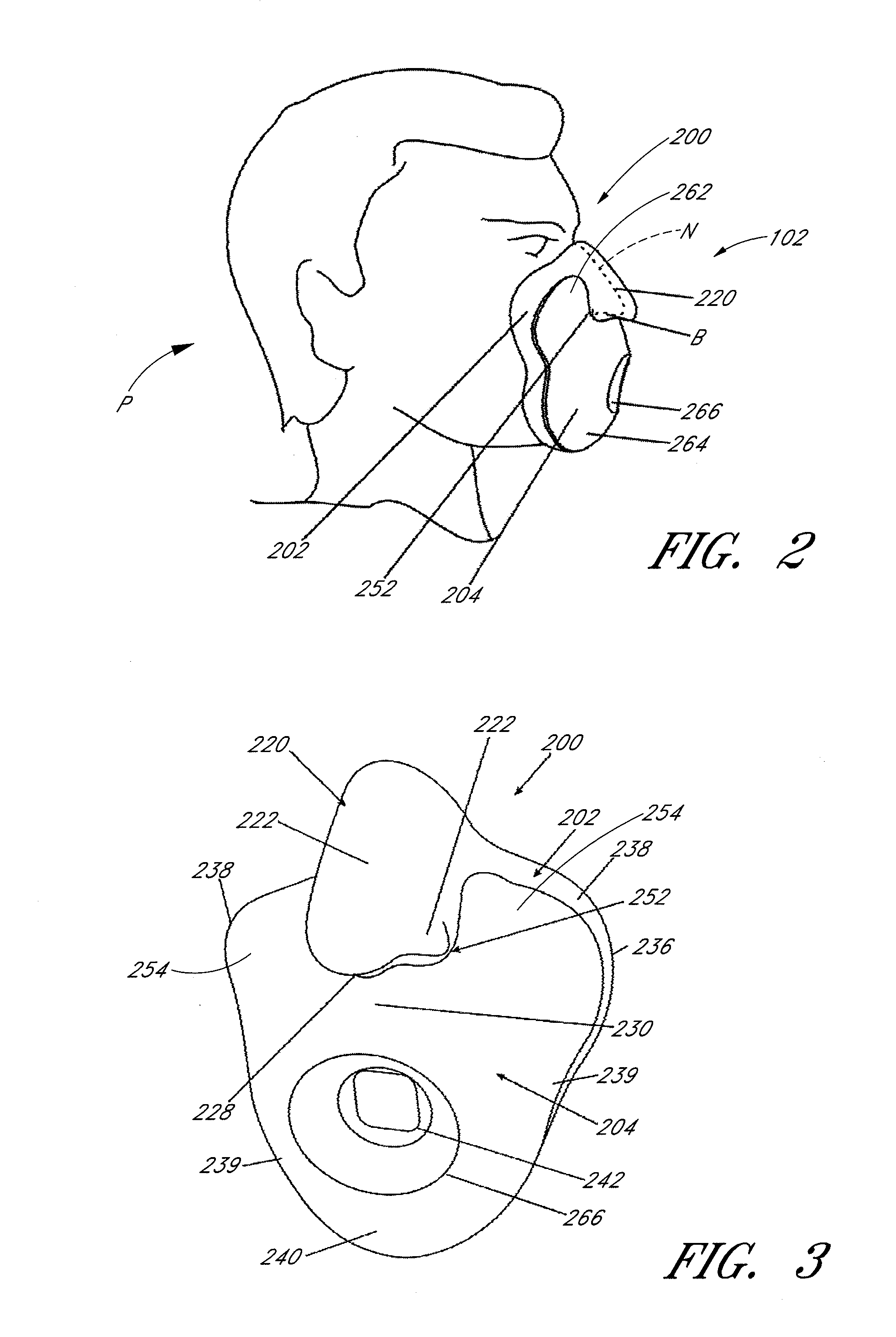
Patient interface and headgear
ActiveUS20130074845A1Improve complianceComfort preferably should be maximizedBreathing masksRespiratory masksElastic componentPatient comfort
Patient interface components and / or associated head gear and adjustment systems improve sealing and / or patient comfort and / or ease of use. The interface comprising an inflating or ballooning seal. The headgear assembly can be connected to the interface with an elastic component and an inelastic component. The elastic component enabling a course fitting of the interface to the patient and the inelastic component enabling a final fitting of the interface to the patient.
Owner:FISHER & PAYKEL HEALTHCARE LTD
Disposable diaper
Here is disclosed a disposable diaper, which includes a liquid-absorbent zone in a crotch region having first through fourth sections. A stiffness of the first and second sections formed from top- and backsheets is lower than a stiffness of the third and fourth sections formed from the top- and backsheets and an absorbent core. Stretchably elastic members extending in a longitudinal direction are contractibly attached to the fourth section. A liquid-absorbent zone in the crotch region is formed with a concavity (feces pocket) as the fourth section contract in the longitudinal direction under a contractile force of the elastic members.
Owner:UNI CHARM CORP
Item of clothing, especially disposable clothing for use once only
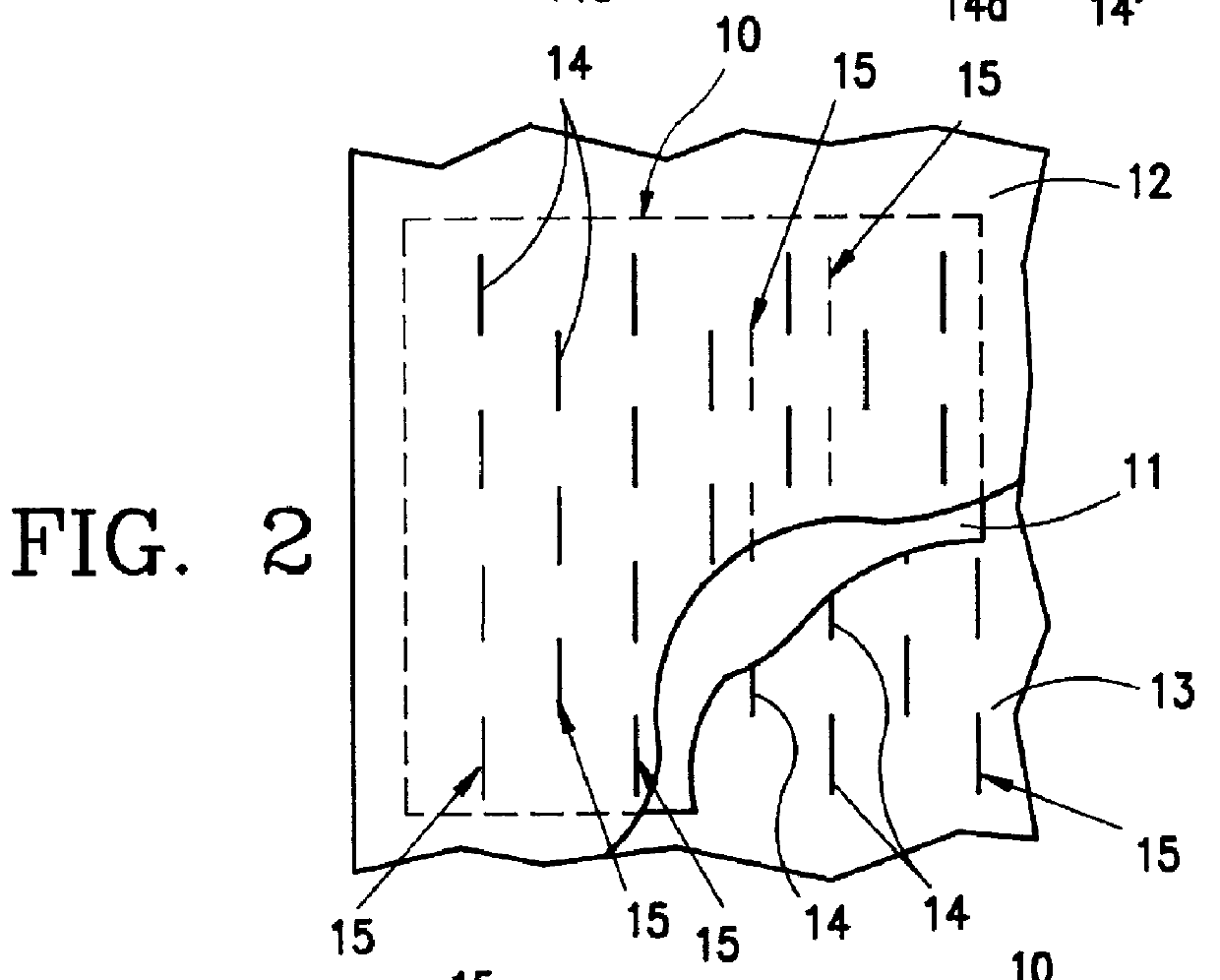
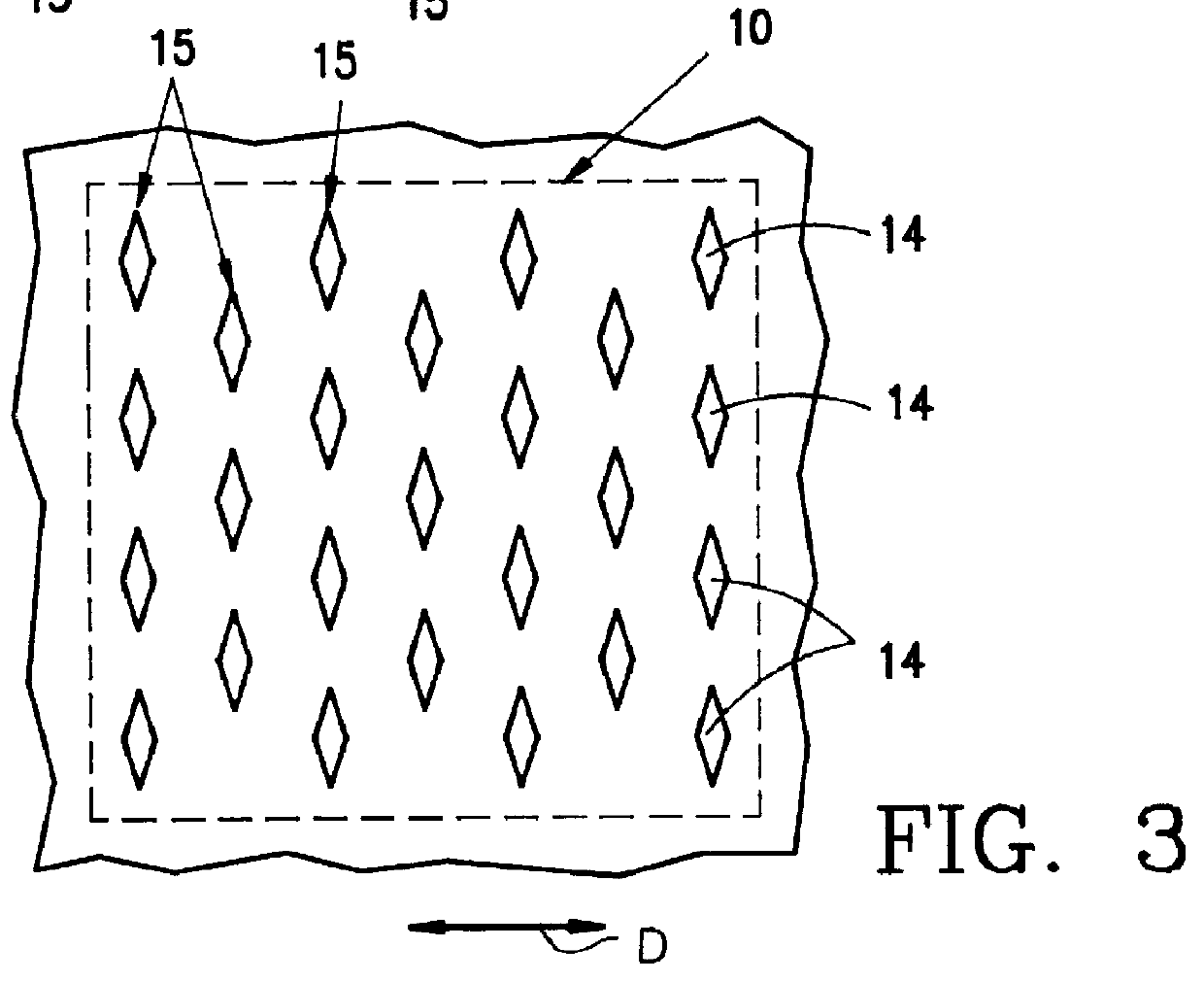
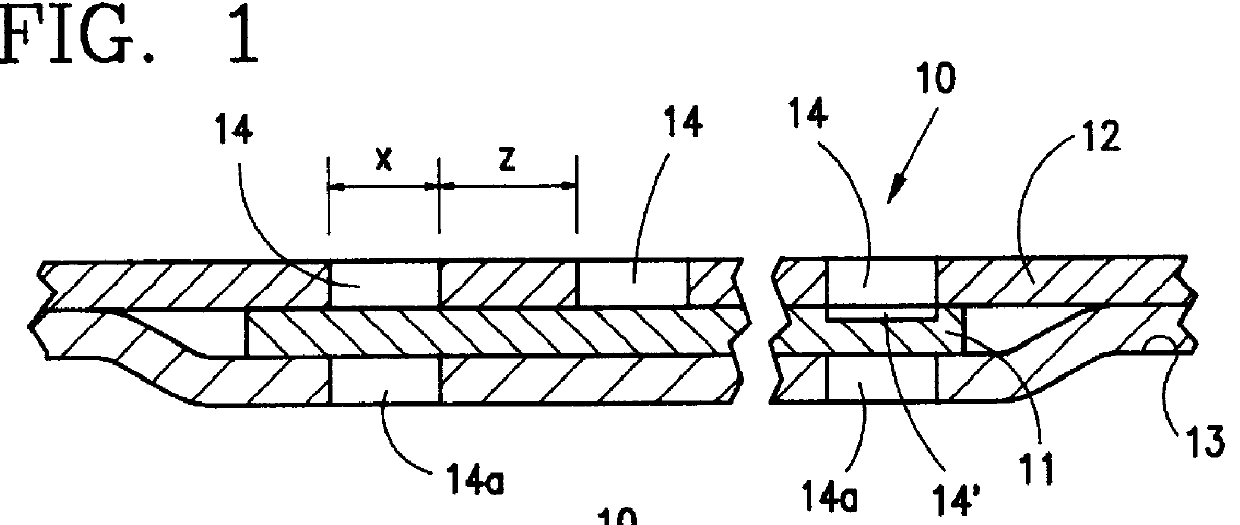
PCT No. PCT / EP96 / 00865 Sec. 371 Date Sep. 11, 1997 Sec. 102(e) Date Sep. 11, 1997 PCT Filed Mar. 1, 1996 PCT Pub. No. WO96 / 29036 PCT Pub. Date Sep. 26, 1996The invention relates to an item of clothing, especially disposable clothing for use once only, with at least one essentially inelastic layer (12) of a soft, flexible and plastic material and a layer (11) of elastic material which extends over at least a partial region of the inelastic layer (12) and is secured thereto to form an elastic component (10). In order to be able to make the elastic component in such an item of clothing easily, there is a plurality of incisions (14) in the inelastic layer (12) in the region of the elastic layer (11) which do not penetrate the elastic layer. The elastic layer may thus also contain absorbent materials.
Owner:PAUL HARTMANN AG
Flexible display panel, production method for same, and flexible display device
ActiveCN103985321AImprove bendabilityAvoid breakingSolid-state devicesIdentification meansElastic componentEngineering
The invention discloses a flexible display panel, a production method for the same, and a flexible display device. The flexible display panel comprises a flexible substrate and a transparent flexible cover plate which are oppositely arranged; the flexible display panel is provided with a display area and a border area surrounding the display area; a rubber layer is arranged in the border area, and used for bonding and fixing the flexible substrate and the transparent flexible cover plate; a plurality of elastic components are arranged in the rubber layer. The flexible display panel is good in bendability. The production method can be used for producing the flexible display panel with good bendability, and the flexible display device is produced by the flexible display panel and good in bendability.
Owner:SHANGHAI TIANMA MICRO ELECTRONICS CO LTD +1
Electric cooker temperature control assembly, electric cooker with same and temperature control method
ActiveCN105455664ARealize the insulation functionAnti-dry functionWarming devicesTime-controlled ignitorsTemperature controlElastic component
The invention provides an electric cooker temperature control assembly, an electric cooker with the same and a temperature control method. A power failure reset snap-action type temperature controller and a simple structure can be used for achieving the functions of temperature limitation, thermal insulation and dry burning prevention of the electric cooker. The electric cooker temperature control assembly comprises the power failure reset snap-action type temperature controller and a heat conduction cover, the open portion of the heat conduction cover extends outwards to be provided with a skirt edge, the power failure reset snap-action type temperature controller is fixed inside the heat conduction cover, the temperature-sensing face of the power failure reset snap-action type temperature controller is tightly attached to the inner top face of the heat conduction cover, and an elastic component is arranged on the lower portion of the heat conduction cover. The electric cooker with the temperature control assembly further comprises a heating disc and an electric cooker inner container, a heat conduction cover installing hole is formed in the heating disc, the heat conduction cover penetrates upwards through the heat conduction cover installing hole, the elastic component abuts against the skirt edge of the heat conduction cover so that the skirt edge of the heat conduction cover can make tight contact with the heating disc, and a heating element of the power failure reset snap-action type temperature controller is connected with a movable contact-fixed contact loop in parallel and then connected with the heating disc in series into a power loop of the electric cooker.
Owner:广东强力科技股份有限公司
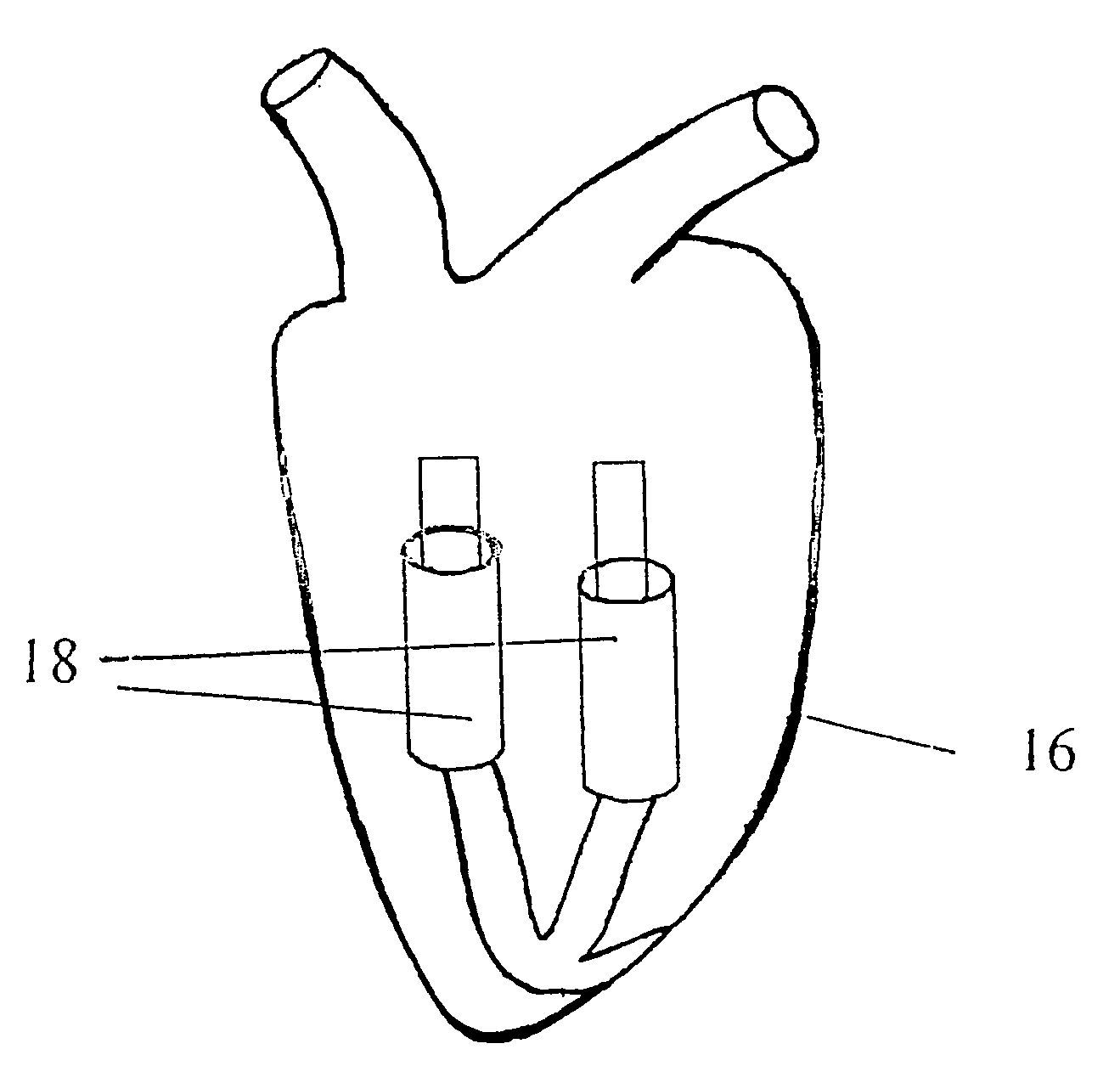
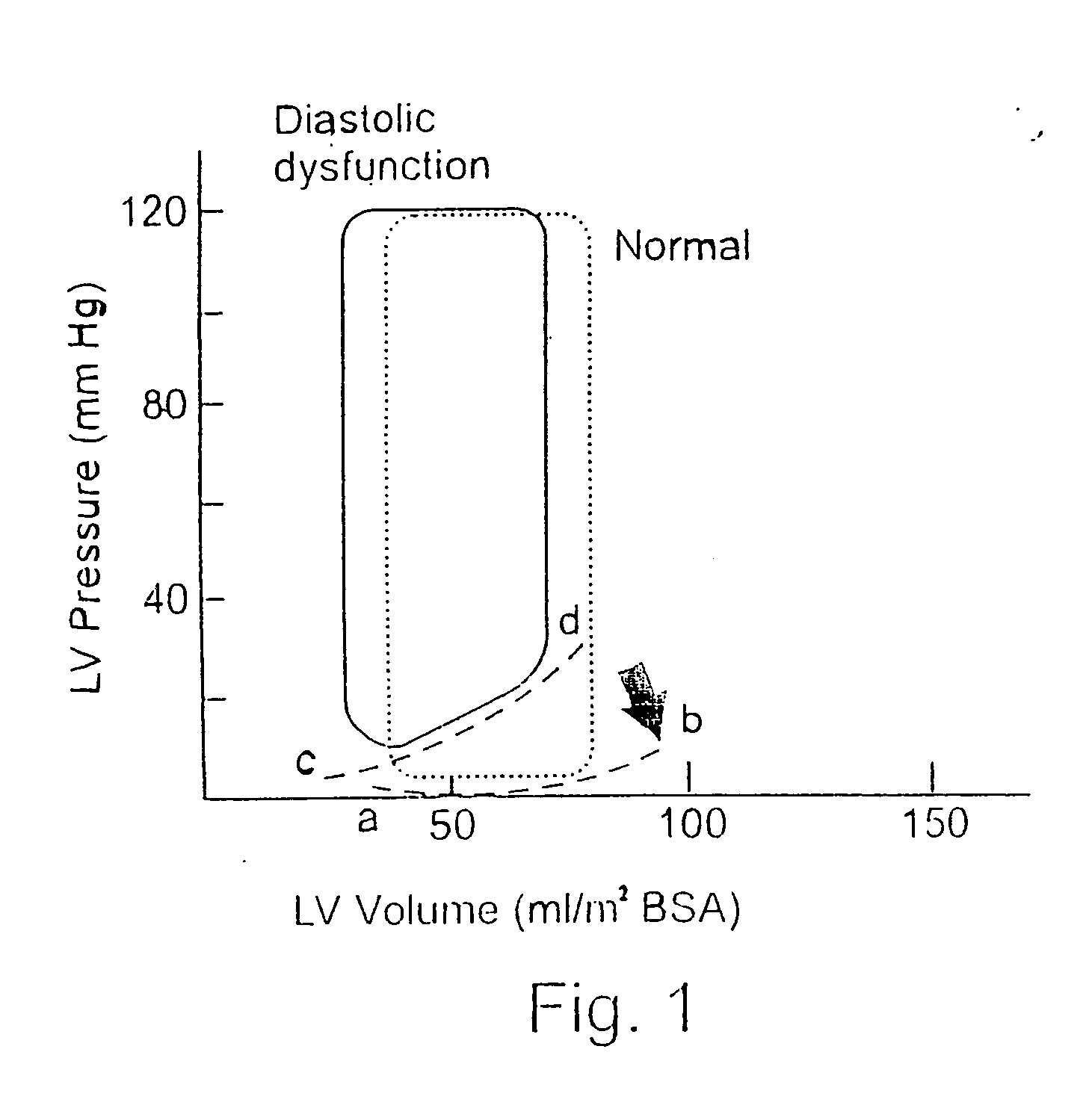
In vivo device for improving diastolic ventricular function
InactiveUS20060241334A1Easy to adaptReduce construction costsSuture equipmentsHeart valvesElastic componentVentricular Functions
The present invention provides an in vivo device for improving diastolic function of the heart, comprising: at least one elastic component that may be operatively connected to the external surface of the left or right ventricle of the heart by means of connecting elements, wherein said elastic component comprises essentially longitudinal members arranged such that the lateral separation therebetween may be increased or decreased in response to elastic deformation of said elastic component, and wherein said essentially longitudinal members are arranged such that said elastic component is curved in both the vertical and horizontal planes, such that its inner surface may be adapted to the curvature of the external ventricular surface of the heart, such that said elastic component is capable of exerting both radially outward expansive and tangentially-directed forces on the external surface of the cardiac ventricle.
Owner:CORASSIST CARDIOVASCULAR LTD
Apparatus for image forming capable of applying a high fixing-nip pressure easily releasable when a recording sheet is stuck in a fixing mechanism
ActiveUS20060002737A1Increase pressureElectrographic process apparatusElastic componentImage formation
An image forming system includes an image forming mechanism and a fixing mechanism. The fixing mechanism includes first and second fixing members configured to face each other to hold recording materials, a lock member, a pressuring lever, a pressure release lever and an elastic member. The pressure release lever turns between a lock position for a regular operation mode and a release position. The elastic member pulls the lock member capturing the pressuring lever to apply pressure to the first and second fixing members at the far end from the instantaneous center of the pressuring lever at the lock position.
Owner:RICOH KK
Insertion connecting component with tension connection function
ActiveCN104878758AReduce movement gapReduce connection gapsBulkheads/pilesElastic componentEngineering
The invention belongs to the technical field of building components, and relates to an insertion connecting component with a tension connection function. By the aid of the insertion connecting component, the technical problem of unreliability in connection in the prior art can be solved. The insertion connecting component comprises a first tension nut and a second tension nut. The first tension nut and the second tension nut are respectively fixed to two ends of a prefabricated member, an insertion rod is detachably connected into the first tension nut, a middle nut is detachably connected into the second tension nut, an elastic component is arranged in the second tension nut, a clamp ring is arranged between the middle nut and the elastic component, the insertion rod can be clamped by the clamp ring when being inserted into the middle nut, the middle nut comprises a nut body, external threads are arranged on the outer wall of the nut body, a channel is arranged inside the nut body, an insertion connector can penetrate the channel, an end of the channel is expanded to form a clamp ring positioning cavity capable of accommodating the clamp ring, a clamp ring limiting cavity is arranged at an end of the clamp ring positioning cavity, and the clamp ring can be blocked by the clamp ring limiting cavity when slipping towards the end of the clamp ring positioning cavity and accordingly can be prevented from slipping out of the clamp ring positioning cavity. The insertion connecting component has the advantages that the insertion connecting component is stable and reliable in insertion procedures, and accordingly the construction work efficiency can be improved.
Owner:周兆弟
Pressure adjusting structure and pressure cooker having same
ActiveCN107136911AAchieve regulationImprove food cookingPressure-cookersElastic componentEngineering
The invention provides a pressure adjusting structure comprising a valve seat, an exhaust tube and a magnetic valve body. The lower end of the exhausting tube passes through the valve seat; the magnetic valve body sleeves the upper end port of the exhausting tube; a magnetic cushion block is arranged between the valve seat and the magnetic valve body; and the magnetic cushion block is connected with the valve seat via a magnetic cushion block adjusting structure adjusting up-down positions of the magnetic cushion block. When a pressure limiting value of a pressure-limiting valve has to be adjusted, the up-down positions of the magnetic cushion block is adjusted by the magnetic cushion block adjusting structure, so a distance from the magnetic gasket to the magnetic valve body can be changed; when the distance changes, magnetic field force between the magnetic cushion block and the magnetic valve body can be changed; the pressure limiting value of the pressure limiting valve is the self weight of the magnetic valve body and the magnetic field force between the pressure limiting valve and the magnetic valve body; the pressure limiting value of the pressure limiting valve can be adjusted and food cooking effect of the pressure cooker can be improved; meanwhile, the use of elastic components such as springs can be avoided in the adjusting structure, so fewer components are required; and the pressure adjusting structure is simply structured, convenient to assembly and has high reliability.
Owner:FOSHAN SHUNDE MIDEA ELECTRICAL HEATING APPLIANCES MFG CO LTD
Plug/unplug moudle base
InactiveUS20070243741A1Simple processDurable and powerful componentEngagement/disengagement of coupling partsTwo-part coupling devicesElastic componentManufacturing technology
Owner:ALL BEST ELECTRONICS
Display Apparatus And Display System
InactiveUS20100220249A1Television system detailsMagnetic/electric field screeningElastic componentDisplay device
A display system comprising: a display apparatus; a frame; an engaging component (81) arranged on the display; an engaged component (82) engages to the engaging component (81) and connected to the frame via an elastic component (84); a preventing component (85) fixed to the engaged component (82), wherein the prevention component (85) prevents the display apparatus from falling ahead.
Owner:SANYO ELECTRIC CO LTD
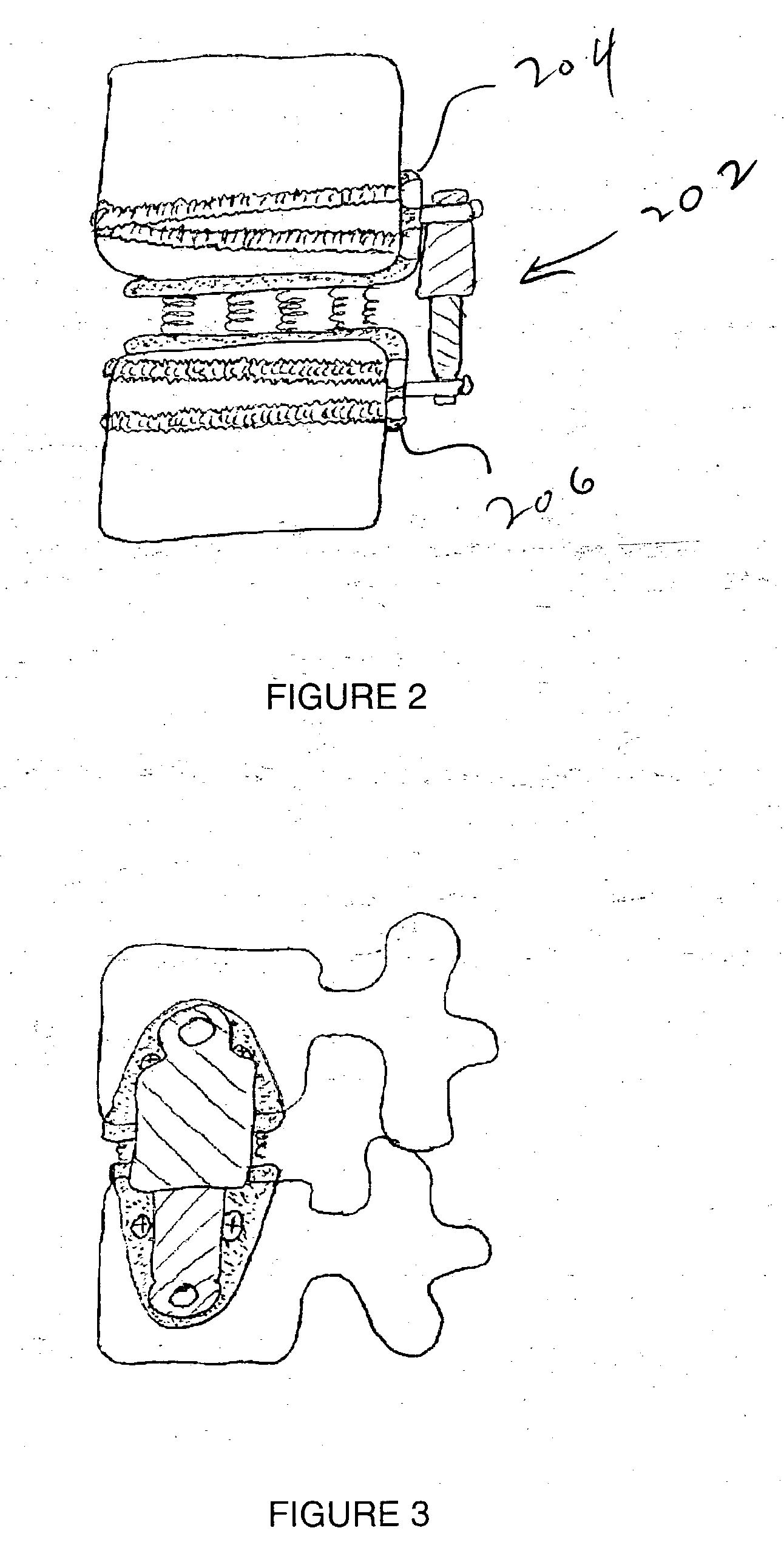
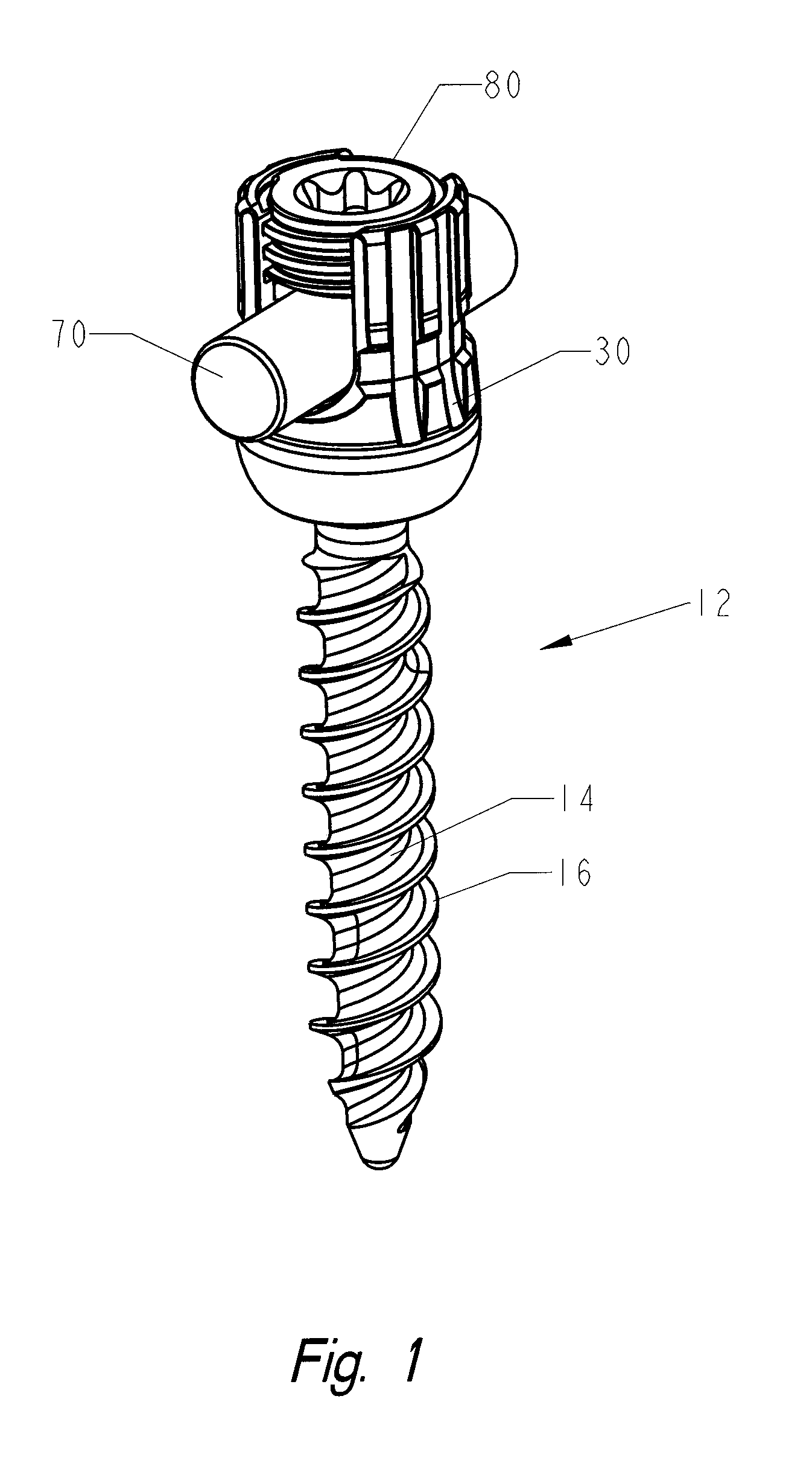
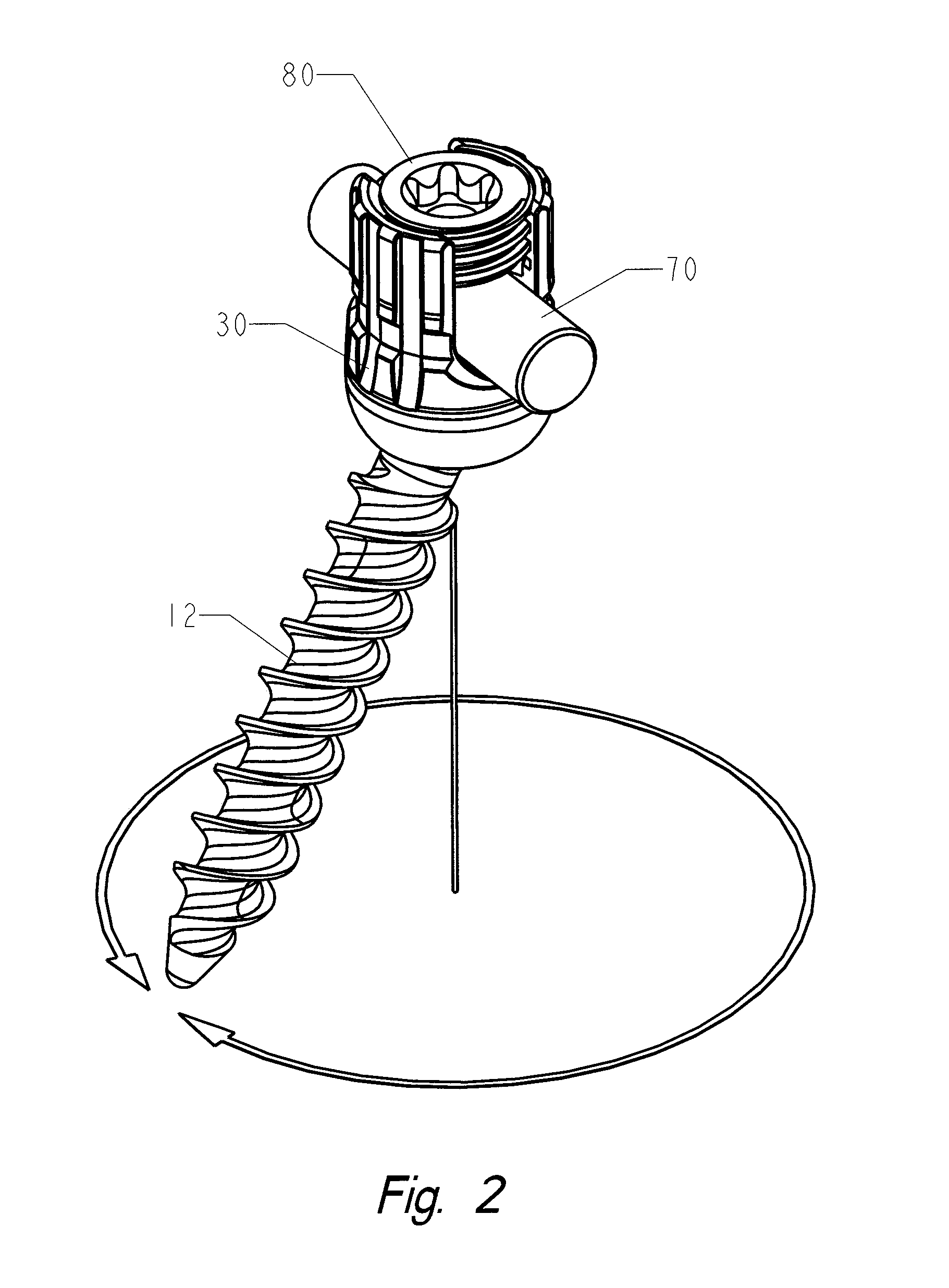
Locking polyaxial ball and socket fastener
ActiveUS20110009911A1Reduce the amount requiredCorrected satisfactorilySuture equipmentsInternal osteosythesisElastic componentEngineering
The fastening system consists of the polyaxial ball and socket joint used in conjunction with a bone screw having threads on one end for use in anchoring to the spine and a spherical connector on the other end operating as a pivot point about which a connecting assembly moves in a polyaxial fashion. A substantially U-shaped connecting assembly has a lower receptacle that operates as a socket for housing an upper retainer ring and a lower split retaining ring. The socket is receptive to the spherical connector which is inserted through the lower split retainer ring causing a momentary displacement thereof which allows for the positioning of the spherical connector between the upper and lower retainer rings. A resilient component positioned between the upper retainer ring and the connecting assembly permits relative predetermined placement and retention of the spherical connector relative to the connector assembly due to the force generated by the resilient component and frictional engagement between the surfaces of spherical connector, the upper and lower retainer rings and the connector assembly. The polyaxial ball and socket can be locked into a fixed position.
Owner:ORTHO INNOVATIONS
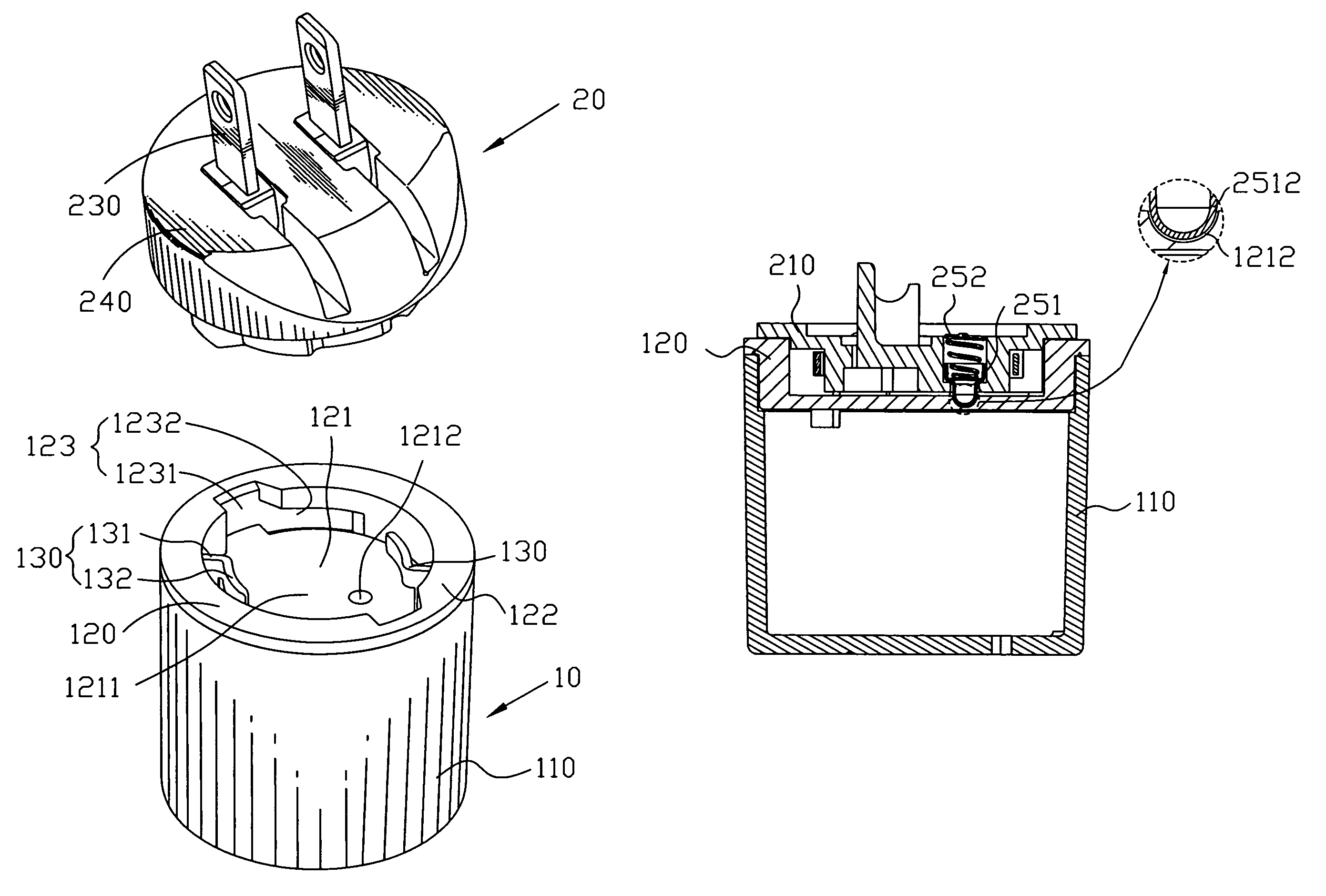
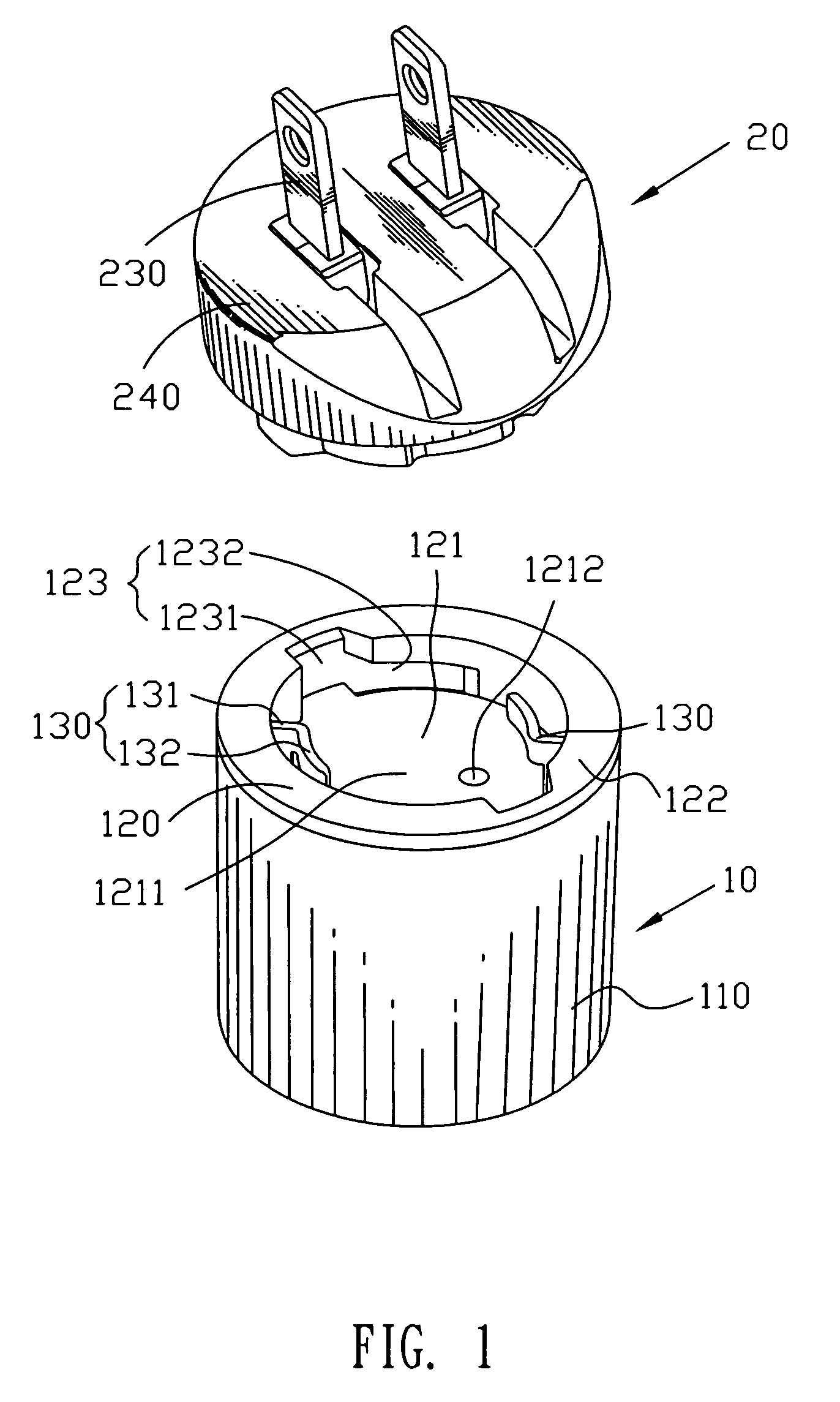
Power adapter
ActiveUS7632119B1Convenient to disassemblePrevent rotationCoupling device detailsClamped/spring connectionsElastic componentEngineering
A power adapter has a main body. A top of the main body defines a recess and an annular sidewall enclosing the recess. The recess has a locating depression arranged at a bottom thereof to be deviated from the center of the bottom. A plug is rotatably and detachably mounted in the recess. An elastic component mounted in the plug defines a contacting portion elastically protruding out of a bottom of the plug. The plug is locked in the recess by rotation so that the contacting portion is pressed against the bottom surface of the recess and compressed back elastically and then the contacting portion engages the locating depression under resilience action of the elastic component for preventing the plug from rotating with respect to the main body.
Owner:CHENG UEI PRECISION IND CO LTD
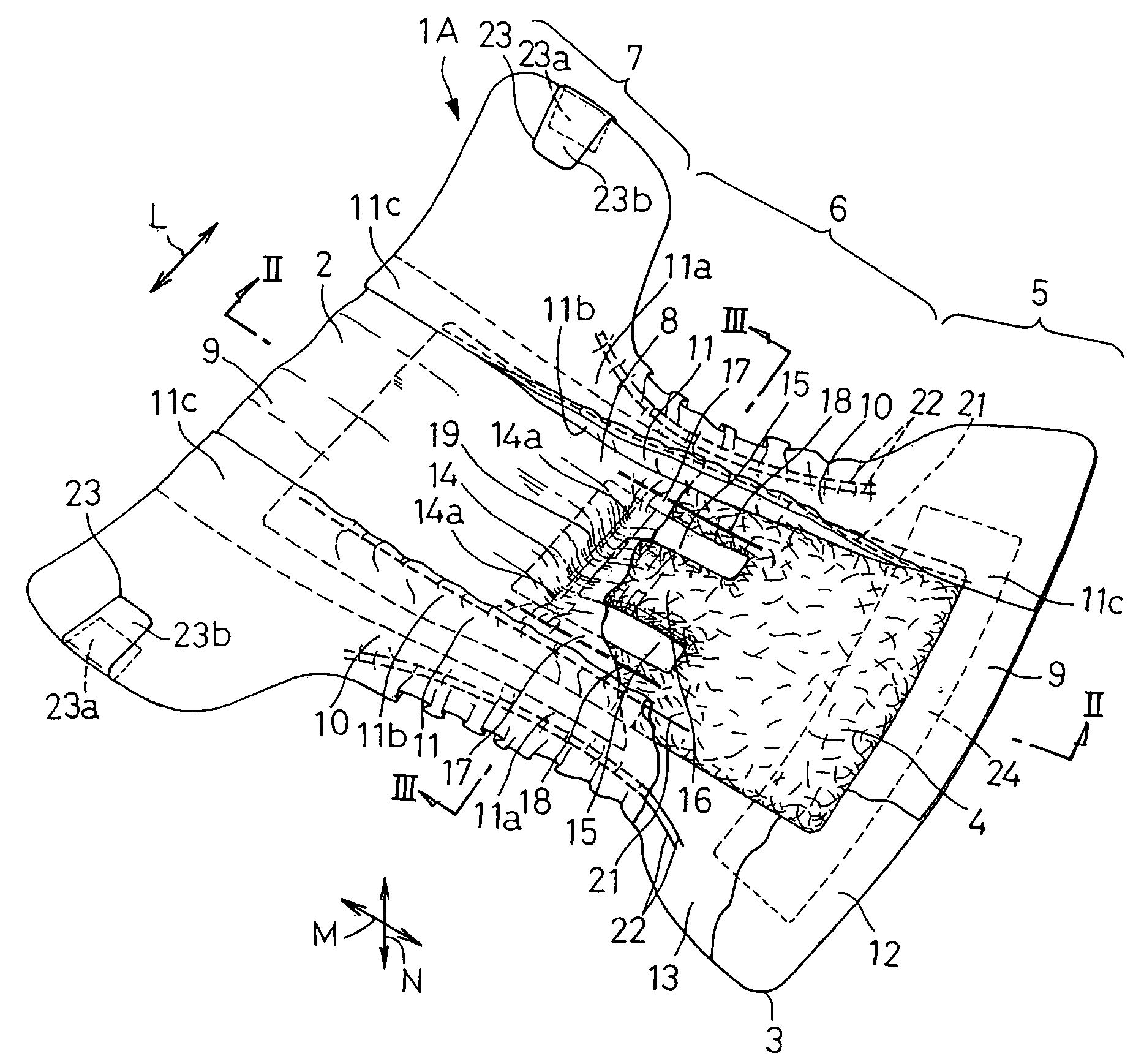
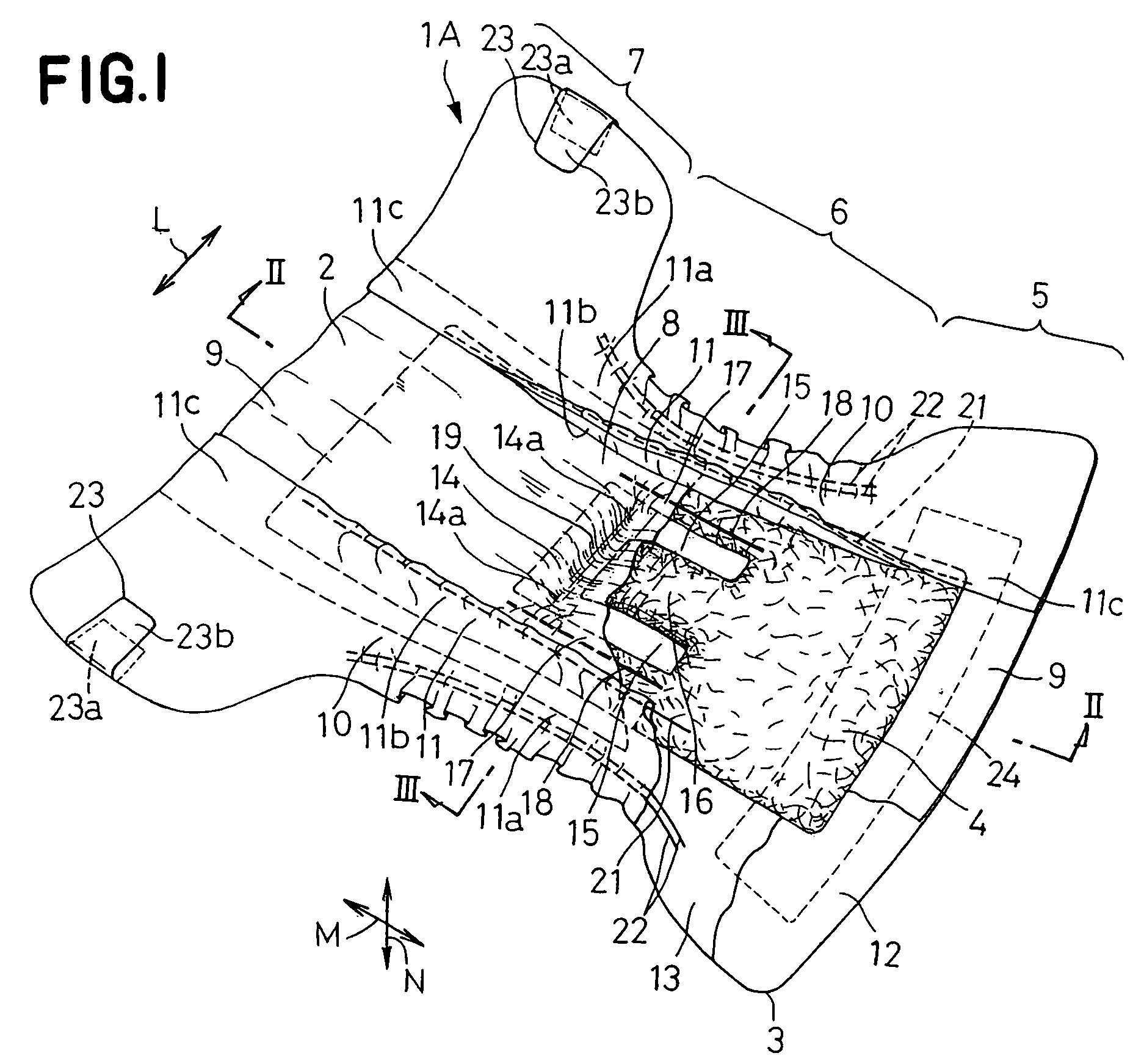
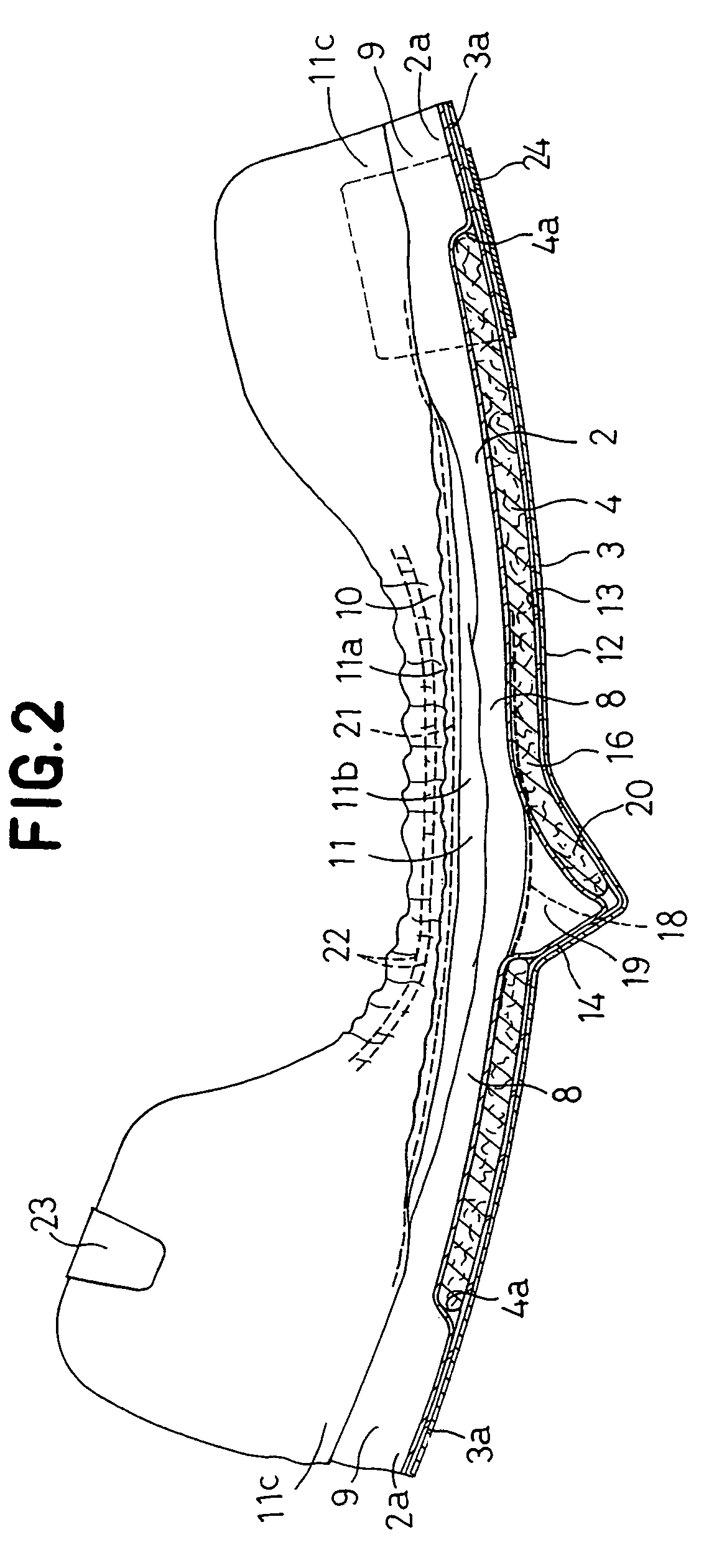
Stretch sheet and process of producing the same
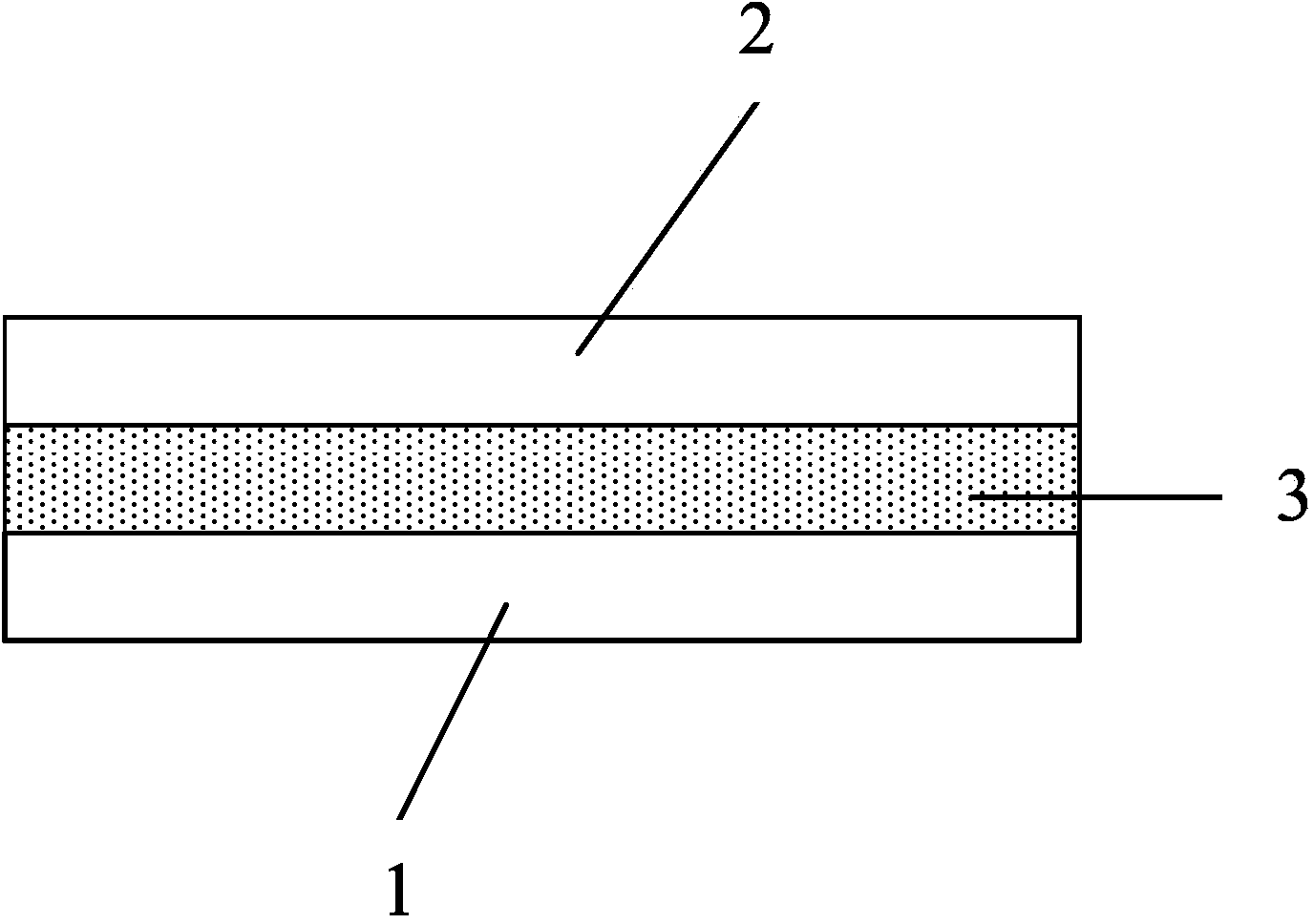
In a process of producing a stretch sheet, a strip-shaped laminate sheet 10A having an elastically stretchable elastic layer 1 and substantially inelastic, inelastic fiber layers 2 and 3 partially joined to each other at bonds 4 or a strip-shaped fibrous sheet containing an elastic component and a substantially inelastic component and having embossed regions formed by embossing in parts is stretched in directions starting from the bonds 4 or the embossed regions to obtain a stretch sheet 10. The stretch sheet has raised ridges and recessed grooves extending in the direction perpendicular to the stretch direction, and the bonds 4 or the embossed regions are present in the ridges.
Owner:KAO CORP
Under-actuated prosthetic hand capable of reproducing hand grasping function
The invention discloses an under-actuated prosthetic hand capable of reproducing a hand grasping function; the under-actuated prosthetic hand is provided with an interphalangeal drive mechanism and a thumb drive mechanism in a palm; finger bodies take pulleys as main parts, and transmission mechanisms arranged in fingers are sequentially and alternately wound on all the pulleys of the fingers by steel wire ropes; joints in the fingers are in flexible coupling by elastic components; the interphalangeal drive mechanism adopts two motors to drive four fingers and multiple degrees of freedom of the fingers in a synergetic way; a thumb body takes a pulley as a main part, the transmission mechanisms in the finger bodies take transmission ropes as transmission mediums, the interphalangeal drive mechanism adopts a gear component for transmission matching, and the four degrees of freedom of the thumb can be controlled by two motors. After the under-actuated prosthetic hand is adopted, self-adaptive grasping of various objects can be accurately completed, and the rich grasping operation function of a human hand can be reproduced; the under-actuated prosthetic hand has the characteristics of being compact in structure, smaller in size, lighter in weight, convenient to control, accurate in grasping action, and the like.
Owner:HUAZHONG UNIV OF SCI & TECH
Pressure-auto-balance hydrodynamic noise silencer
ActiveCN103062569ACompact structureStrong adjustable noise cancellation frequencyPipe elementsElastic componentEngineering
The invention aims to provide a pressure-auto-balance hydrodynamic noise silencer comprising an inner perforated tube, an end face flange and a barrel casing. The end face flange is respectively hermetically connected with the inner perforated tube and the barrel casing to form a cavity. A compressible elastic component is filled in the cavity. One end of the inner perforated tube is connected with an inlet tube, and the other end of the inner perforated tube is connected with an outlet tube. The inner perforated tube is provided with perforated holes. The pressure-auto-balance hydrodynamic noise silencer is compact in structure, and can automatically balance along with changes of system static pressure during working, and a conventional energy accumulator inflating and deflating device is omitted. Besides, the noise silencer combines advantages of a resistant noise silencer and an energy accumulator, and is high in adjustability of silencing frequency and capable of effectively suppressing broadband hydrodynamic noise.
Owner:HARBIN ENG UNIV
Sensors carrier for in-tube inspection scraper
InactiveUS7240574B2Improve efficiencyReduce the amount requiredDetection of fluid at leakage pointMeasurement of fluid loss/gain rateElastic componentKinematics
A sensor carrier for an in-tube inspection scraper has setting places for installation of monitoring sensors responsive to any diagnostic parameters of a pipeline being inspected. The carrier includes a set of kinematically interconnected sensor supports that are subjected to an elastic force in the radial direction from the carrier axis. The supports are made as elastic ring-shaped elements or as rows of interconnected elements adjoining the pipe wall or are fixed on elastic components such as rings or sectors of sleeves, and / or are connected to a housing capable of bending. Installed on the supports between the sensors and the internal surface of the pipeline are elastic gaskets. The carrier allows one to increase the efficiency of the flaw detection in pipelines having defects of geometry in the cross section.
Owner:WEATHERFORD TECH HLDG LLC +1
Tunable Actuator Joint Modules Having Energy Recovering Quasi-Passive Elastic Actuators for Use within a Robotic System
ActiveUS20180193172A1Minimize power consumptionReduce the required powerProgramme-controlled manipulatorJointsRobotic systemsStored energy
A tunable actuator joint module of a robotic assembly comprises an output member and an input member, where the output member is rotatable about an axis of rotation. A primary actuator (e.g., a motor) is operable to apply a torque to rotate the output member about the axis of rotation. A quasi-passive elastic actuator (e.g., rotary or linear pneumatic actuator) comprising an elastic component is tunable to a joint stiffness value and is operable to selectively release stored energy to apply an augmented torque to assist rotation of the output member and to minimize power consumption of the primary actuator. The tunable actuator joint module comprises a control system having a valve assembly controllably operable to switch the quasi-passive elastic actuator between an elastic state and an inelastic state during respective portions of movement of the robotic assembly (e.g., a hip or knee joint of an exoskeleton). Associated systems and methods are provided.
Owner:SARCOS CORP
Suspension packaging system
InactiveUS20060102515A1Improve buffering effectContainers to prevent mechanical damageRigid containersCushioningElastic component
A suspension packaging system can include a retention member having at least one pocket and a relatively more rigid member configured to be inserted into the pocket and folded into a state in which a tension in the retention member is increased. The more rigid member can be in the form of a tray and can include protrusions over which the pocket is placed. The protrusions can then be moved into engagement with apertures thereby engaging the resilient member between the protrusion and the aperture. Additionally, flaps of a box can be inserted into pockets of a resilient member and rotated so as to generate attention in the retention member so as to provide further cushioning of an article to be packaged therein.
Owner:CLEARPAK
Residence structures and related methods
ActiveUS20170106099A1Facilitate dissociationTetracycline active ingredientsMedical devicesStimulantDissolution
Residence structures, systems, and related methods are generally provided. Certain embodiments comprise administering (e.g., orally) a residence structure to a subject (e.g., a patient) such that the residence structure is retained at a location internal to the subject for a particular amount of time (e.g., at least about 24 hours) before being released. The residence structure may be, in some cases, a gastric residence structure. In some embodiments, the structures and systems described herein comprise one or more materials configured for high levels of active substances (e.g., a therapeutic agent) loading, high active substance and / or structure stability in acidic environments, mechanical flexibility and strength in an internal orifice (e.g., gastric cavity), easy passage through the GI tract until delivery to at a desired internal orifice (e.g., gastric cavity), and / or rapid dissolution / degradation in a physiological environment (e.g., intestinal environment) and / or in response to a chemical stimulant (e.g., ingestion of a solution that induces rapid dissolution / degradation). In certain embodiments, the structure has a modular design, combining a material configured for controlled release of therapeutic, diagnostic, and / or enhancement agents with a structural material necessary for gastric residence but configured for controlled and / or tunable degradation / dissolution to determine the time at which retention shape integrity is lost and the structure passes out of the gastric cavity. For example, in certain embodiments, the residence structure comprises a first elastic component, a second component configured to release an active substance (e.g., a therapeutic agent), and, optionally, a linker. In some such embodiments, the linker may be configured to degrade such that the residence structure breaks apart and is released from the location internally of the subject after a predetermined amount of time.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC +1
Electronic device with sheath structure
InactiveUS7104814B1Connection portion can be preventedEasy to doDigital data processing detailsLive contact access preventionElastic componentFixed position
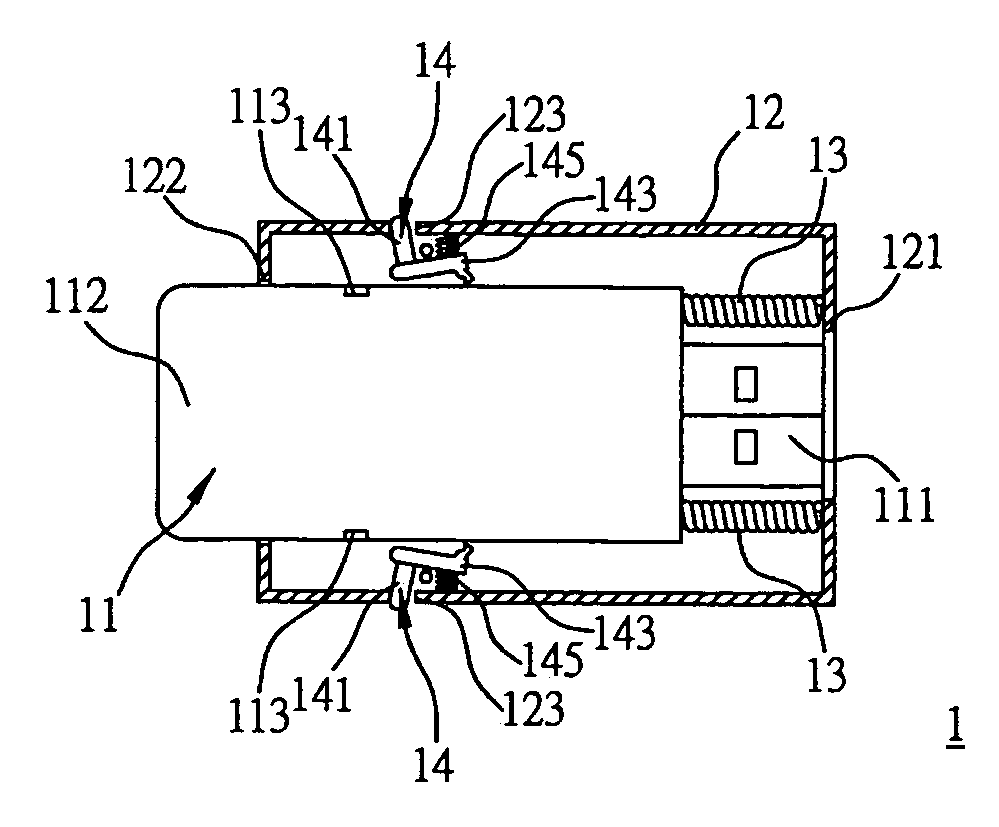
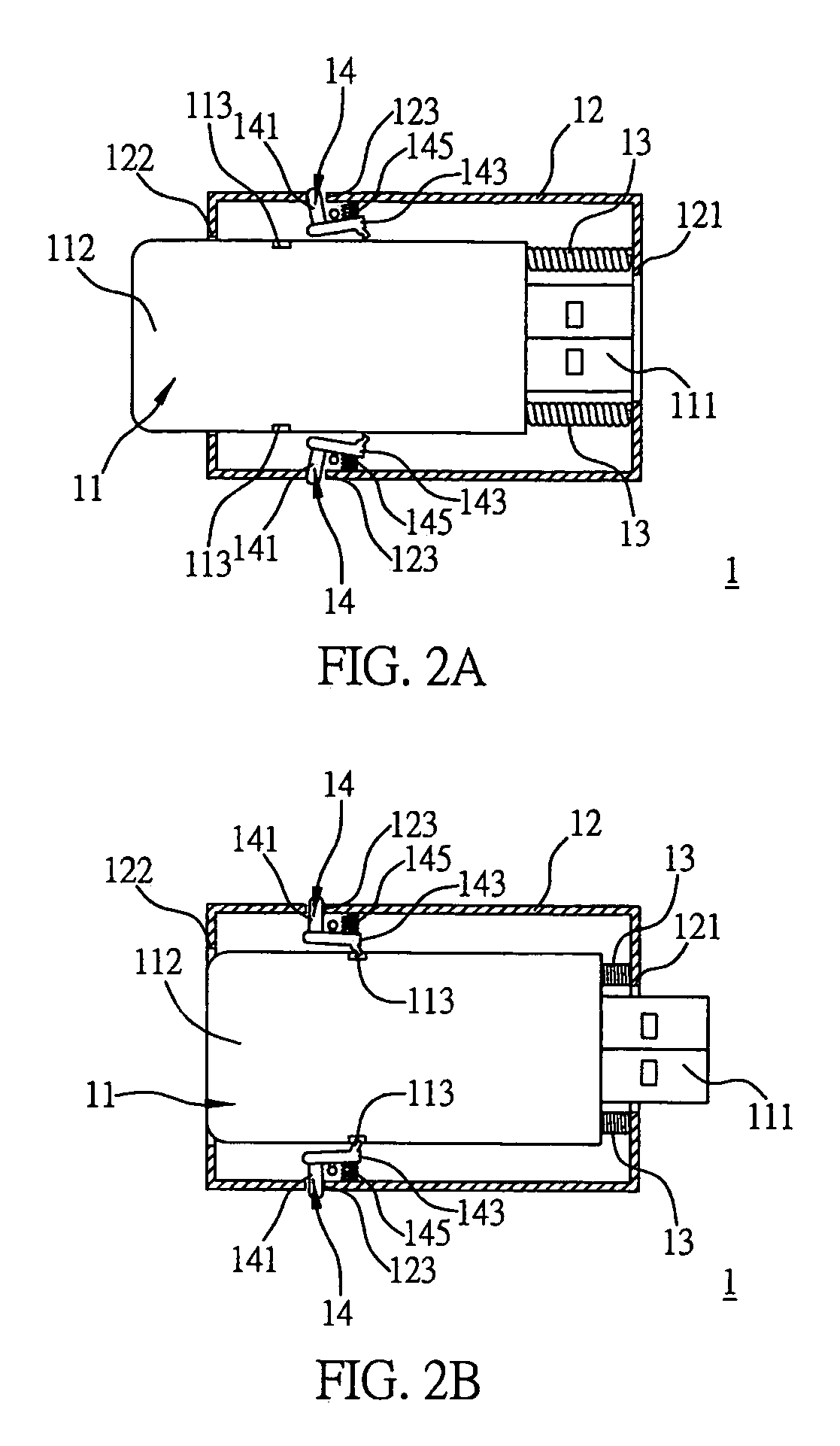
An electronic device includes a main body having a connection portion for electrically connecting the electronic device to an external device; a sheath for the main body to be slidably received therein; at least one positioning component provided in the sheath, for fixing the main body when the main body is sliding toward a first terminal of the sheath and arrives at a fixing position such that the connection portion is protruded from a first opening formed at the first terminal in order to use the electronic device; and at least one first elastic component provided in the sheath, for pressing the main body to move toward a second terminal of the sheath when the main body is released from the fixing position and is sliding toward the first terminal of the sheath, such that the connection portion is received in the sheath in order to store the electronic device.
Owner:INVENTEC CORP
Flex chair
A chair having a seat, a first support base and an upright elongated support element. The upright elongated support element has a first point and a second point, with the first point connecting to the seat and the second point connecting to the first support base. The first support base is bolstered by a resilient component since the resilient component is disposed beneath the first support base. The resilient component is capable of providing cushioning to the first support base is also capable of providing a full axial rotation to said upright elongated support element.
Owner:PRODS OF TOMORROW
Temperature regulator
ActiveCN103867692APrevent leakageReduce leakageOperating means/releasing devices for valvesGear lubrication/coolingElastic componentHeat sensitive
The invention relates to a temperature regulator. The temperature regulator comprises a valve body and a thermodynamic element, wherein a cavity is formed in the valve body, and the thermodynamic element is mounted in the cavity; the cavity is communicated with the outside through at least three valve ports, namely a first valve port, a second valve port and a third valve port, the two ends of the thermodynamic element are respectively mounted in the valve body through elastic components, a flow cutoff ring which is of an annular component is mounted in the cavity in a sealed and fixed manner, and a thermodynamic element body is located between the third valve port and the flow cutoff ring. When the temperature of fluid is relatively low, a heat-sensitive material in the thermodynamic element contracts, and the thermodynamic element body moves towards the flow cutoff ring and closes an internal hole of the flow cutoff ring; when the temperature of the fluid is relatively high, the heat-sensitive material in the thermodynamic element expands, and the thermodynamic element body moves towards the third valve port until the third valve port is closed by the thermodynamic element body. The temperature regulator provided by the invention has the advantage that the internal leakage of temperature regulators in the prior art is lowered greatly.
Owner:ZHEJIANG SANHUA AUTOMOTIVE COMPONENTS CO LTD
Rubber crawler
InactiveUS20050168069A1Small thicknessImprove joint strengthDriving beltsAlighting gearElastic componentEngineering
Owner:SUMITOMO RUBBER IND LTD
Camera assembly structure
InactiveUS20060170817A1Small sizeEasy to carryTelevision system detailsColor television detailsElastic componentLocal structure
The present invention is a camera assembly structure having a body and a base. The body, having a front cover, a back cover and a focus ring clamped between the front and back covers, is connected at the base and can rotate in relation to the base. The base has a front base, an elastic component, and a back base. The front base has two side troughs for inserting the elastic component, and the elastic component can move along the side troughs. The back base engaging with partial structure of the front cover is connected at the elastic component and can use the elastic component as an axis so as to rotate downward.
Owner:BEHAVIOR TECH COMPUTER
System for inductively charging vehicles, comprising an electronic positioning aid
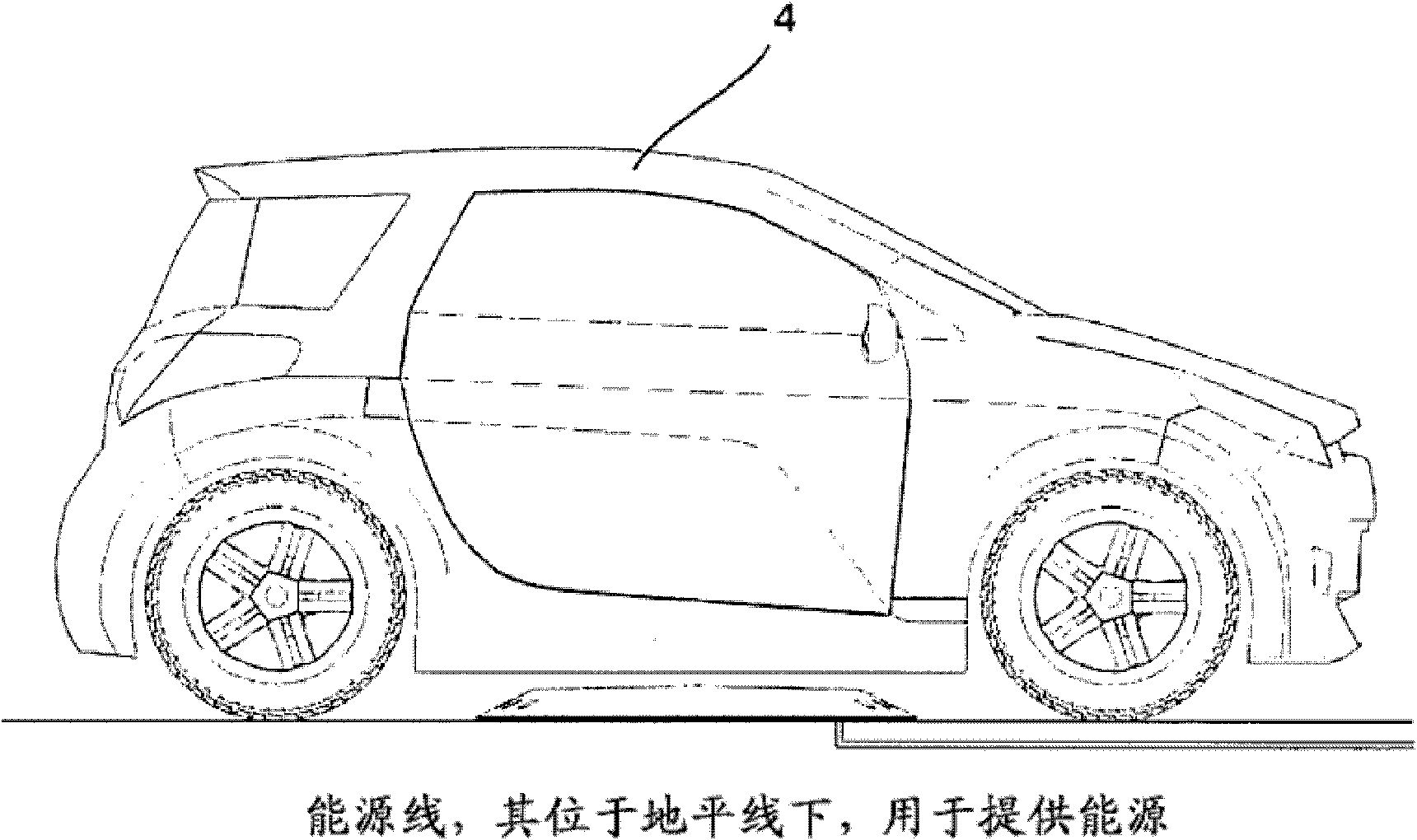
InactiveCN102741083AImprove convenienceFrequent connectionCharging stationsOperating modesElectricityDriver/operator
The present invention relates to a system that ensures a self-guiding, electronic positioning of a secondary coil at a vehicle side, without the aid of indicators or kinematic or mechanical aids, in relation to a primary coil that is fixed at a a ground side, in order to guarantee a transfer of energy with over 90% efficiency without the disadvantages of moving, frictional and elastic components in terms of energy consumption, functional safety and wear. To achieve this aim, the coil housing at the ground side fulfils the role of an electronics housing, reflective element and cooling element thanks to the choice of material used, the surface and the inner supports and can thus be retrofitted, as a single installation at the ground side in the form of an operation-ready complete package, to any flat base with an electric connection. The vehicle can be used both for transporting passengers and loads and can be steered by a vehicle driver or can be operated without a driver, for example for cleaning areas, for natural protection or for intralogistics.
Owner:CONDUCTIX WAMPFLER
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com