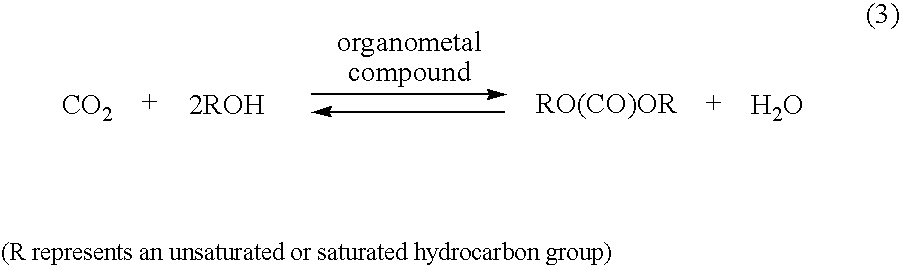
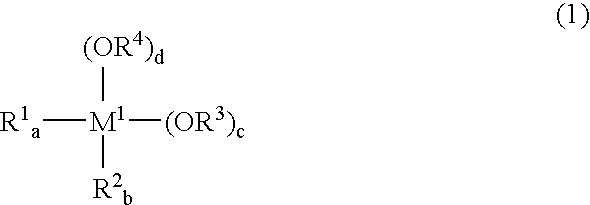Since
phosgene used in this method is extremely harmful and highly corrosive, this method is disadvantageous in that the transportation and storage of
phosgene need detailed care and, also, there is a large cost for the maintenance of production equipment and for assuring safety.
Further, this method poses a problem in that it is necessary to dispose of
hydrochloric acid produced as a waste by-product.
In this method,
carbon monoxide (which is extremely harmful) is used under
high pressure; therefore, this method is disadvantageous in that there is a large cost for the maintenance of production equipment and for assuring safety.
Therefore, in the case of these methods, a carbonic ester obtained contains a trace amount of a
halogen which cannot be completely removed by a simple purification step.
However, this method poses the following problems.
Therefore, the method using the equilibrium reaction has a problem in that, for producing a carbonic ester in high yield, it is necessary that the carbonic ester and water as products be removed from the
reaction system.
Further, there is also a problem in that the water formed decomposes a catalyst, so that not only is the reaction hindered, but also the number of turnovers of the catalyst (i.e., the number of cycles of regeneration and reuse) is only 2 or 3.
This method has a problem in that the dehydrating agent after use cannot be regenerated, resulting in the occurrence of a large amount of a waste derived from the dehydrating agent.
Thus, this method is as defective as the above-mentioned methods.
The water is then reacted with an
acetal.”) However, these patent documents do not teach or suggest a method for efficiently producing an
acetal without forming a waste.
Further, the methods disclosed in these patent documents have a problem in that, when an
acetal is used as a dehydrating agent, large amounts of by-products, such as a
ketone and an
aldehyde, are formed as wastes.
However, an organic dehydrating agent is consumed in a stoichiometric amount in accordance with the formation of a carbonic ester (and water as a by-product), so that a large amount of an organic dehydrating agent is consumed, thus forming a large amount of a degeneration product of the organic dehydrating agent.
Further, in
spite of the use of an organic dehydrating agent in a large amount, the possibility still remains that deactivation of a catalyst occurs.
However, this method has a problem in that the
solid dehydrating agent cannot be regenerated, thus forming a large amount of a waste.
Thus, this method has problems in that it is necessary to consume an extremely large amount of energy for cooling and heating, and a large amount of a
solid dehydrating agent is needed.
However, in the production of a carbonic ester from carbon dioxide and an
alcohol, wherein the equilibrium of the reaction is largely biased toward the original
system, this method cannot be suitably used because this method poses a serious problem that it is necessary to repeat the above-mentioned operation which needs a very large consumption of energy for cooling and heating.
Further, for regenerating a degenerated dehydrating agent which has adsorbed water to saturation, it is generally necessary to calcine the degenerated dehydrating agent at several hundreds ° C., thus rendering this method commercially disadvantageous.
Furthermore, in this method, only one (water) of the two products of an equilibrium reaction is removed and, therefore, there is a problem in that, when the equilibrium reaction progresses to increase the carbonic ester concentration of the
reaction system, the reaction becomes unlikely to progress any more, that is, this method is still under the restriction of an equilibrium reaction.
Therefore, when the reaction mixture is cooled to
room temperature in a cooling step, the reaction mixture turns into a white
slurry, thus causing a problem in that, in a subsequent
dehydration step performed using a packed column containing a dehydrating agent, the
slurry causes clogging of the packed column.
However, in the field of the production of a carbonic ester from carbon dioxide and an
alcohol, although “Study Report of Asahi Glass Association for Promotion of
Industrial Technology (Asahi Garasu Kogyogijutsu Shoreikai Kenkyu Hokoku)”, Vol. 33, 31-45 (1978) states that “
dehydration by
distillation is now being studied”, there have been no reports or the like which state that a
dehydration method using
distillation has been completed.
There has been a report which mentions a
distillation separation of a carbonic ester from a reaction mixture containing a
metal alkoxide, wherein the reaction mixture is obtained by reacting carbon dioxide and an alcohol with each other in the presence of a
metal alkoxide catalyst; however, it is known in the art that, when a
metal alkoxide catalyst is used, a distillation separation causes a reverse reaction, thus rendering it difficult to recover a carbonic ester by distillation separation (see “Journal of the Chemical Society of Japan (Nippon Kagaku Kaishi)”, No. 10, 1789-1794 (1975)).
Especially, no method is known by which a carbonic ester having a high
boiling point can be separated in high yield from a reaction mixture containing a
metal alkoxide.
On the other hand, a
metal alkoxide is so unstable that it is susceptive to deactivation due to the
moisture in the air.
A metal alkoxide catalyst is an expensive compound, and no technique is known for regenerating a deactivated metal alkoxide catalyst.
This method has a problem in that, although
dibutyltin oxide which is charged into the
reaction system is stable, the dibutyltin
oxide is converted, during the reaction, into a dibutyltin dialkoxide, which is unstable.
Therefore, this method cannot solve the above-mentioned problem of the
instability of a metal alkoxide catalyst.
Specifically, this method has a defect in that, once the reaction mixture is removed from the reaction
system for isolating the carbonic ester obtained as a
reaction product, the unstable dibutyltin dialkoxide is deactivated and cannot be regenerated by a
conventional technique.
Therefore, in this method, there is no other choice but to discard the dibutyltin dialkoxide catalyst (which is expensive) as a waste after the reaction.
It is difficult (or substantially impossible) to regenerate a dialkyltin dialkoxide having excellent activity from the trialkyltin alkoxide.
Further, the formation of such degraded compound (i.e., an unregenerable unreactive compound) poses a problem in that, when a metal alkoxide is reused as a catalyst, the content of an active catalyst in the metal alkoxide is decreased and, hence, the
reaction rate and the yield of a carbonic ester are decreased, rendering a stable production of a carbonic ester difficult.
However, this method poses a problem in that, when the addition of a fresh metal alkoxide is performed while leaving the deterioration product formed during the reaction as it is in the reaction
system, the deterioration product, which has a low catalyst activity, accumulates in a large amount in the reaction system.
As apparent also from the above, there is no conventional method in which a metal alkoxide is effectively reused as a catalyst; in any of the conventional methods for producing a carbonic ester, there is no other choice but to discard the metal alkoxide as a waste after the reaction, thus rendering the production of a carbonic ester disadvantageously costly.
Thus, in the conventional methods for producing a carbonic ester by using a metal alkoxide, carbon dioxide and an alcohol, when the metal alkoxide (which is expensive) has lost its catalyst activity due to
hydrolysis or the like, there is no way to easily and effectively regenerate and reuse the metal alkoxide.
Therefore, the conventional methods for producing a carbonic ester is disadvantageous in that it is necessary to use a large amount of an organic dehydrating agent or a solid dehydrating agent in combination with a small amount of a metal alkoxide.
As described hereinabove, the prior art techniques for producing a carbonic ester have many problems and, therefore, have not been put to practical use.
However, even this method still poses a problem in that an unregenerable unreactive organometal compound is formed during the reaction and accumulates in the reaction system.
 Login to View More
Login to View More