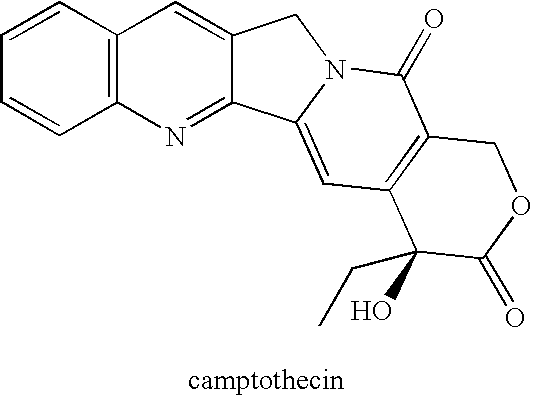
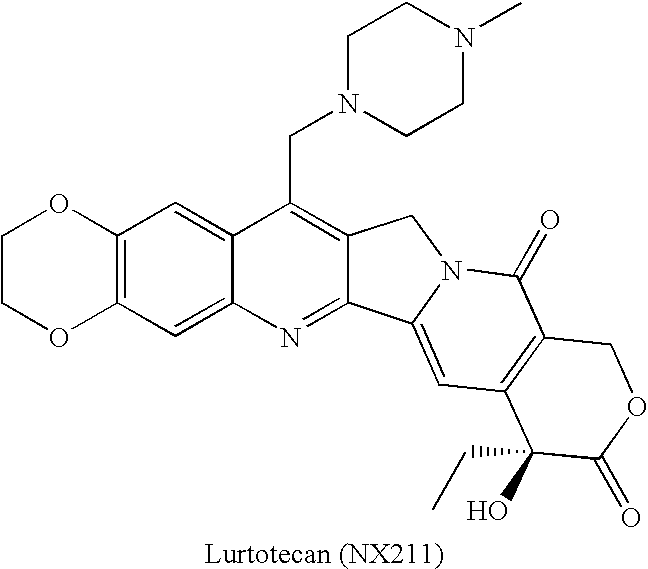
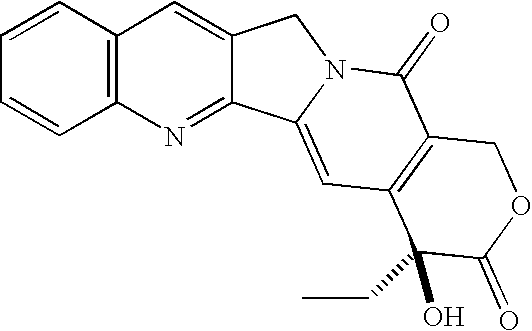
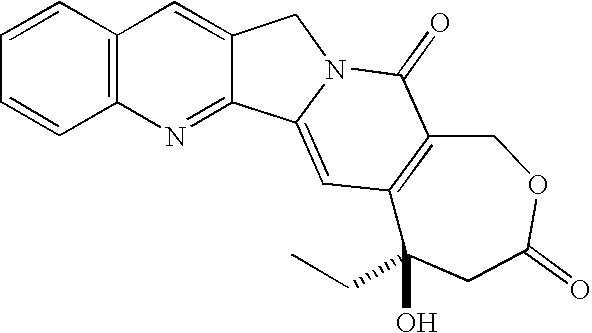
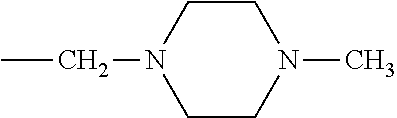
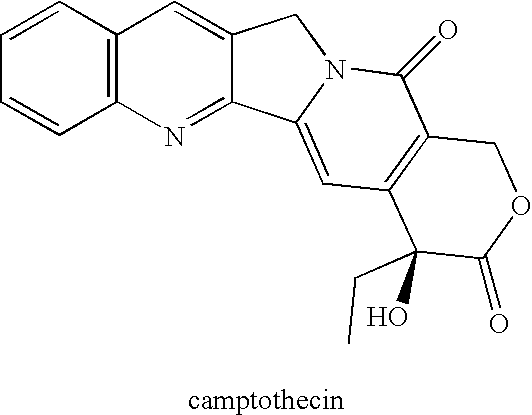
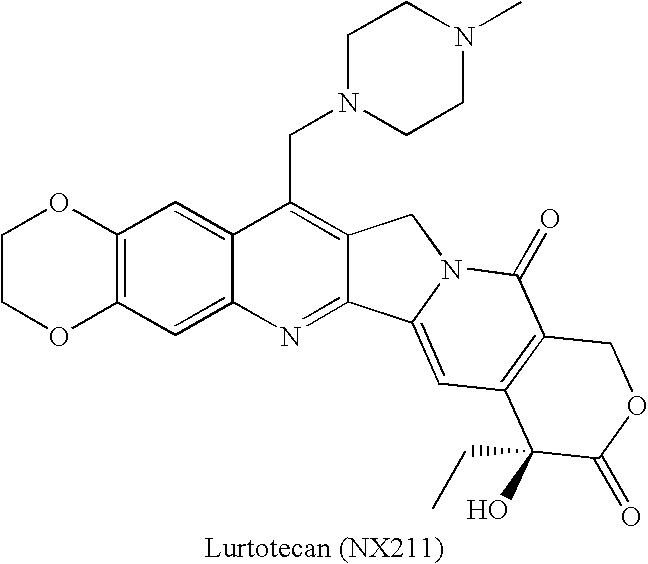
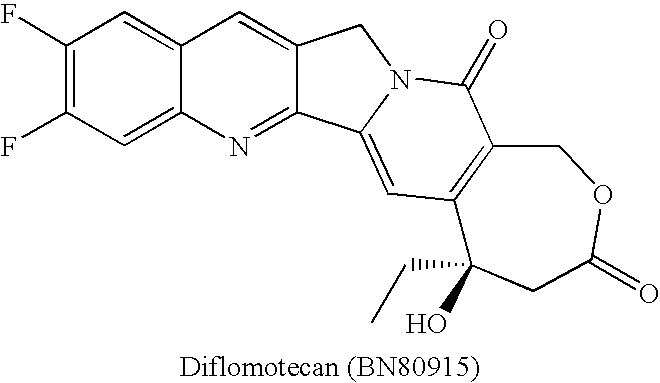
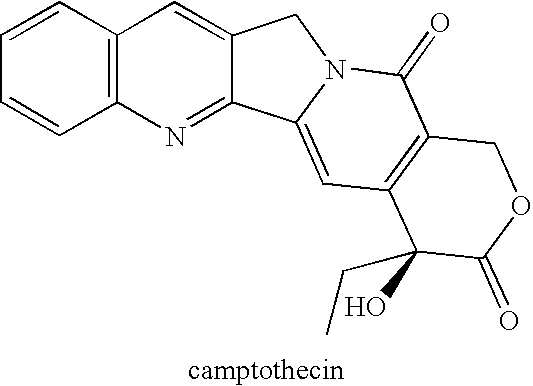
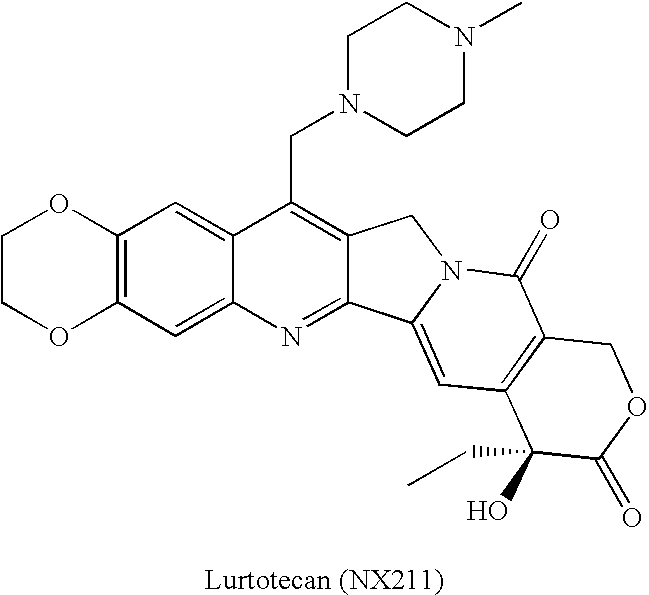
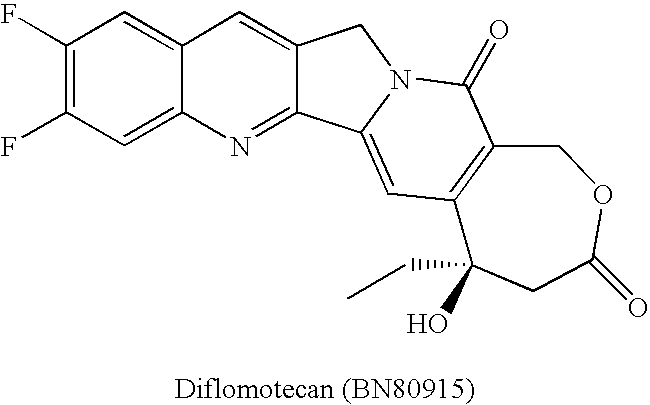
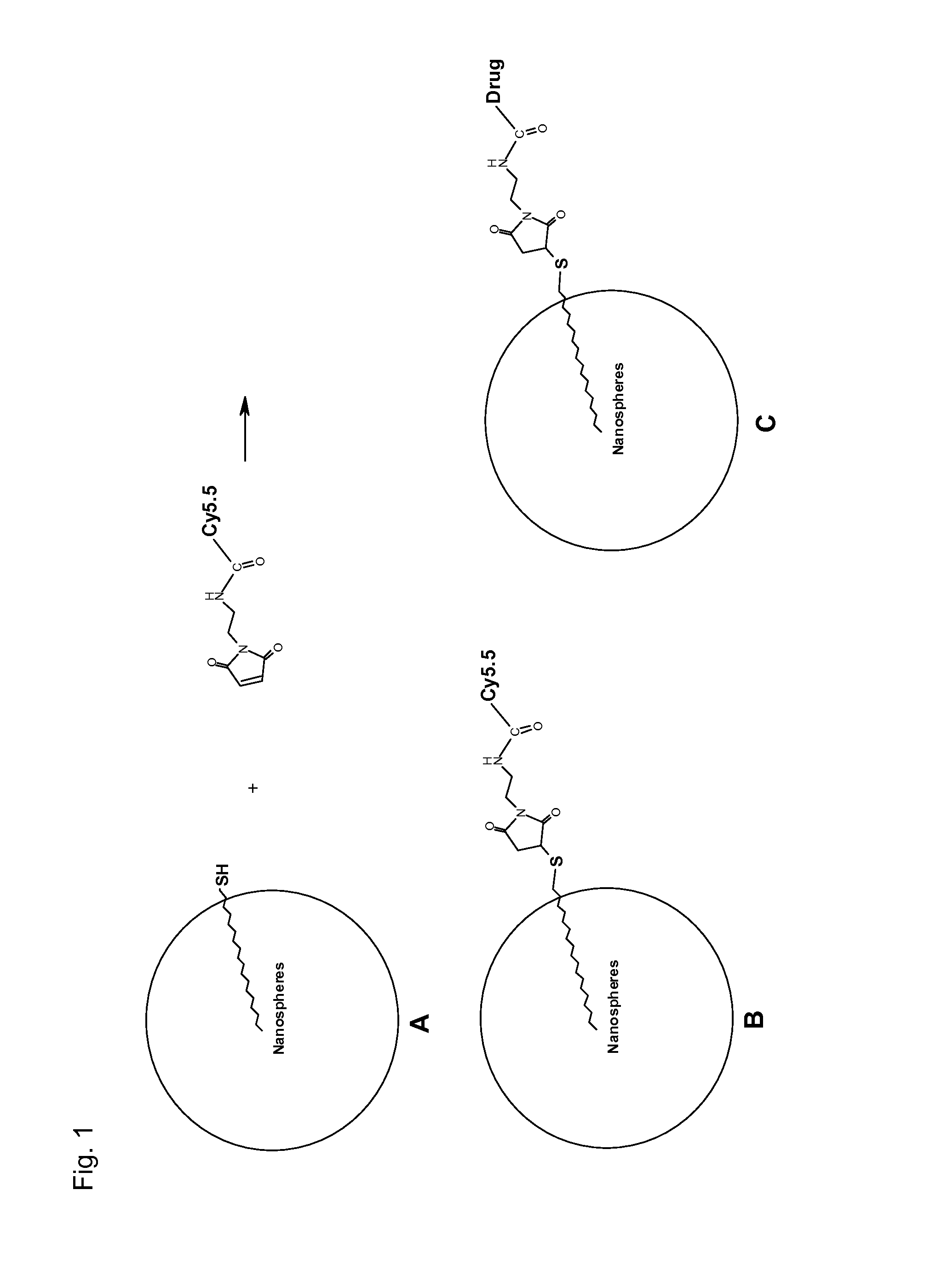
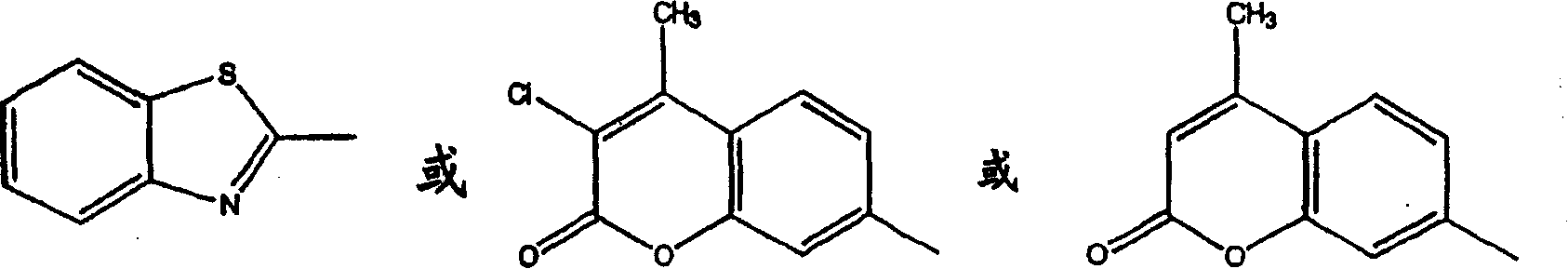
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
34 results about "Camptothecin Analog" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
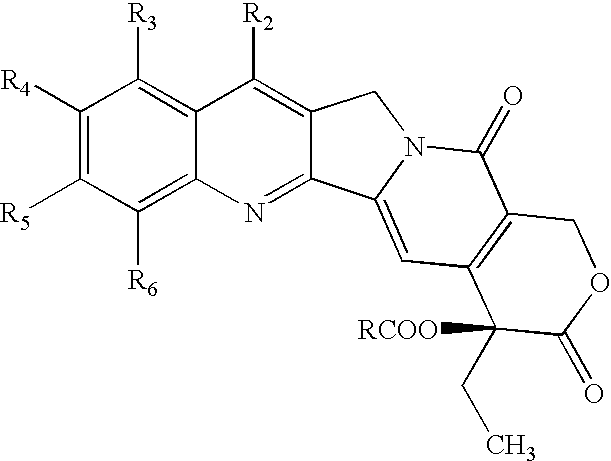
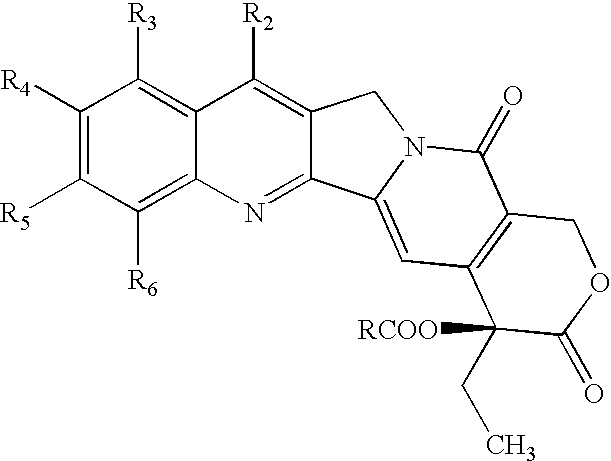
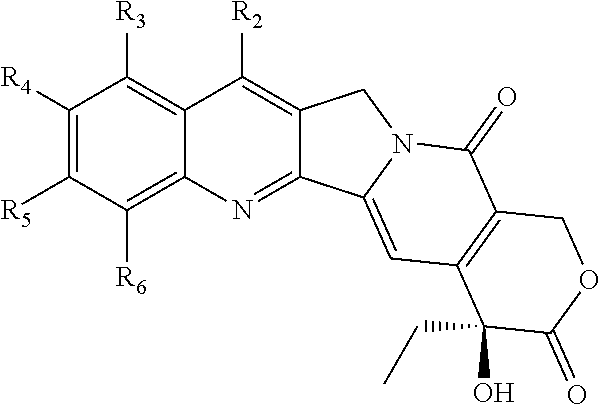
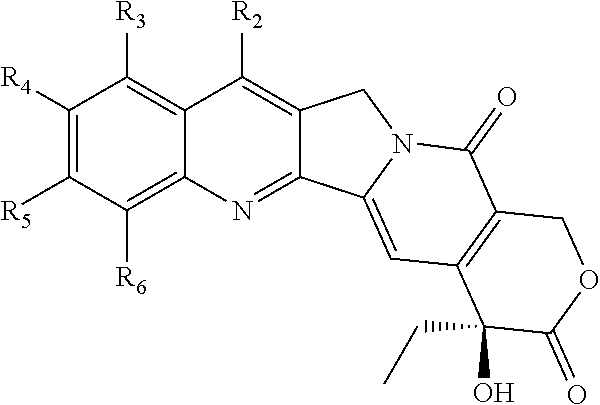
Nitrogen-based camptothecin derivatives
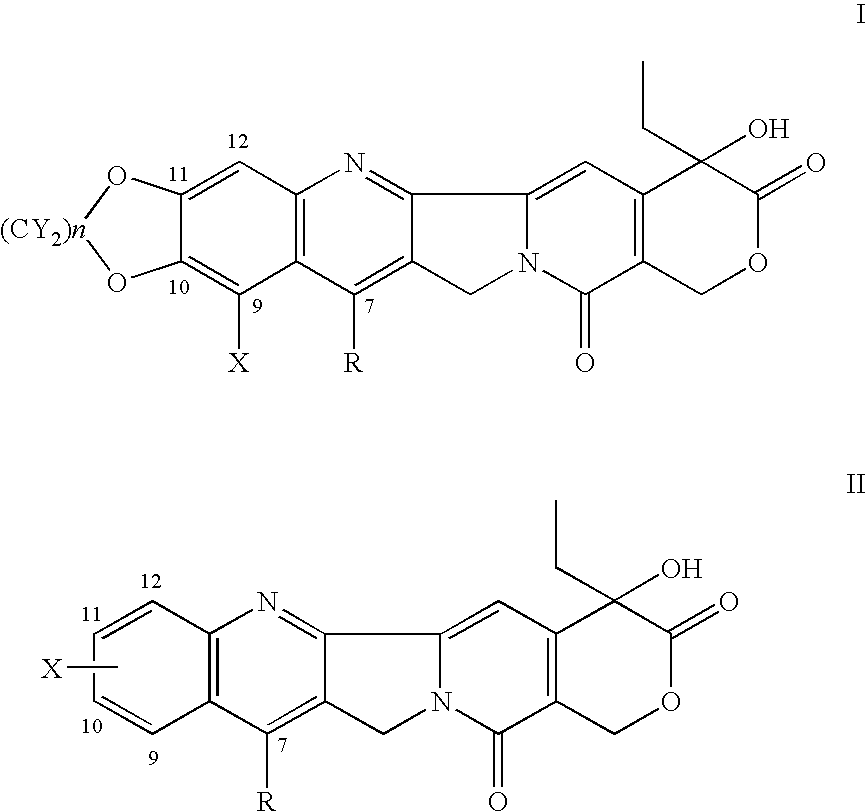
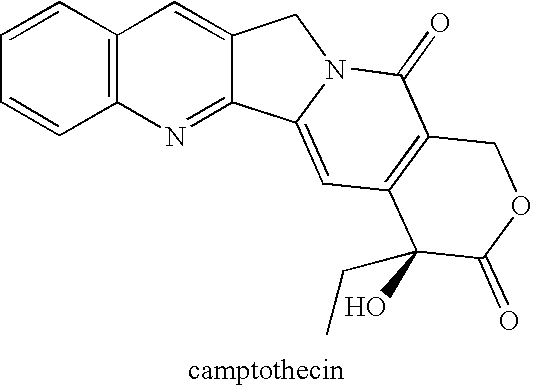
(20S) esters of camptothecin analogs are provided. The compounds are (20S) esters of an aminoalkanoic acid or an imidoalkanoic acid and camptothecin, which is optionally substituted at the 7, 9, 10, 11, and 12 positions of the camptothecin ring. The compounds are useful for treating cancer.
Owner:SUTTER WEST BAY HOSPITALS +1
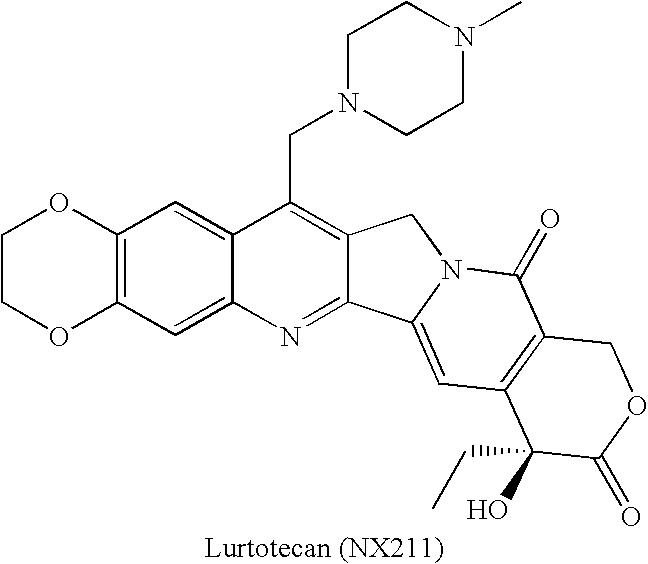
Novel Analogs of Camptothecin
The present invention provides novel conjugates of camptothecin and camptothecin analogs with a linker and an HSA-binding moiety. The novel conjugates are prodrug forms of the camptothecin or camptothecin analogs and can be used to treat mammalian cell proliferative diseases, such as cancer.
Owner:ZHUHAI BEIHAI BIOTECH CO LTD
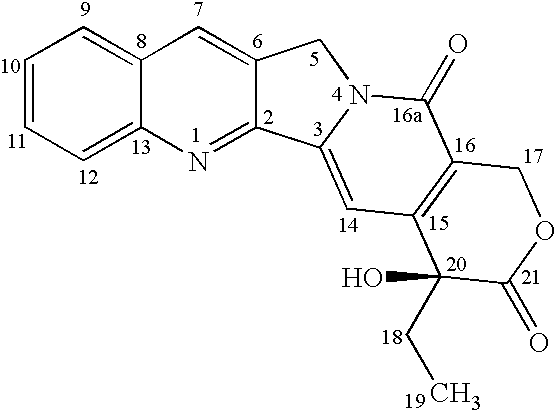
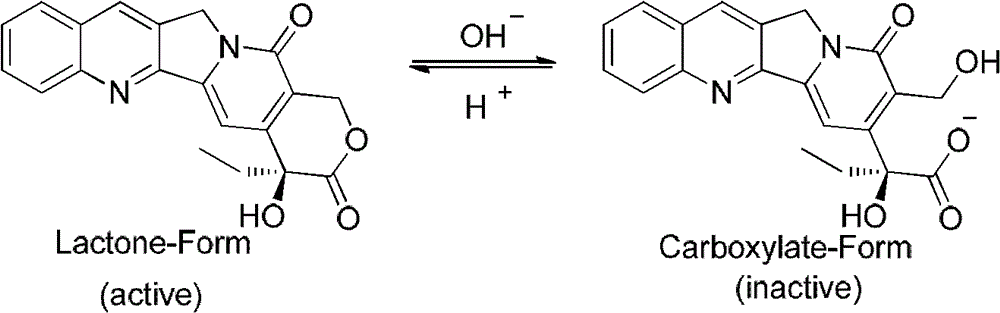
Camptothecin-analog with a novel, "flipped" lactone-stable, E-ring and methods for making and using same
InactiveUS20080261919A1Beneficial chemotherapeutic effectImprove lipophilicityBiocideGroup 8/9/10/18 element organic compoundsLactone formationChemistry
The present invention discloses: (i) a novel, lactone-stable, “flipped” E-ring camptothecin, pharmaceutically-acceptable salts, and / or analogs thereof; (ii) methods of synthesis of said novel, lactone-stable, “flipped” E-ring camptothecin, pharmaceutically-acceptable salts, and / or analogs thereof; (iii) pharmaceutically-acceptable formulations comprising said novel, lactone-stable, “flipped” E-ring camptothecin, pharmaceutically-acceptable salts, and / or analogs thereof, and, optionally, one or more additional chemotherapeutic agents; (iv) methods of administration of said novel, lactone-stable, “flipped” E-ring camptothecin, pharmaceutically-acceptable salts, and / or analogs thereof, and, optionally, one or more additional chemotherapeutic agents, to subjects in need thereof; and (v) devices for the administration of said novel, lactone-stable, “flipped” E-ring camptothecin, pharmaceutically-acceptable salts, and / or analogs thereof, and, optionally, one or more chemotherapeutic agents, to subjects in need thereof.
Owner:CROWN BIOSCIENCE INC
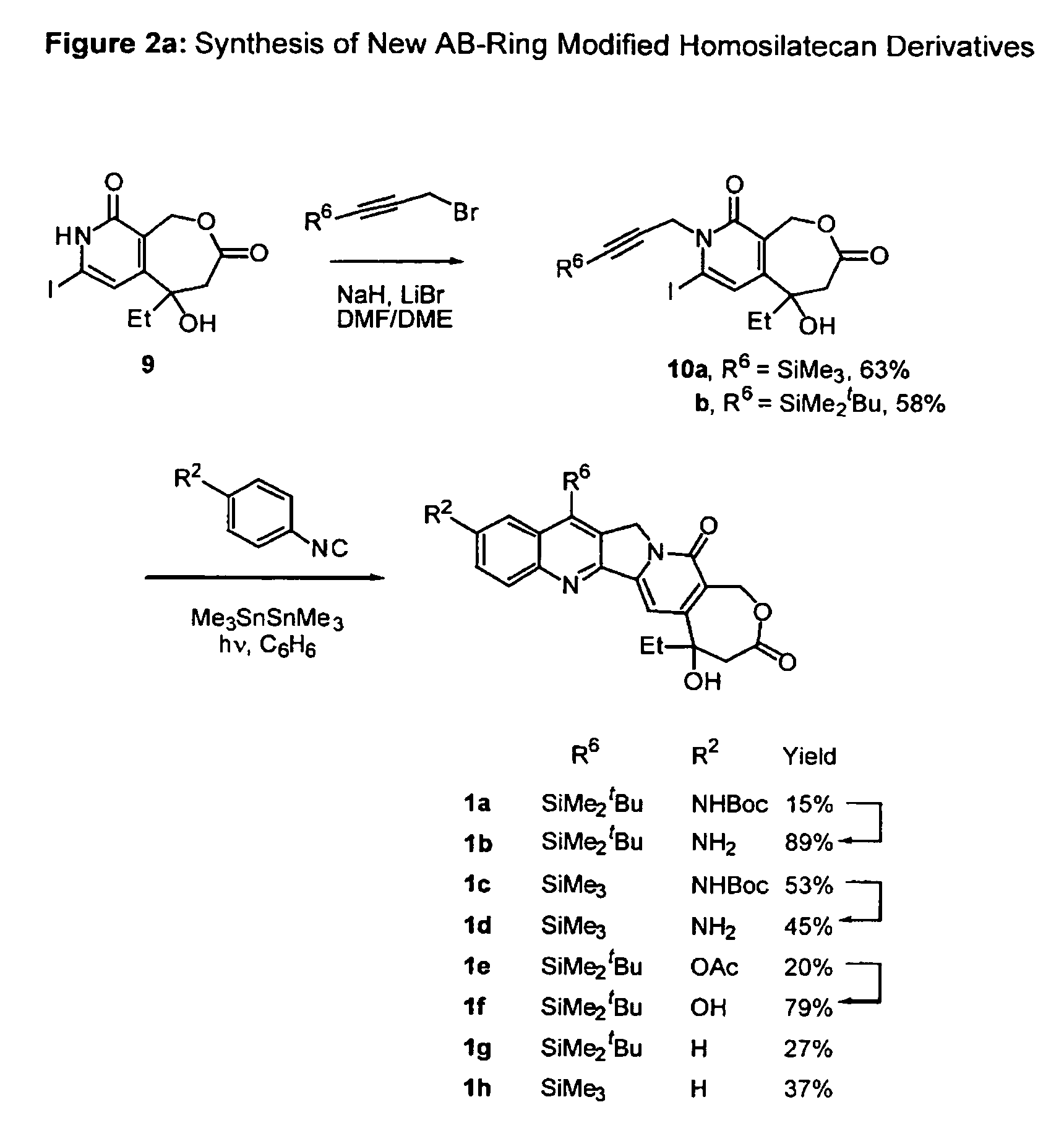
Nitrogen-based homo-camptothecin derivatives
(20) esters of camptothecin analogs are provided. The compounds are (20) esters of an aminoalkanoic acid or an imidoalkanoic acid and homocamptothecin, which is optionally substituted at the 7, 9, 10, 11, and 12 positions of the homocamptothecin ring. The compounds are useful for treating cancer.
Owner:CATHOLIC HEALTHCARE WEST ST JOSEPHS HOSPITAL +1
Soluble complexes of drug analogs and albumin
ActiveUS20150366984A1Organic active ingredientsPeptide/protein ingredientsWater solubleSerum albumin
The present invention provides novel, non-covalently bound complexes of serum albumin and analogs of poorly soluble drugs, such as camptothecin. The novel complexes are significantly more water-soluble than the camptothecin analogs and are useful as prodrug forms of the camptothecin analogs for the treatment of mammalian cell proliferative diseases, such as cancer.
Owner:ZHUHAI BEIHAI BIOTECH CO LTD
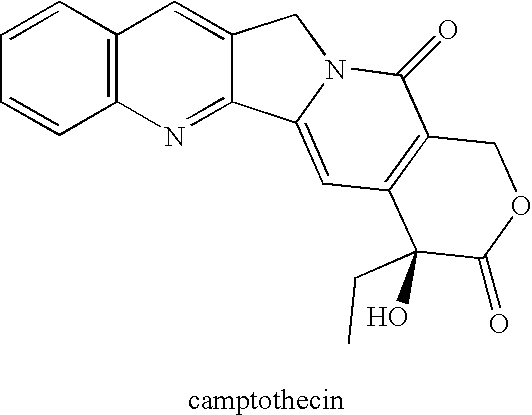
Analogs of camptothecin
Owner:ZHUHAI BEIHAI BIOTECH CO LTD
C7-substituted camptothecin analogs
InactiveUS7687496B2Highly lipophilicSubstantial lactone stabilitySilicon organic compoundsBiocideGlucuronidationTopoisomerase
The novel C7-modified camptothecin analogs, and pharmaceutically-acceptable salts thereof, of the present invention: (i) possess potent antitumor activity (i.e., in nanomolar or subnanomolar concentrations) for inhibiting the growth of human and animal tumor cells in vitro; (ii) are potent inhibition of Topoisomerase I; (iii) lack of susceptibility to MDR / MRP drug resistance; (iv) require no metabolic drug activation: (v) lack glucuronidation of the A-ring or B-ring; (vi) reduce drug-binding affinity to plasma proteins; (vii) maintain lactone stability; (viii) maintain drug potency; and (ix) possess a low molecular weight (e.g., MW<600).
Owner:CROWN BIOSCIENCE INC
C10-substituted camptothecin analogs
InactiveUS20090099166A1Highly lipophilicSubstantial lactone stabilitySilicon organic compoundsBiocidePharmacologyGlucuronidation
The novel C10-modified camptothecin analogs, and pharmaceutically-acceptable salts thereof, of the present invention: (i) possess potent antitumor activity (i.e., in nanomolar or subnanomolar concentrations) for inhibiting the growth of human and animal tumor cells in vitro; (ii) are potent inhibition of Topoisomerase I; (iii) lack of susceptibility to MDR / MRP drug resistance; (iv) require no metabolic drug activation: (v) lack glucuronidation of the A-ring or B-ring; (vi) reduce drug-binding affinity to plasma proteins; (vii) maintain lactone stability; (viii) maintain drug potency; and (ix) possess a low molecular weight (e.g., MW<600).
Owner:CROWN BIOSCIENCE INC
Antioxidant, neuroprotective and antineoplastic nanoparticles comprising a therapeutic agent on an amphiphilic spacer or an amphiphilic polymer
InactiveUS20140140931A1Reduce the possibilityLower cholesterol levelsUltrasonic/sonic/infrasonic diagnosticsPowder deliveryNanoparticleMedicine
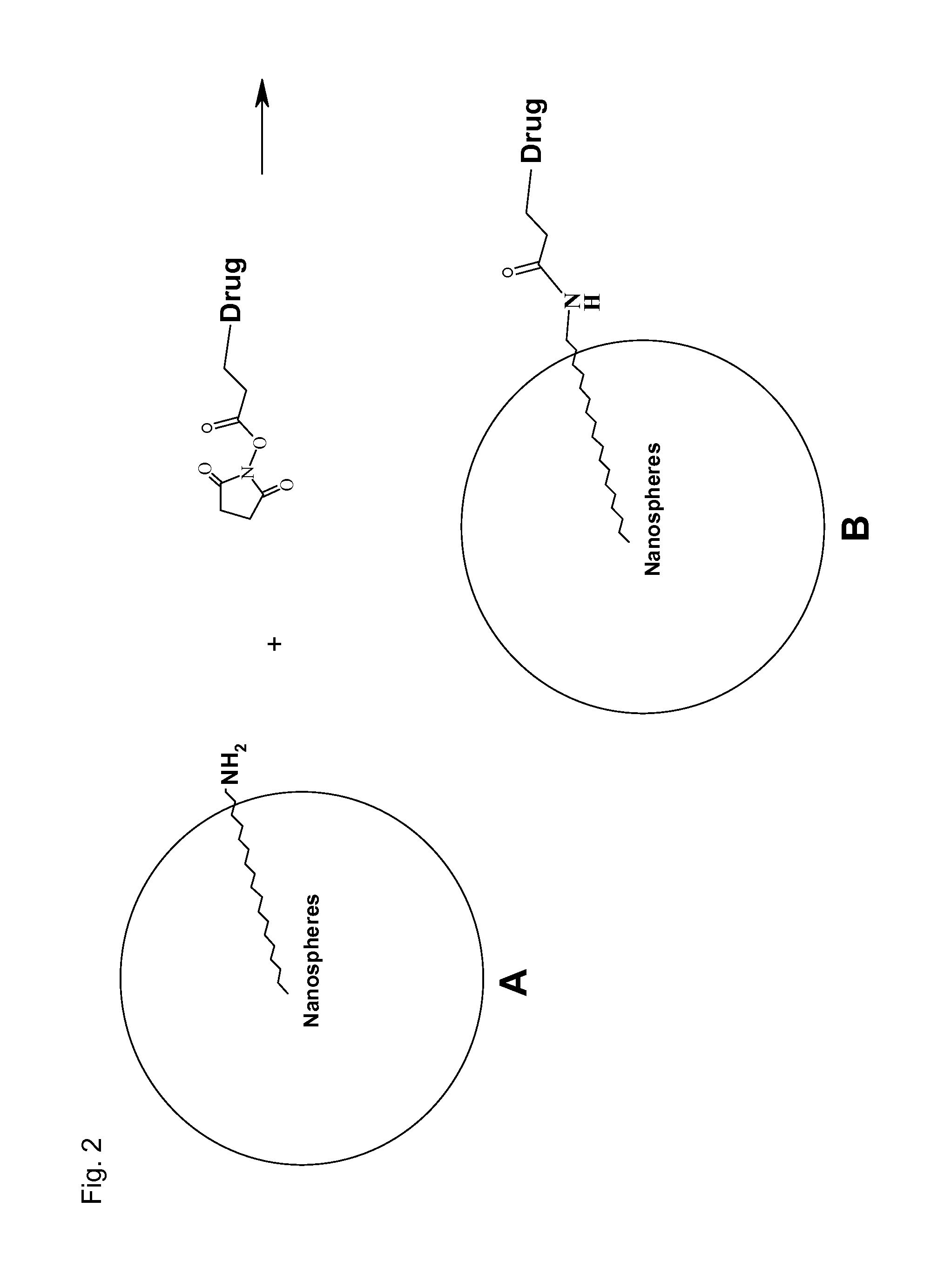
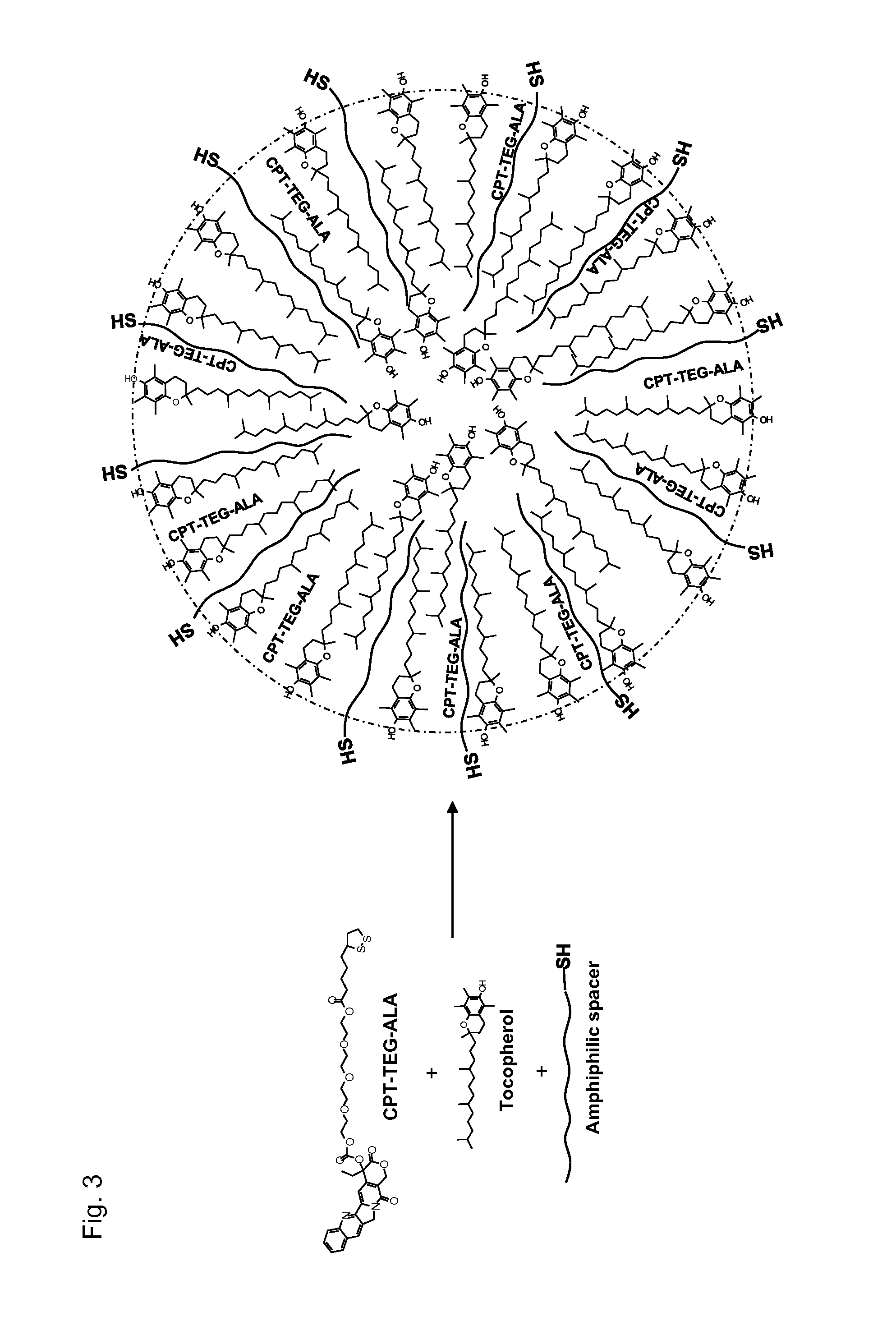
This invention relates to antioxidant, neuroprotective and antineoplastic nanoparticles comprising a therapeutic agent on an amphiphilic spacer or an amphiphilic polymer. Methods of synthesizing the antioxidant derivatives of camptothecin and anti-oxidant derivatives of camptothecin analogs, NSAIDs and statins, spontaneous emulsification or nanoprecipitation thereof to produce antioxidant, neuroprotective and anti-neoplastic nanoparticles comprising a therapeutic agent on an amphiphilic spacer or an amphiphilic polymer and their use in treating cancerous diseases are also provided. A further aspect of this invention is the use of these neuroprotective and anti-neoplastic nanoparticles for the preparation of delivery devices of other pharmaceuticals and / or drugs.
Owner:CEDARS SINAI MEDICAL CENT
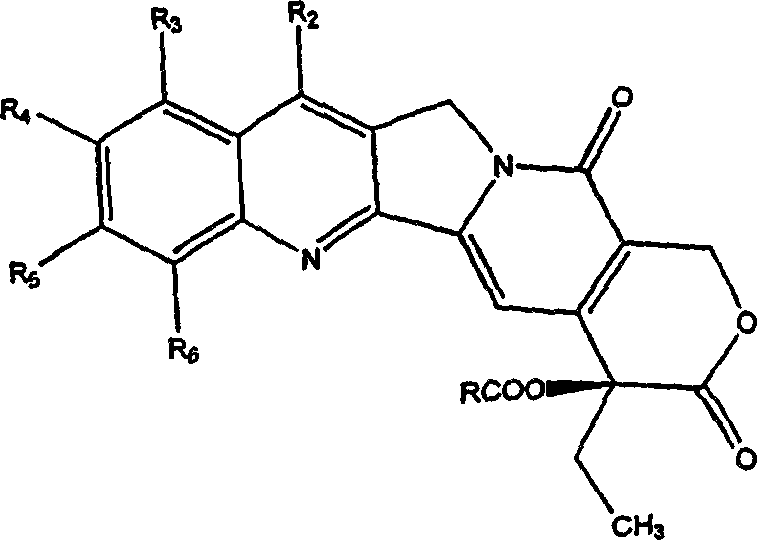
Camptothecin analogs and methods of preparation thereof
InactiveCN1352646AHighly lipophilicHigh activitySilicon organic compoundsSilicon compound active ingredientsArylHalogen
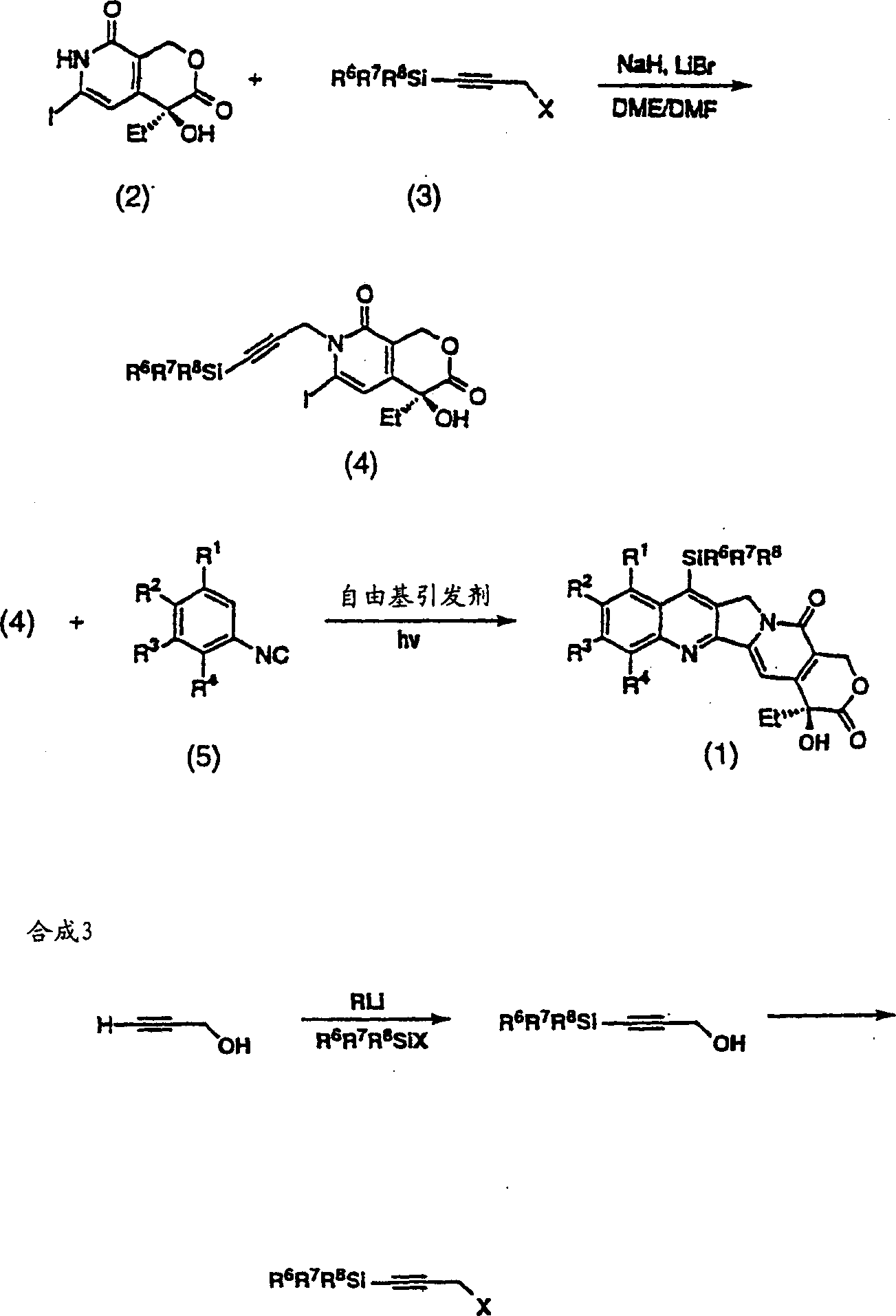
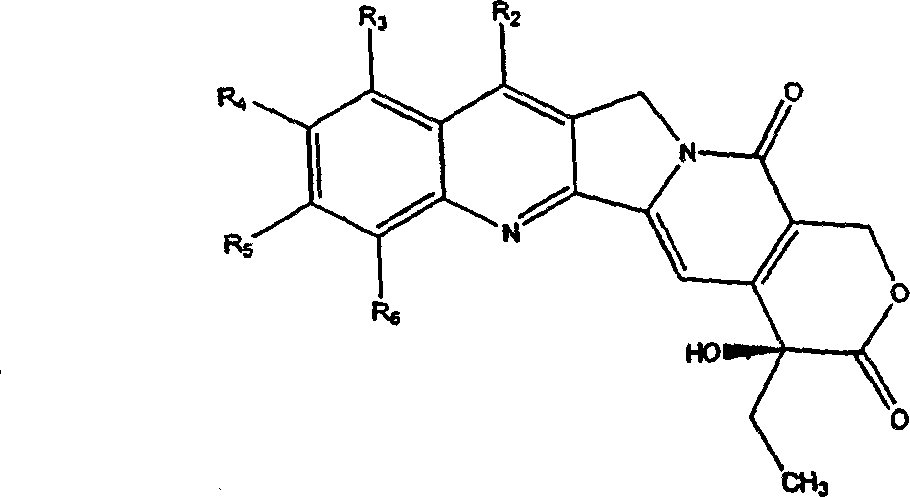
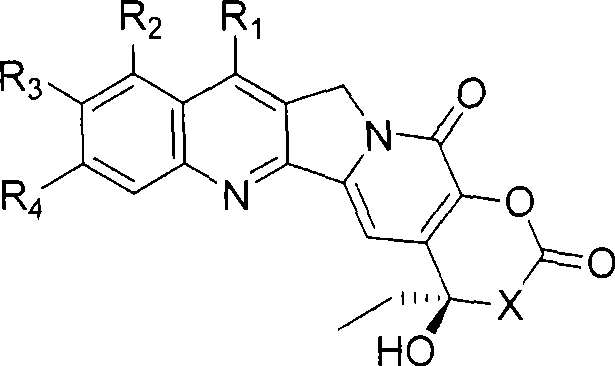
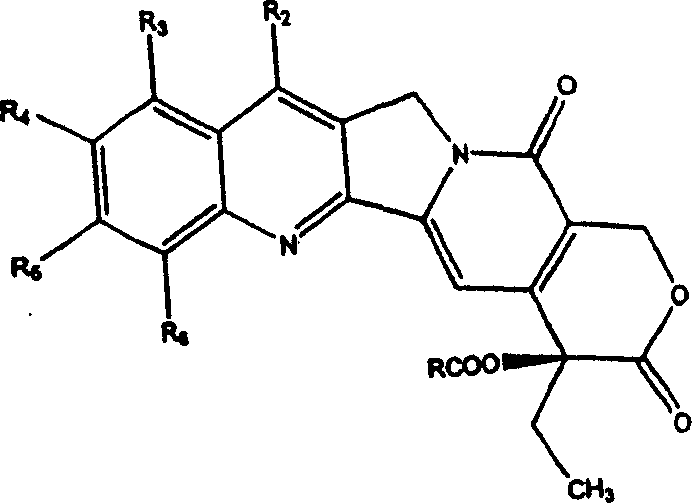
A compound of general formula (1) and pharmaceutically acceptable salts thereof and their preparation method: in the formula, R 1 and R 2 Each is the same or different, hydrogen, alkyl, alkenyl, benzyl, alkynyl, alkoxy, aryloxy, acyloxy, -OC(O)OR d , where R d Is alkyl, carbamoyloxy, halogen, hydroxyl, nitro, cyano, azido, formyl, hydrazino, acyl, amino, -SR c , where R c is hydrogen, acyl, alkyl or aryl, or R 1 and R 2 together to form the formula -O(CH 2 ) n O-group, where n represents an integer 1 or 2; R 3 is H, F, a halogen atom, nitro, amino, hydroxyl or cyano; or R 2 and R 3 can also be combined to form the formula -O(CH 2 ) n O-group, where n represents an integer 1 or 2; R 4 Is H, trialkylsilyl, F, Cl-3 alkyl, C2-3 alkenyl, C2-3 alkynyl, or C1-3 alkoxy; R 5 Is C1-10 alkyl, enbenzyl or propargyl; R 6 , R 7 and R 8 Each is C1-10 alkyl, C2-10 alkenyl, C2-10 alkynyl, aryl or -(CH 2 ) N R 9 group, where N is an integer ranging from 1 to 10, and R 9 is hydroxyl, alkoxy, amino, alkylamino, dialkylamino, halogen atom, cyano or nitro; R 11 is an alkylene or alkenylene group.
Owner:UNIVERSITY OF PITTSBURGH
7-Substituted camptothecin and camptothecin analogs and methods for producing the same
Methods of forming camptothecin compounds which are effective anti-tumor compounds are disclosed. These compounds inhibit the enzyme topoisomerase I and may alkylate DNA of the associated topoisomerase I-DNA cleavable complex.
Owner:RES TRIANGLE INST
Camptothecin analogs and methods of preparation thereof
The present invention provides generally a compound having the following general formula (1):wherein R1 and R2 are independently the same or different and are hydrogen, an alkyl group, an alkenyl group, a benzyl group, an alkynyl group, an alkoxyl group, an aryloxy group, an acyloxy group, a carbonyloxy group, a carbamoyloxy group, a halogen, a hydroxyl group, a nitro group, a cyano group, an azido group, a formyl group, a hydrazino group, an acyl group, an amino group, —SRc, wherein, Rc is hydrogen, an acyl group, an alkyl group, or an aryl group, or R1 and R2 together form a group of the formula —O(CH2)nO— wherein n represents the integer 1 or 2; R3 is H, F, a halogen atom, a nitro group, an amino group, a hydroxyl group, or a cyano group; or R2 and R3 together form a group of the formula —O(CH2)nO— wherein n represents the integer 1 or 2; R4 is H, F, a C1-3 alkyl group, a C2-3 alkenyl group, a C2-3 alkynyl group, or a C1-3 alkoxyl group; R5 is a C1-10 alkyl group, or a propargyl group; and R6, R7 and R8 are independently a C1-10 alkyl group, a C2-10 alkenyl group, a C2-10 alkynyl group, an aryl group or a —(CH2)NR9 group, wherein N is an integer within the range of 1 through 10 and R9 is a hydroxyl group, alkoxy group, an amino group, an alkylamino group, a dialkylamino group, a halogen atom, a cyano group or a nitro group; and pharmaceutically acceptable salts thereof.
Owner:UNIVERSITY OF PITTSBURGH
Antioxidant, neuroprotective and antineoplastic nanoparticles comprising a therapeutic agent on an amphiphilic spacer or an amphiphilic polymer
Owner:CEDARS SINAI MEDICAL CENT
C7- substituted camptothecin analogs
InactiveUS20090099224A1Substantial lactone stabilityLow affinityBiocideSilicon organic compoundsGlucuronidationTopoisomerase
The novel C7-modified camptothecin analogs, and pharmaceutically-acceptable salts thereof, of the present invention: (i) possess potent antitumor activity (i.e., in nanomolar or subnanomolar concentrations) for inhibiting the growth of human and animal tumor cells in vitro; (ii) are potent inhibition of Topoisomerase I; (iii) lack of susceptibility to MDR / MRP drug resistance; (iv) require no metabolic drug activation: (v) lack glucuronidation of the A-ring or B-ring; (vi) reduce drug-binding affinity to plasma proteins; (vii) maintain lactone stability; (viii) maintain drug potency; and (ix) possess a low molecular weight (e.g., MW<600).
Owner:CROWN BIOSCIENCE INC
Camptothecin derivatives
InactiveCN1553802AOrganic chemistry methodsAntineoplastic agentsAklanonic acidCamptothecin derivative
(20 S )esters of camptothecin analogs are provided. The compounds are (20 S ) esters of an oxyalkanoic acid and camptothecin, which is optionally substituted at the 7, 9, 10, 11, and 12 positions of the camptothecin ring. The compounds are useful for treating cancer.
Owner:CALIFORNIA PACIFIC MEDICAL CT +1
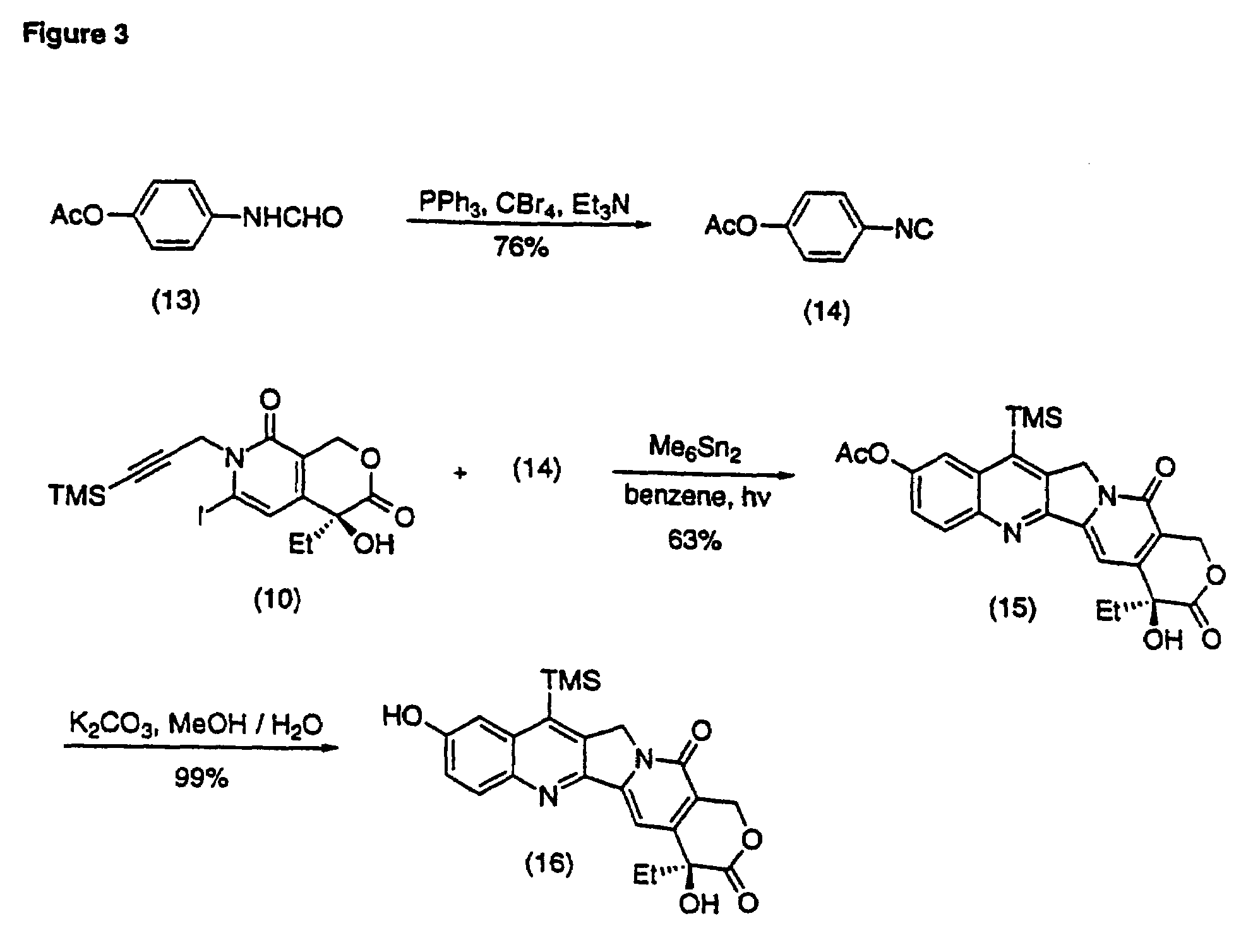
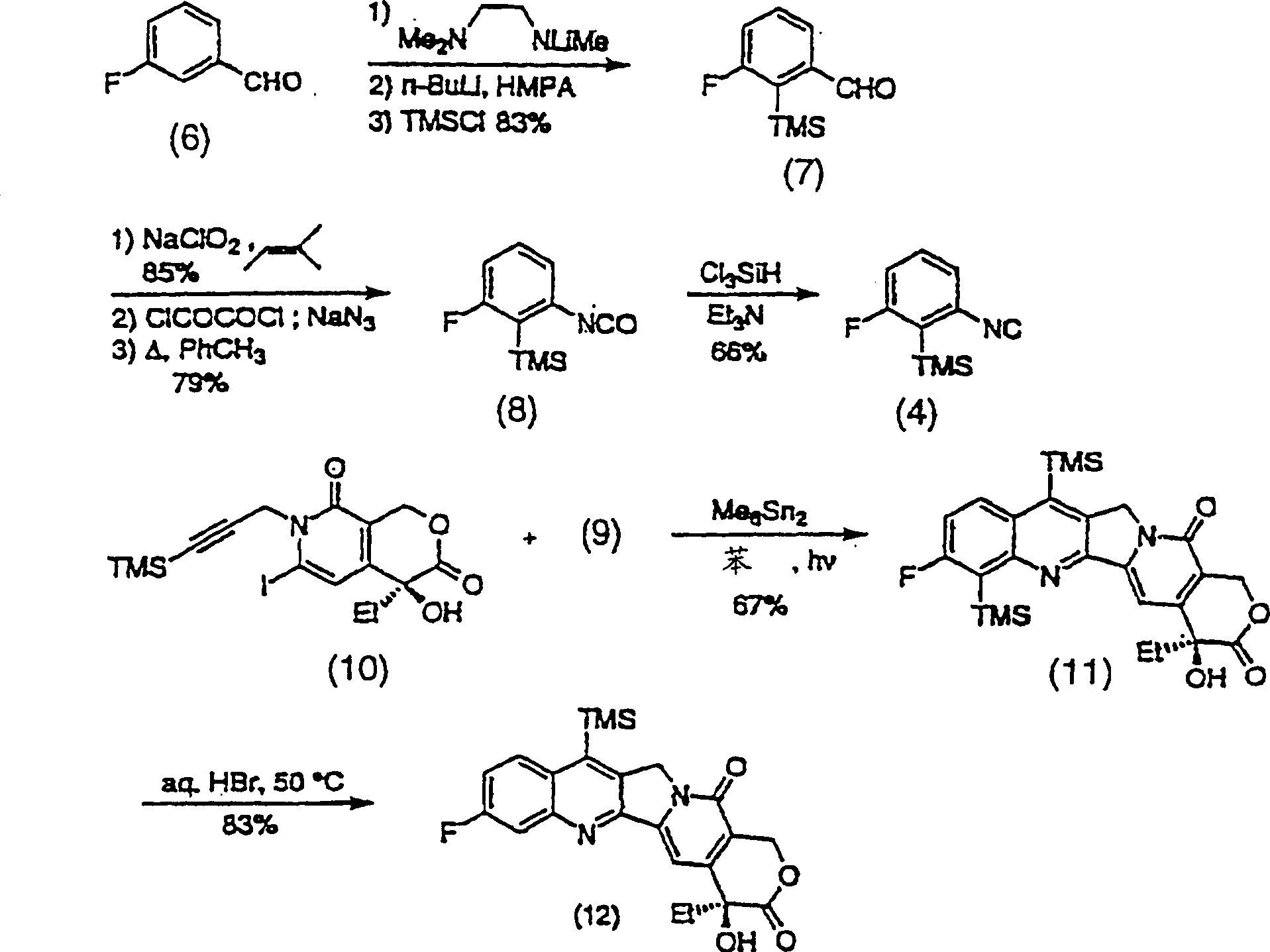
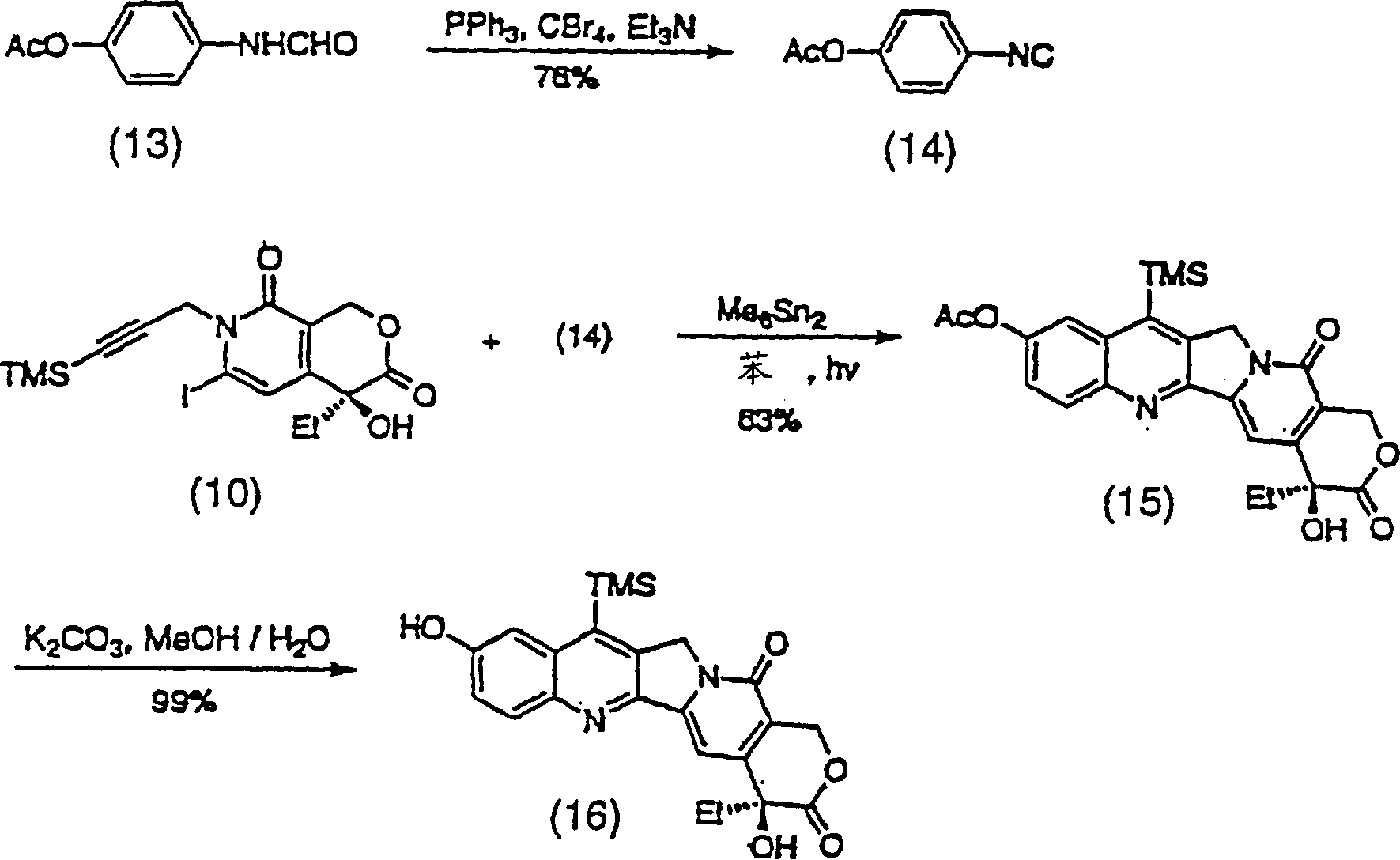
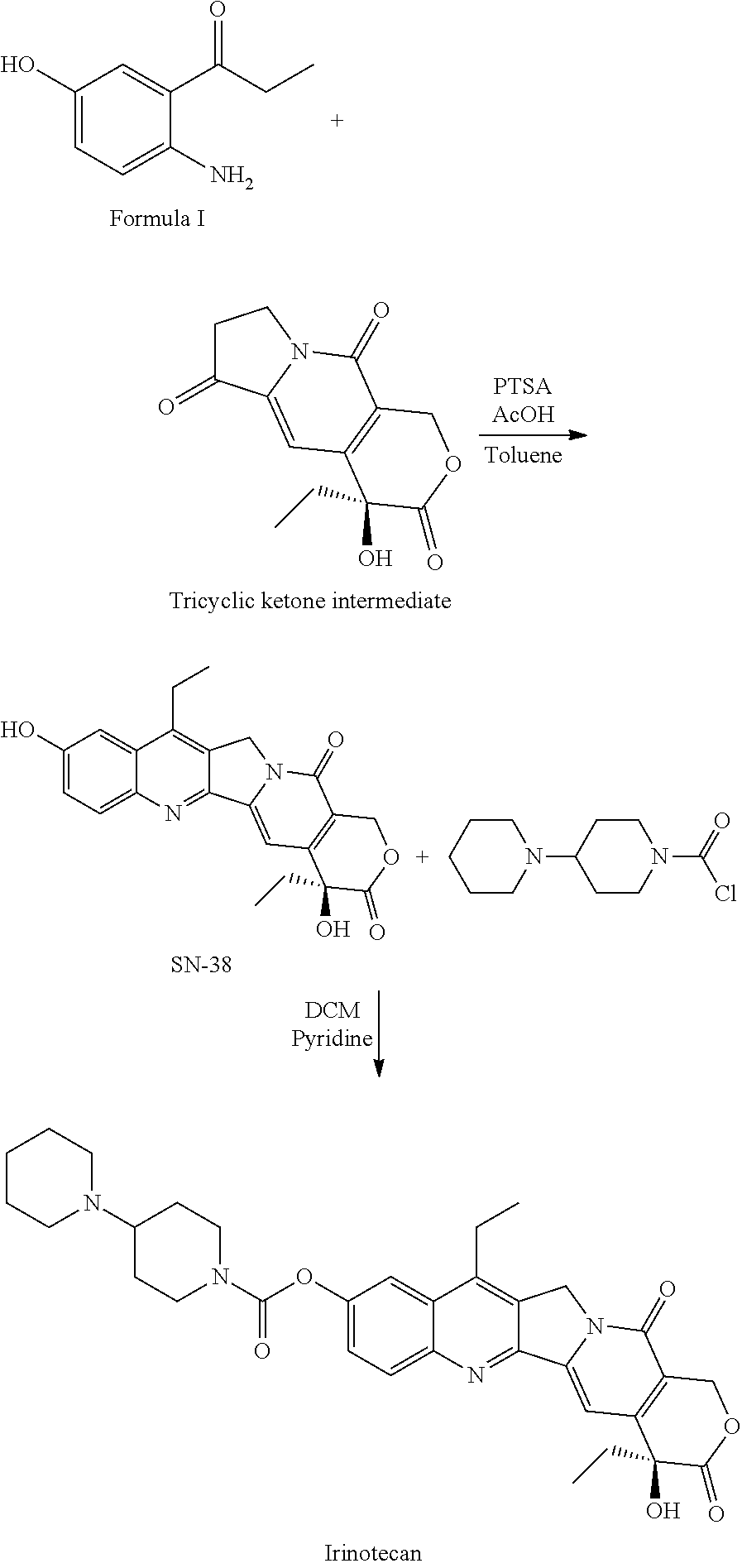
Method of synthesizing key intermediates for the production of camptothecin derivatives
ActiveUS7608740B2Easy to operatePractical utilityOrganic compound preparationHydroxy compound preparationCamptothecin derivativeMedicinal chemistry
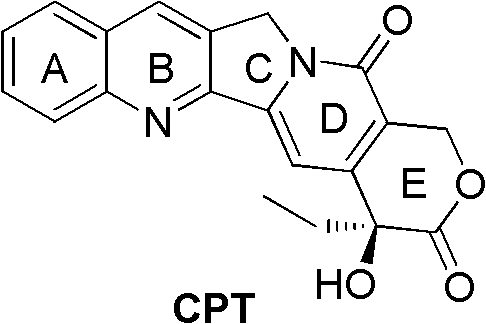
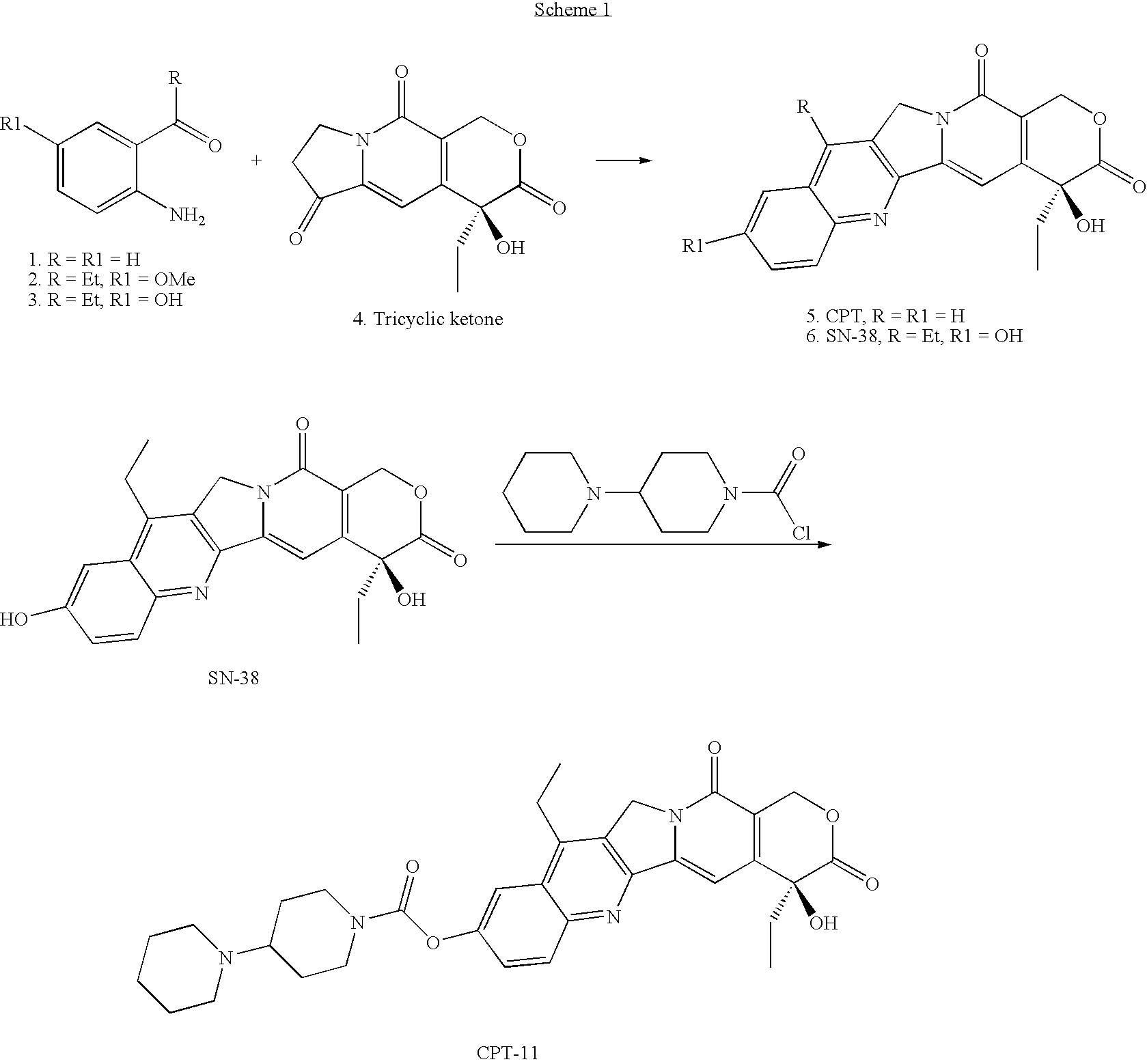
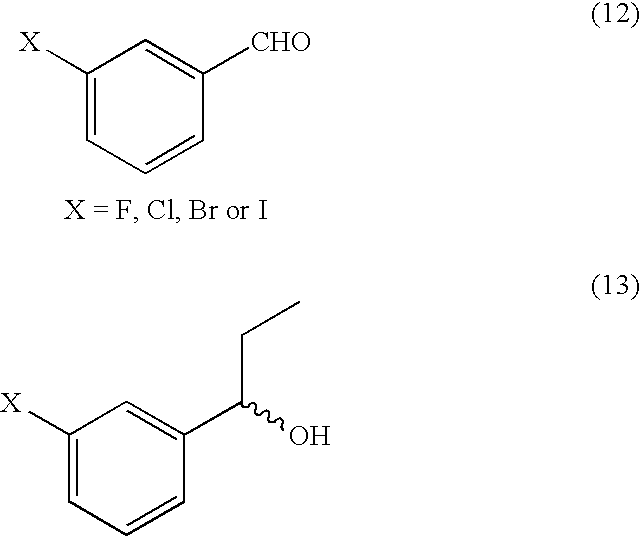
The present invention discloses a process for efficient production of 2-amino-5-hydroxypropiophenone corresponding to the AB ring part of camptothecin (CPT) skeleton, which is a key intermediate useful for the total synthesis of camptothecin analogs including 7-Ethyl-10-hydroxy camptothecin and novel intermediates thereof.
Owner:AVRA LAB PVT
Camptothecin analogs and methods of preparation thereof
InactiveUS7220860B2Promoting lactone interactionImprove stabilitySilicon organic compoundsOrganic active ingredientsArylChemical compound
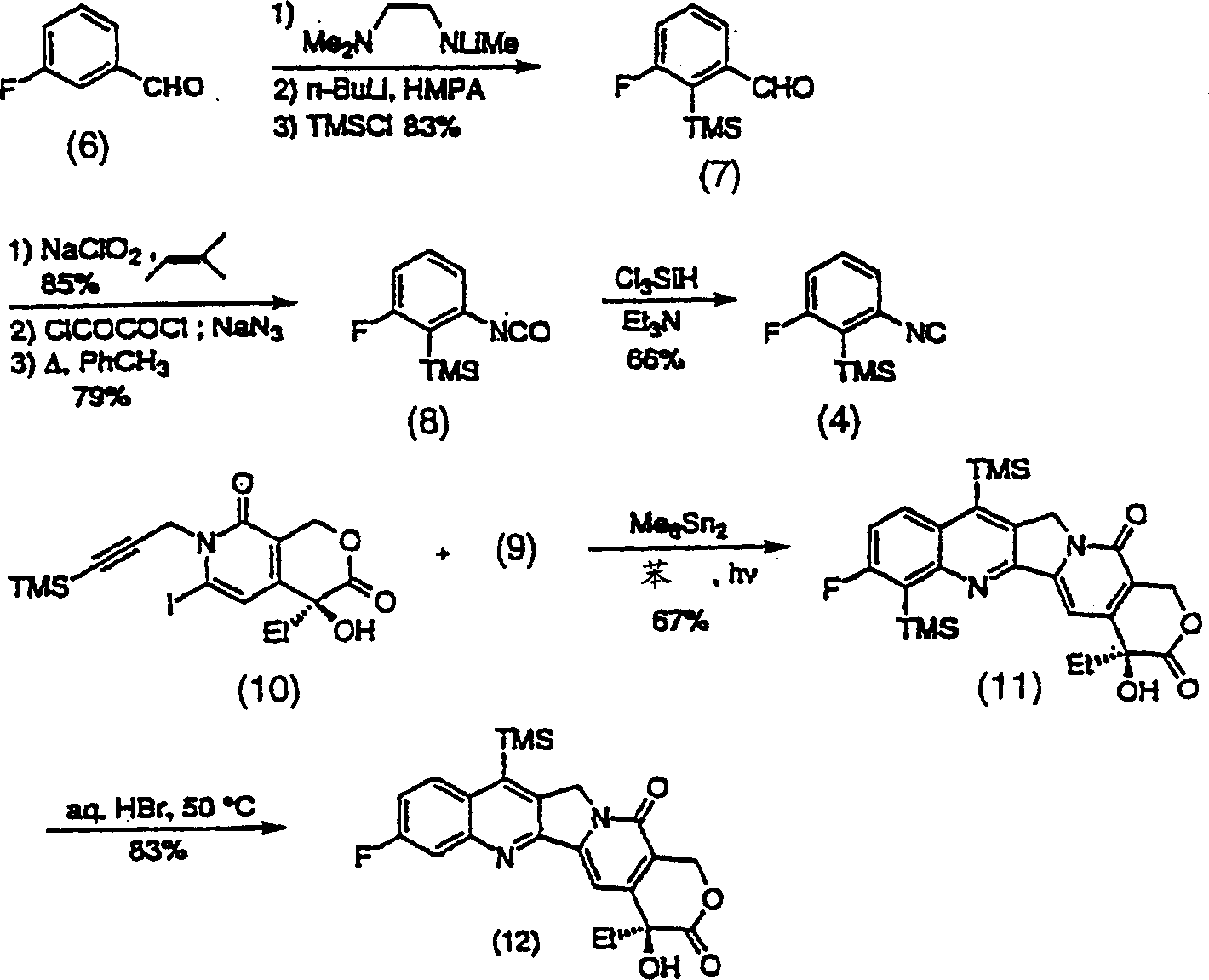
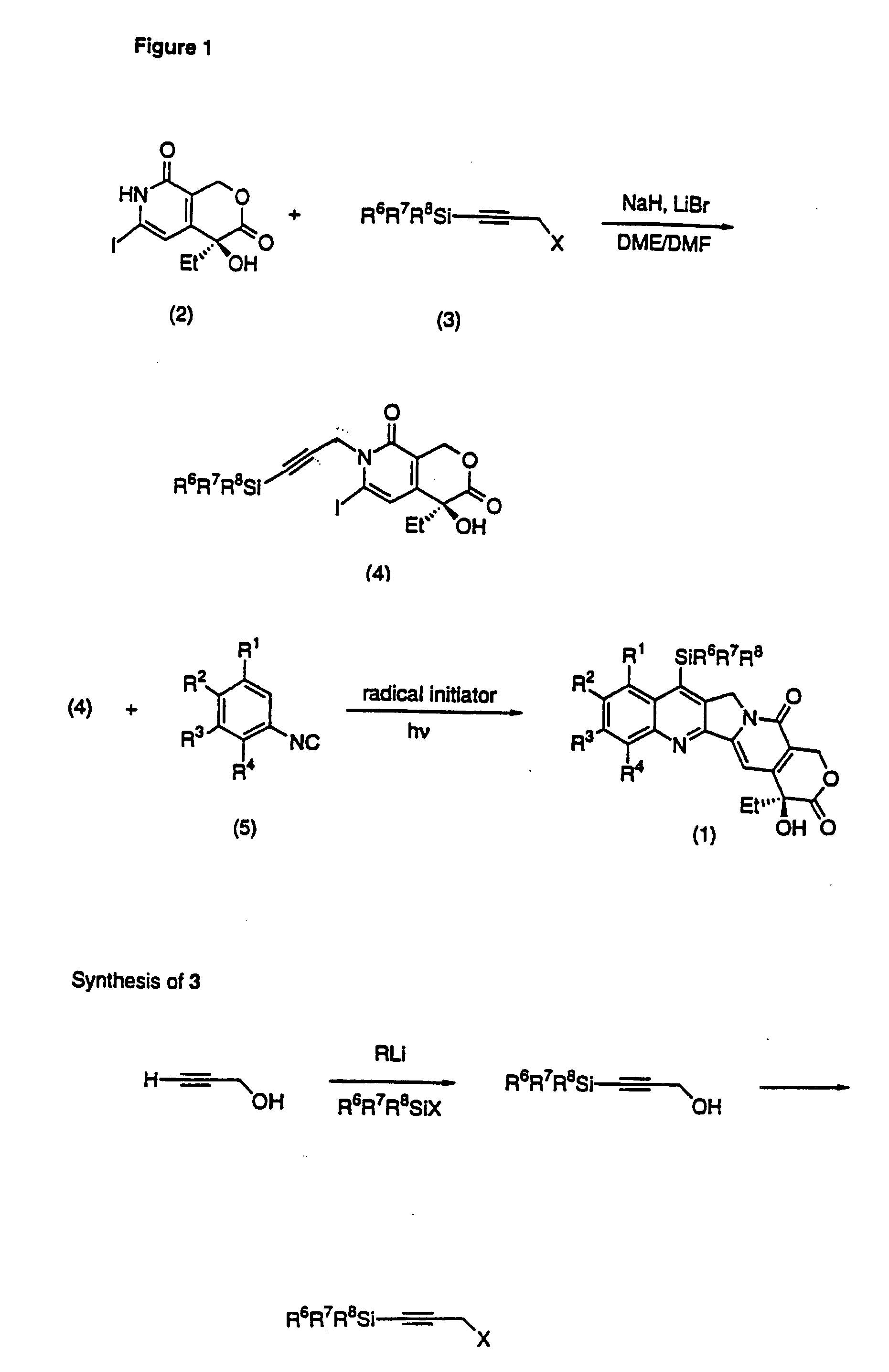
A compound has the formulain racemic form, enantiomerically enriched form or enantiomerically pure form. R6 is preferably —Si(R8R9R10) or —(R7)Si(R8R9R10), wherein R7 is an alkylene group, an alkenylene group, or an alkynylene group; and R8, R9 and R10 are independently a C1-10 alkyl group, a C2-10 alkenyl group, a C2-10 alkynyl group, an aryl group or a —(CH2)NR11 group, wherein N is an integer within the range of 1 through 10 and R11 is a hydroxy group, alkoxy group, an amino group, an alkylamino group, a dialkylamino group, a halogen atom, a cyano group, —SRc or a nitro group. R1–R4 can be broadly substituted. R5 is preferably a C1-10 alkyl group, an alkenyl group, an alkynyl group, or a benzyl group. R13 is preferably H, F or —CH3. R16 is R16 is —C(O)Rf or H. The E-ring (the lactone ring) may be opened. A method of synthesis of compound (1) and intermediates in the synthesis thereof are provided.
Owner:UNIV OF KENTUCKY RES FOUND +1
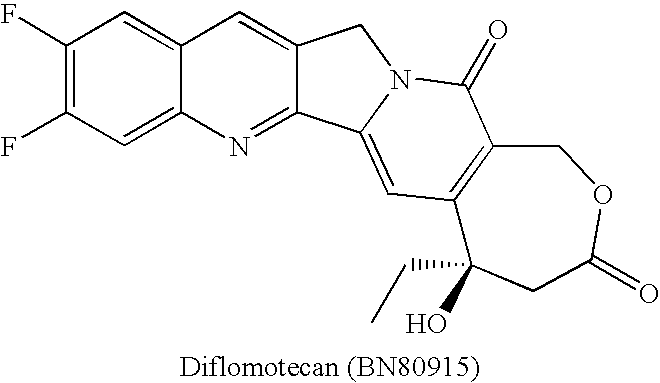
Fluorine-substituted e-ring camptothecin analogs and their use as medicines
InactiveCN103288842BHigh activityGood metabolic stabilityOrganic active ingredientsOrganic chemistryPharmaceutical drugMedicinal chemistry
The invention relates to the technical field of medicines and in particular relates to fluoro-substituted E cyclocamptothecin analogues and use thereof as a drug. The structure of the compounds is shown in a formula I; and the compounds exist in forms of raceme, diastereoisomer as well as any mixture of the forms or medical salts thereof. The compounds disclosed by the invention have the effect of restraining the activity of the topoisomerase I and can be used for preparing anti-tumor drugs and also can be used for preparing drugs for preventing virus and fungal infection.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Nanospheres comprising tocopherol, an amphiphilic spacer and a therapeutic or imaging agent
InactiveUS20140105822A1Lower cholesterol levelsReduce the possibilityCompounds screening/testingOrganic active ingredientsDiseaseImaging agent
This invention relates to a nanosphere comprising tocopherol, an amphiphilic spacer and a therapeutic agent, an imaging agent, a hydrophobic antioxidant, a hydrophobic nonsteroidal anti-inflammatory drug (NSAID) derivative, a hydrophobic antioxidant and anti-inflammatory derivative of a nonsteroidal anti-inflammatory drug (NSAID), a statin lactone derivative, an antioxidant derivative of camptothecin or camptothecin analog, or a combination thereof. Methods of synthesizing the nanospheres and their use in treating, detecting or diagnosing diseases are also provided.
Owner:CEDARS SINAI MEDICAL CENT
Camptothecin-analog with a novel, flipped lactone-stable, E-ring and methods for making and using same
The present invention discloses: (i) a novel, lactone-stable, ''flipped'' E-ring camptothecin, pharmaceutically-acceptable salts, and / or analogs thereof; (ii) methods of synthesis of said novel, lactone-stable, ''flipped'' E-ring camptothecin, pharmaceutically-acceptable salts, and / or analogs thereof; (iii) pharmaceutically-acceptable formulations comprising said novel, lactone-stable, ''flipped'' E-ring camptothecin, pharmaceutically-acceptable salts, and / or analogs thereof, and, optionally, one or more additional chemotherapeutic agents; (iv) methods of administration of said novel, lactone-stable, ''flipped'' E-ring camptothecin, pharmaceutically-acceptable salts, and / or analogs thereof, and, optionally, one or more additional chemotherapeutic agents, to subjects in need thereof; and (v) devices for the administration of said novel, lactone-stable, ''flipped'' E-ring camptothecin, pharmaceutically-acceptable salts, and / or analogs thereof, and, optionally, one or more chemotherapeutic agents, to subjects in need thereof.
Owner:BIONUMERIK PHARMA INC
Method for determining predisposition to a physiological reaction in a patient
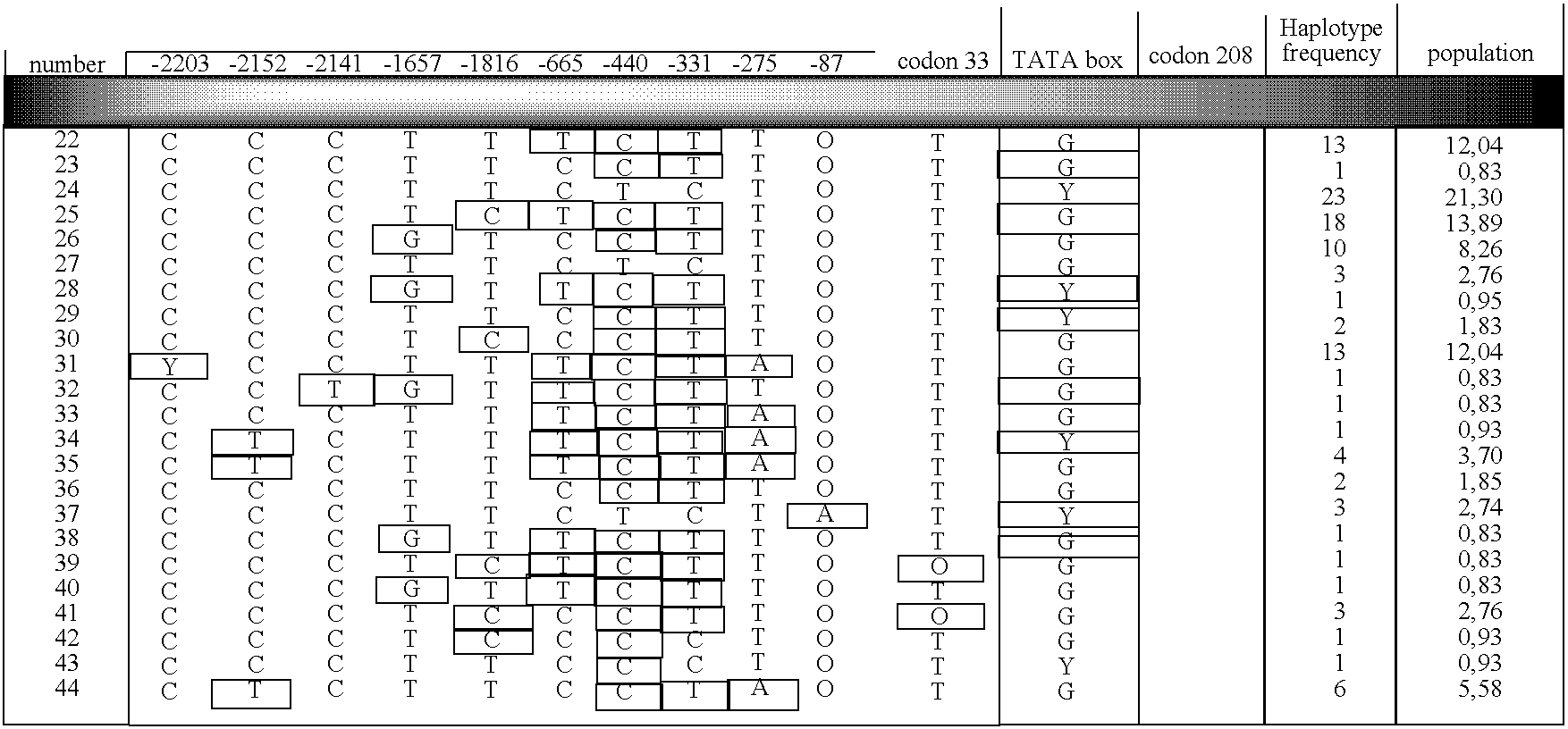
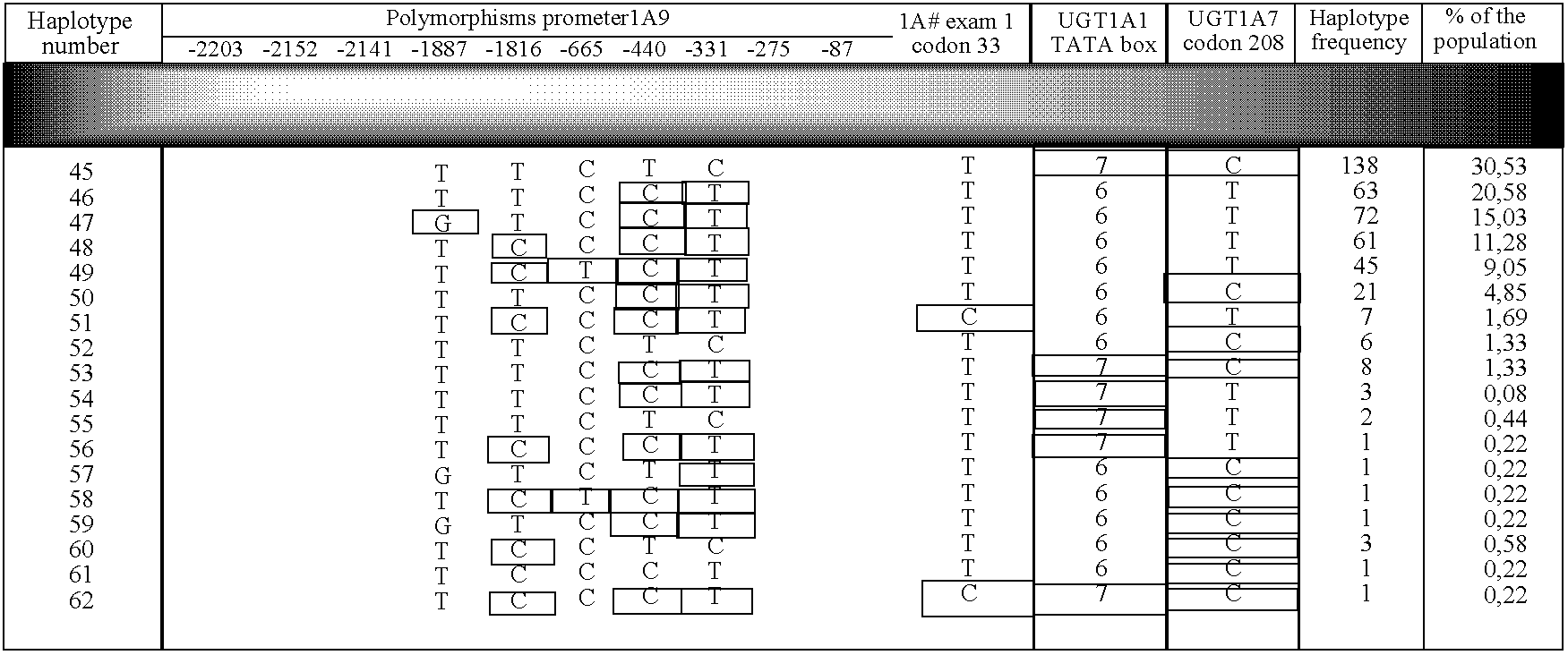
InactiveUS20060183119A1Significant positive effectMicrobiological testing/measurementMycophenolic acidHaplotype
The present invention relates to a method for determining predisposition to a physiological reaction in a patient. Particularly, the present invention relates to a method for determining a predisposition to toxicity induced by a camptothecin analog or to an immunosuppressive mycophenolic acid-based therapy. This method comprises the characterization of nucleic acid sequences from the patient. The nucleic acid sequence encodes for an amino acid sequence or regulates the expression of UGT1A1, UGT1A7, UGT1A9 or their polymorphic variants. The method also comprises the analysis of haplotypic variation within these genes.
Owner:UNIV LAVAL
Method of Synthesizing Key Intermediates for the Production of Camptothecin Derivatives
ActiveUS20080221358A1Easy to operatePractical utilityOrganic compound preparationHydroxy compound preparationCamptothecin derivativeMedicinal chemistry
The present invention discloses a process for efficient production of 2-amino-5-hydroxypropiophenone corresponding to the AB ring part of camptothecin (CPT) skeleton, which is a key intermediate useful for the total synthesis of camptothecin analogs including 7-Ethyl-10-hydroxy camptothecin and novel intermediates thereof.
Owner:AVRA LAB PVT
Camptothecin analogs and methods of preparation thereof
InactiveUS20050014775A1Decrease preferential carboxylate over lactone bindingImprove lipophilicityBiocideSilicon organic compoundsArylCombinatorial chemistry
A compound has the formula in racemic form, enantiomerically enriched form or enantiomerically pure form. R6 is preferably —Si(R8R9R10) or —(R7)Si(R8R9R10), wherein R7 is an alkylene group, an alkenylene group, or an alkynylene group; and R8, R9 and R10 are independently a C1-10 alkyl group, a C2-10 alkenyl group, a C2-10 alkynyl group, an aryl group or a —(CH2)NR11 group, wherein N is an integer within the range of 1 through 10 and R11 is a hydroxy group, alkoxy group, an amino group, an alkylamino group, a dialkylamino group, a halogen atom, a cyano group, —SRc or a nitro group. R1-R4 can be broadly substituted. R5 is preferably a C1-10 alkyl group, an alkenyl group, an alkynyl group, or a benzyl group. R13 is preferably H, F or —CH3. R16 is R16 is —C(O)Rf or H. The E-ring (the lactone ring) may be opened. A method of synthesis of compound (1) and intermediates in the synthesis thereof are provided.
Owner:UNIV OF KENTUCKY RES FOUND +1
Camptothecin analogs and methods of preparation thereof
The present invention provides generally a compound having the following general formula (1): wherein R1 and R2 are independently the same or different and are hydrogen, an alkyl group, an alkenyl group, a benzyl group, an alkynyl group, an alkoxyl group, an aryloxy group, an acyloxy group, a carbonyloxy group, a carbamoyloxy group, a halogen, a hydroxyl group, a nitro group, a cyano group, an azido group, a formyl group, a hydrazino group, an acyl group, an amino group, —SRc, wherein, Rc is hydrogen, an acyl group, an alkyl group, or an aryl group, or R1 and R2 together form a group of the formula —O(CH2)nO— wherein n represents the integer 1 or 2; R3 is H, F, a halogen atom, a nitro group, an amino group, a hydroxyl group, or a cyano group; or R2 and R3 together form a group of the formula —O(CH2)nO— wherein n represents the integer 1 or 2; R4 is H, F, a C1-3 alkyl group, a C2-3 alkenyl group, a C2-3 alkynyl group, or a C1-3 alkoxyl group; R5 is a C1-10 alkyl group, or a propargyl group; and R6, R7 and R8 are independently a C1-10 alkyl group, a C2-10 alkenyl group, a C2-10 alkynyl group, an aryl group or a —(CH2)NR9 group, wherein N is an integer within the range of 1 through 10 and R9 is a hydroxyl group, alkoxy group, an amino group, an alkylamino group, a dialkylamino group, a halogen atom, a cyano group or a nitro group; and pharmaceutically acceptable salts thereof.
Owner:UNIVERSITY OF PITTSBURGH
C10-substituted camptothecin analogs
InactiveUS7687497B2Highly lipophilicSubstantial lactone stabilityBiocideSilicon organic compoundsDNA underwindingGlucuronide metabolism
The novel C10-modified camptothecin analogs, and pharmaceutically-acceptable salts thereof, of the present invention: (i) possess potent antitumor activity (i.e., in nanomolar or subnanomolar concentrations) for inhibiting the growth of human and animal tumor cells in vitro; (ii) are potent inhibition of Topoisomerase I; (iii) lack of susceptibility to MDR / MRP drug resistance; (iv) require no metabolic drug activation: (v) lack glucuronidation of the A-ring or B-ring; (vi) reduce drug-binding affinity to plasma proteins; (vii) maintain lactone stability; (viii) maintain drug potency; and (ix) possess a low molecular weight (e.g., MW<600).
Owner:CROWN BIOSCIENCE INC
Camptothecin analogs and methods of preparation thereof
Owner:UNIVERSITY OF PITTSBURGH
Process for preparation of 2-Amino-5-hydroxy propiophenone
ActiveUS11434196B2Organic compound preparationPreparation from phosgene or haloformatesEthyl groupCombinatorial chemistry
The present invention relates to a process for preparation of 2-Amino-5-hydroxy propiophenone, a key intermediate for the synthesis of camptothecin analogs including 7-Ethyl-10-hydroxycamptothecin (SN-38).
Owner:LAURUS LABS
Camptothecin derivatives
(20S) esters of camptothecin analogs are provided. The compounds are (20S) esters of an oxyalkanoic acid and camptothecin, which isare optionally substituted at the 7, 9, 10, 11, and 12 positions of the camptothecin ring. The compounds are useful for treating cancer.
Owner:SUTTER WEST BAY HOSPITALS +1
Camptothecin derivatives
InactiveCN100406013COrganic chemistry methodsAntineoplastic agentsAklanonic acidCamptothecin derivative
(20 S )esters of camptothecin analogs are provided. The compounds are (20 S ) esters of an oxyalkanoic acid and camptothecin, which is optionally substituted at the 7, 9, 10, 11, and 12 positions of the camptothecin ring. The compounds are useful for treating cancer.
Owner:CALIFORNIA PACIFIC MEDICAL CT +1
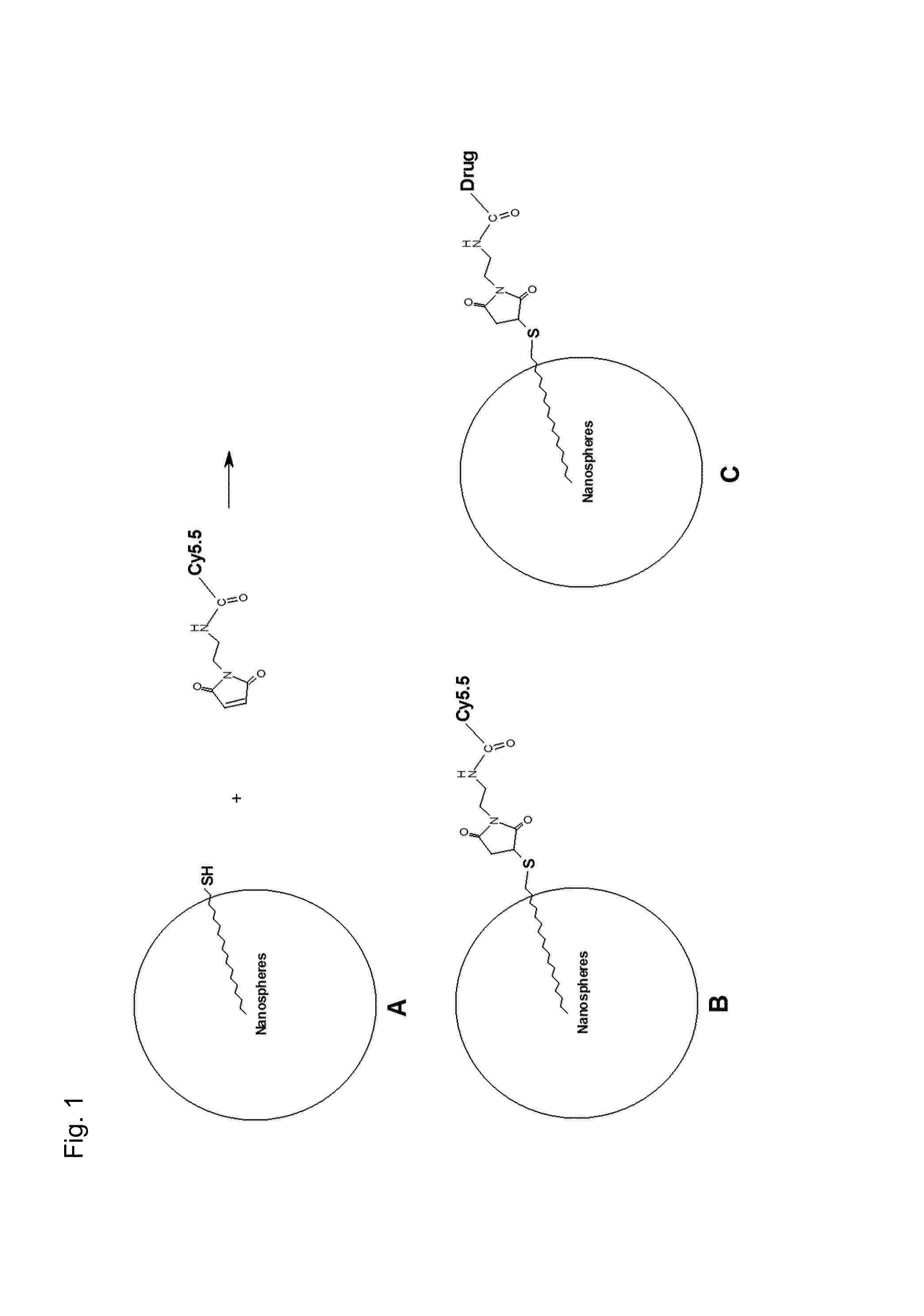
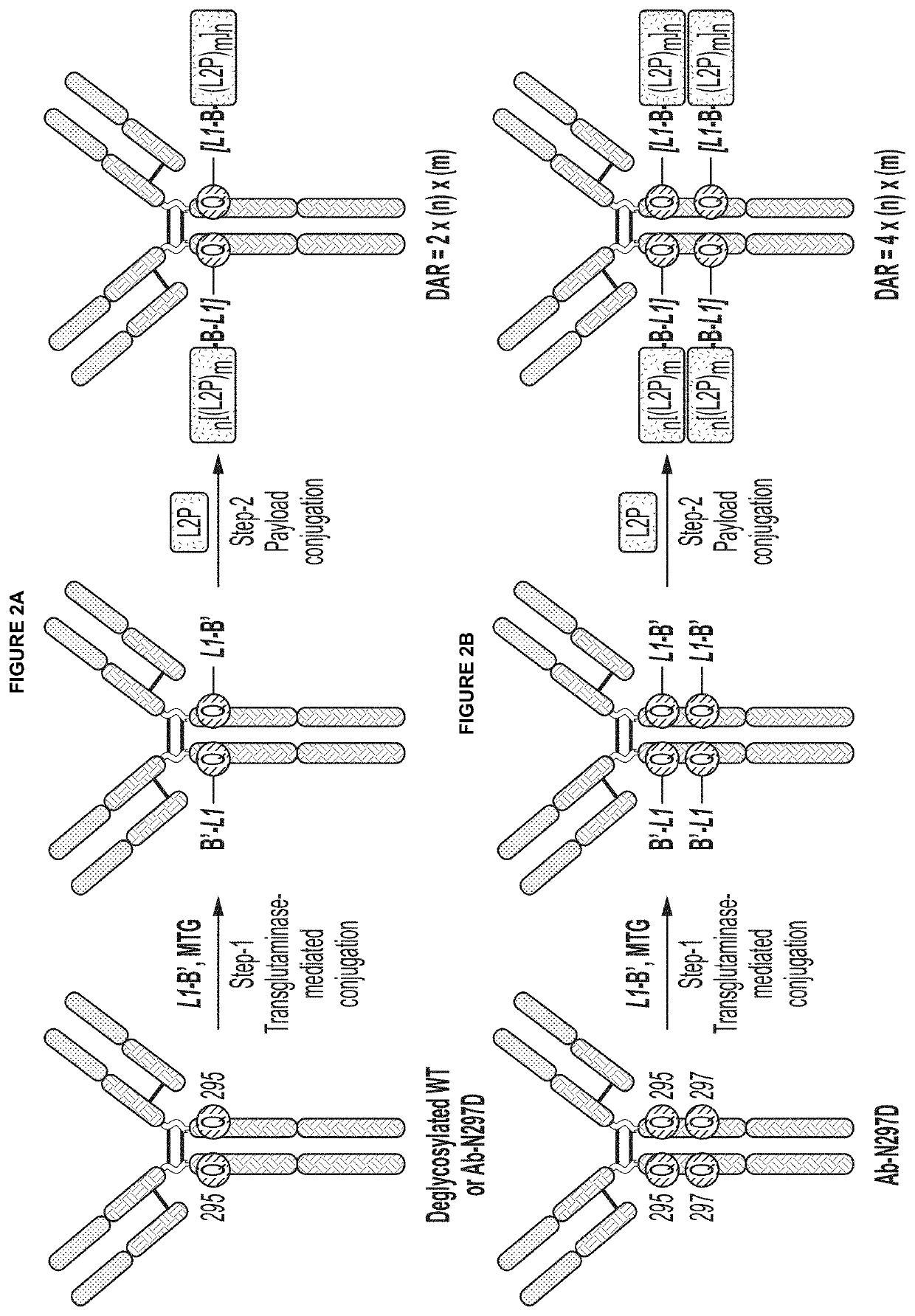
Protein-drug conjugates comprising camptothecin analogs and methods of use thereof
PendingUS20220072141A1Pharmaceutical non-active ingredientsAntineoplastic agentsWAS PROTEINDrug conjugation
Described herein are protein-drug conjugates and compositions thereof that are useful, for example, for target-specific delivery of therapeutic moieties, e.g., camptothecin analogs and / or derivatives. In certain embodiments, provided are specific and efficient methods for producing protein-drug constructs (e.g., antibody-drug conjugates) utilizing a combination of transglutaminase and 1,3-cycloaddition techniques. Camptothecin analogs, antibody-drug conjugates, and compositions which comprise glutaminyl-modified antibodies and camptothecin analog payloads and are provided.
Owner:REGENERON PHARM INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com