Fast quench reactor and method
a reactor and fast technology, applied in the field of fast quench reactor and method, can solve the problems of high cost, relatively low titanium utilization, and shortfall in sponge availability, and achieve the effect of minimizing back reactions and rapid cooling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1-titanium
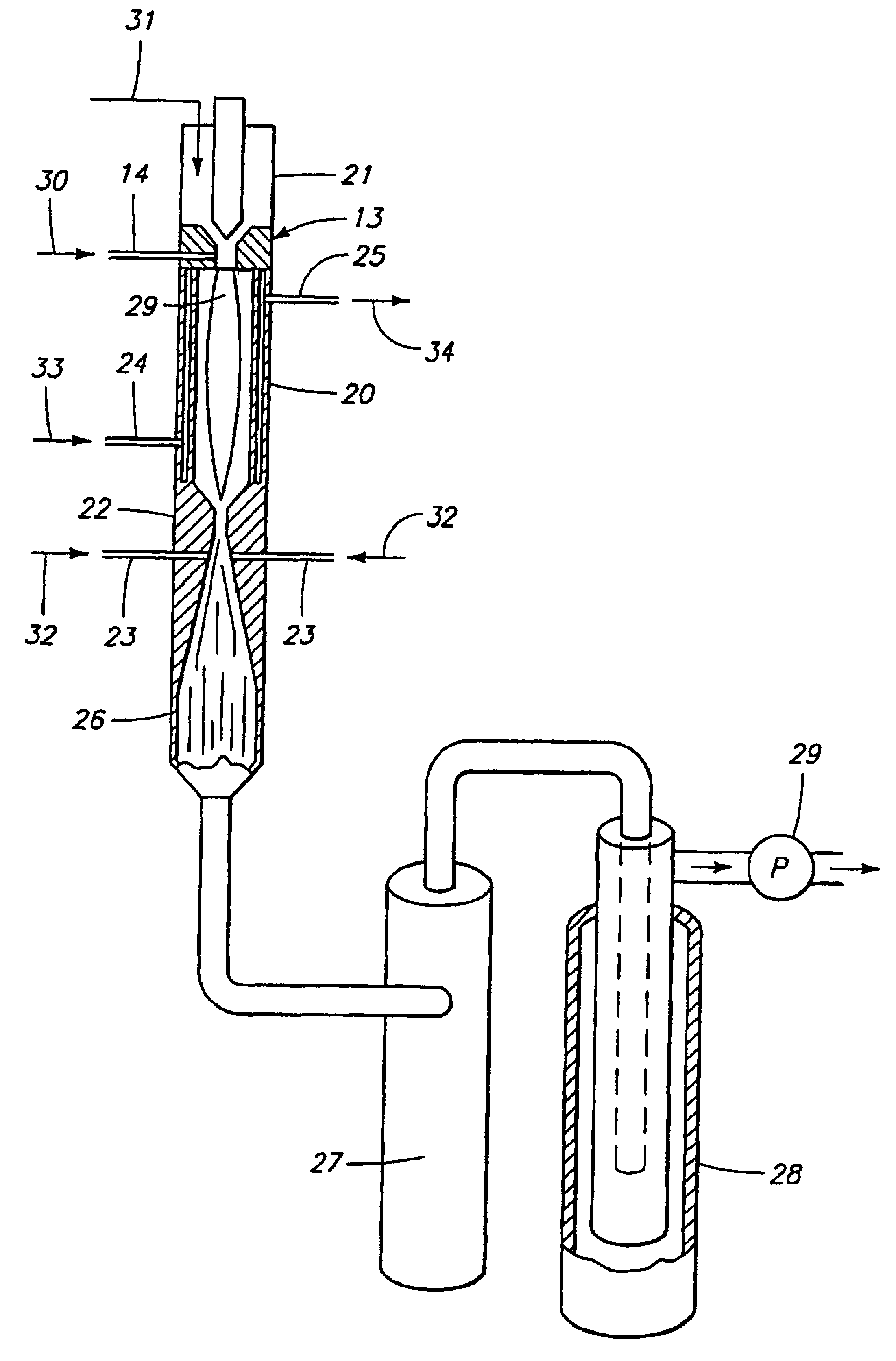
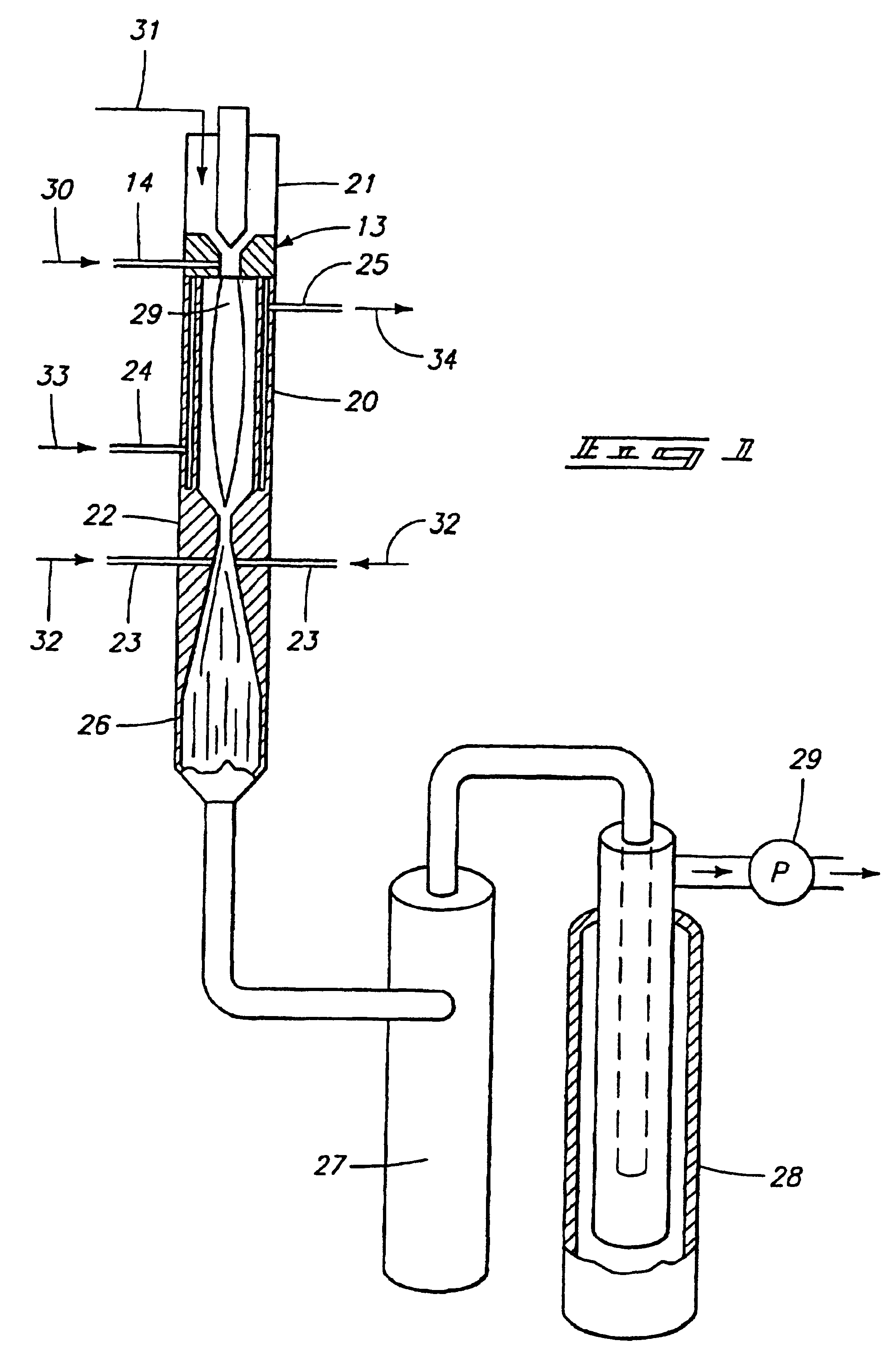
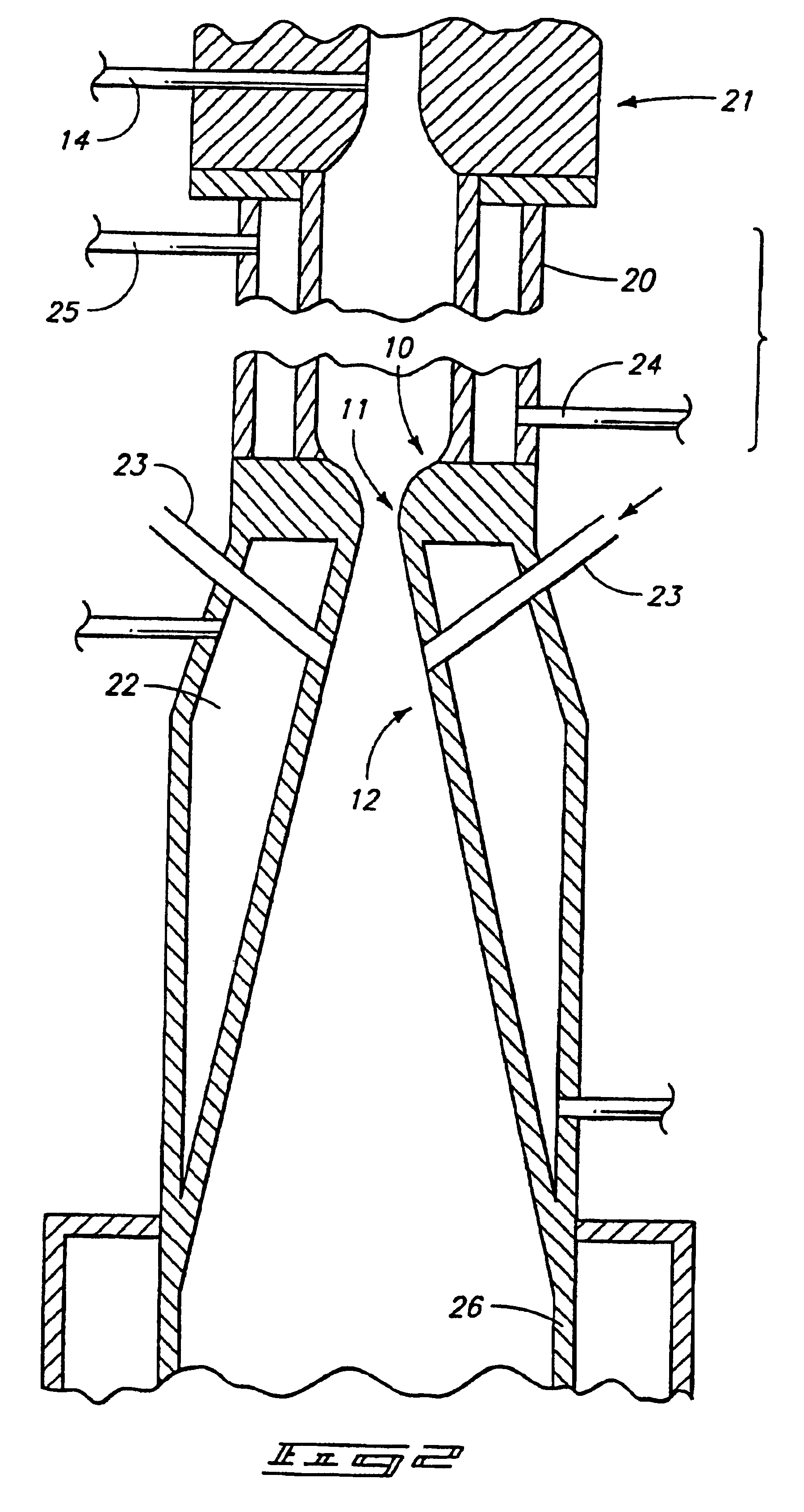
The preferred method for producing titanium from titanium tetrachloride (TiCl.sub.4 involves directing titanium tetrachloride vapor and .[.a.]. hydrogen into a hot plasma torch operated at 12 kW with a mixture of argon and hydrogen as the plasma gas (95% Argon; 5% Hydrogen, by volume) to decompose it to titanium and chlorine, followed by rapid expansion of the resulting hot gases and cooling with additional hydrogen to retain the titanium in an elemental solid metal state.
The diameter and length (6.0 mm.times.700.0 mm) of the reaction chamber was chosen to obtain maximum mixing while maintaining a minimum of 4000 K temperature at the entrance of the nozzle throat. The reaction chamber, converging / diverging nozzle were constructed from nickel 200 alloy to reduce corrosion. Standard equations were used to calculate the dimensions of the bell-shaped converging nozzle, nozzle throat diameter diverging angle, and diverging nozzle exit diameter. Reactants: Titanium tetrachloride liquid an...
example 2 -
EXAMPLE 2-TITANINUM DIOXIDE
TiO.sub.2 production in the 50 nm range can also be carried out in the existing facilities. For this purpose, TiCl.sub.4 is injected into an argon plasma and mixed with O.sub.2 just before the quench zone. Most of the TiO.sub.2 produced today is used in the paint industry and a 50 nm size (rather than the present 200 nm) is advantageous. There is also some interest in fine TiO.sub.2 as a ceramic. Preliminary results at low TiCl.sub.4 (-1 gm / hr) flow are encouraging.
The resulting titanium dioxide has a rutile structure, which has superior properties in blocking ultraviolet light. Titanium dioxide particles can be produced with average diameters of 10 nanometers or less in the narrow size ranges as defined, which can find use as a sun blocking agent for protecting human skin against harmful effects of sunlight.
The process meets all requirements for titanium production, in that it provides downstream reduction in a kinetically controlled reactor to remove hal...
example 3--
Methane conversion to acetylene in a high temperature reactor follows the theoretical chemical reaction: 2CH.sub.4.fwdarw.C.sub.2 H.sub.2 +3H.sub.2. In principle, under careful kinetic studies on the pyrolysis of methane it has been shown that it is possible to obtain high yields of acetylene where the main by-product is hydrogen, instead of tars and acetylene black. Such studies also showed that pyrolysis in the presence of hydrogen suppressed carbon formation.
In practice, a range of other hydrocarbons, specifically the light olefins and solid carbon, are always formed as byproducts with acetylene if the reaction condition is not well controlled. Equilibrium thermodynamic calculations predict a yield of acetylene at 38%, but plasma conversion experiments indicate acetylene yields are as high as 70-85%. Solid carbon formation can be as low as 10%.
Experiments using the fast quench system of this disclosure have revealed that the methane decomposition to acetylene is kinetics...
PUM
| Property | Measurement | Unit |
|---|---|---|
| included angle | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com