Antioxidant, neuroprotective and antineoplastic nanoparticles comprising a therapeutic agent on an amphiphilic spacer or an amphiphilic polymer
An antioxidant and nanosphere technology, applied in antitumor drugs, animal repellents, plant growth regulators, etc., can solve the problem that the molecular mechanism of antitumor effect is not clear.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
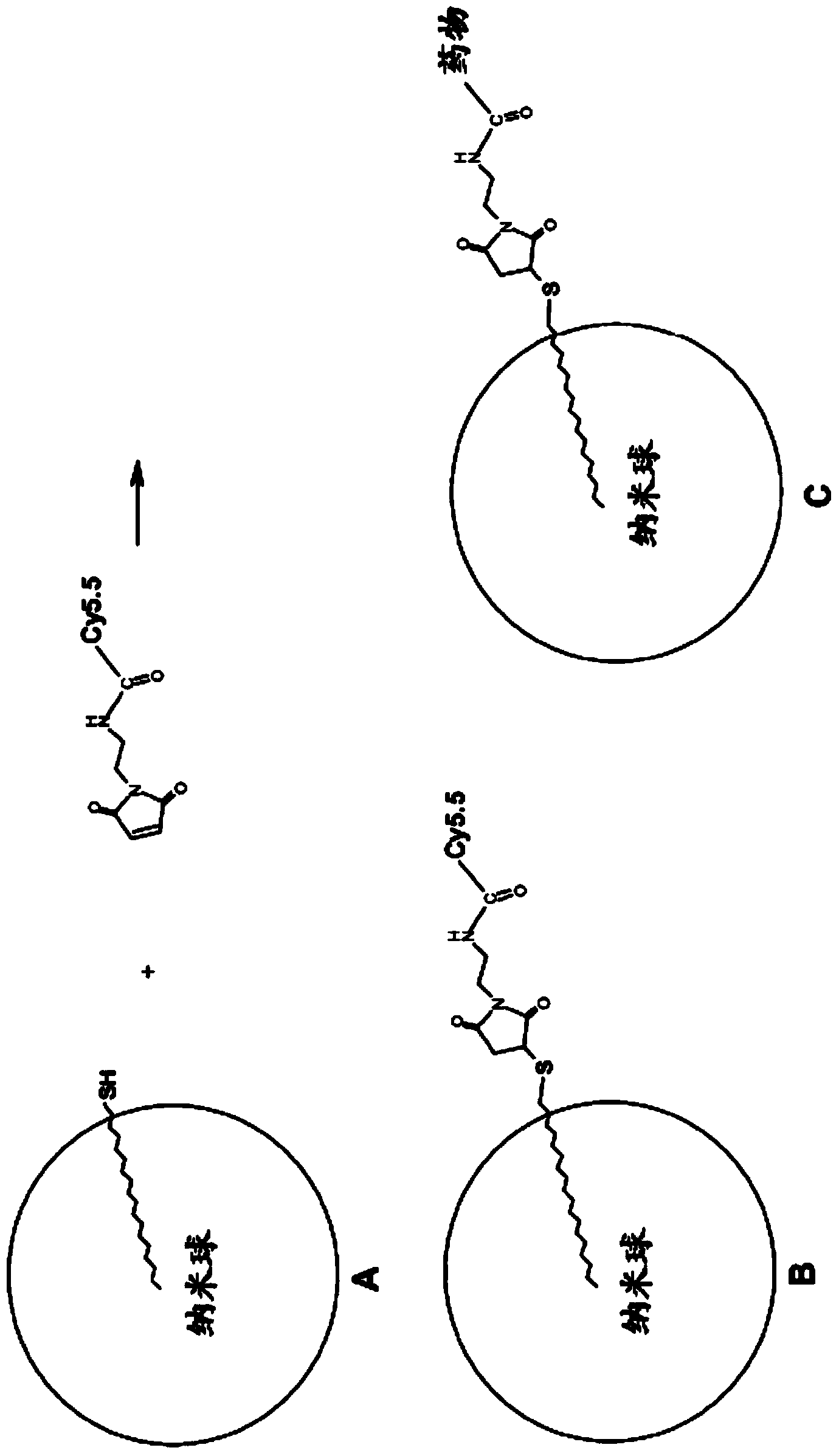
[0336] In order to prepare cy3 / cy5 / cy5.5-labeled antioxidant-antitumor nanospheres, antioxidant antitumor nanospheres were prepared by the same procedure as described in Example A-Example D below, except that the 0.1-2 mg of 1-octadecanethiol (Aldrich, code O1858) was added to the organic phase ( figure 1 in A).
[0337] Add 500 μL of 10× PBS and 1.5 molar equivalents of Cy3 / Cy5 / Cy5.5 maleimide to 3 mL of the suspension of antioxidant-antitumor nanospheres containing 1-octadecanethiol ( figure 1 in B). Such as figure 1 As shown in C in , this intermediate can be used to modify the carrier drug to have a maleimide group.
[0338] Such as figure 2 As shown, the SH-maleimide pair can be replaced by NH 2 - NHS pair or other substitution.
Embodiment A
[0339] Embodiment A-antioxidant-preparation of anti-tumor nanospheres
[0340] Nanospheres were prepared according to the method using spontaneous emulsification with slight modifications. Briefly, 15 mg of the mixture (camptothecin derivatives and ALA 2(a mixture of 1,12-dodecanediol)) was dissolved in acetone (5 mL, 0.1% polysorbate 80). The organic solution was poured into the aqueous phase prepared by dissolving 25 mg of Pluronic F68 in 10 mL of double distilled water (0.25% w / v) with gentle stirring on a magnetic plate. After magnetic stirring for 15 minutes, the acetone was removed under reduced pressure at room temperature. Nanospheres were filtered through a 0.8 μm hydrophilic syringe filter and stored at 4 °C. Hydrodynamic size measurements and size distributions of nanospheres were performed by dynamic light scattering (DLS) using a Coulter N4-Plus Particle Sizer (Coulter Corporation, Miami, FL).
[0341] Separately, 25 mg of a mixture (an antioxidant camptothe...
Embodiment B
[0345] Example B-Preparation of antioxidant and anti-tumor nanospheres
[0346] According to the method described in Example 5, nanospheres were prepared from 25 mg of the mixture (a mixture of camptothecin derivative and α-tocopherol) using spontaneous emulsification. In the absence of camptothecin derivatives, by α-tocopherol or Ibu 2 TEG to prepare control nanospheres.
[0347] Table 2. Size and Polydispersity Index (P.I.):
[0348] Antioxidant-Anti-tumor Nanosphere II
[0349] α-tocopherol (mg)
Compound C-10(mg)
Size (nm)
P.I.
25
1
128±45
0.25
25
0
121±40
0.20
[0350] Example C- Preparation of anti-inflammatory-anti-tumor nanospheres
[0351] According to the method described in Example 5, nanospheres were prepared from 25 mg of a mixture (a mixture of camptothecin derivatives, non-steroidal anti-inflammatory drug (NSAID) derivatives and alpha-tocopherol) using spontaneous emulsification. Contr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com