Chemical vapor deposition and powder formation using thermal spray
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example i
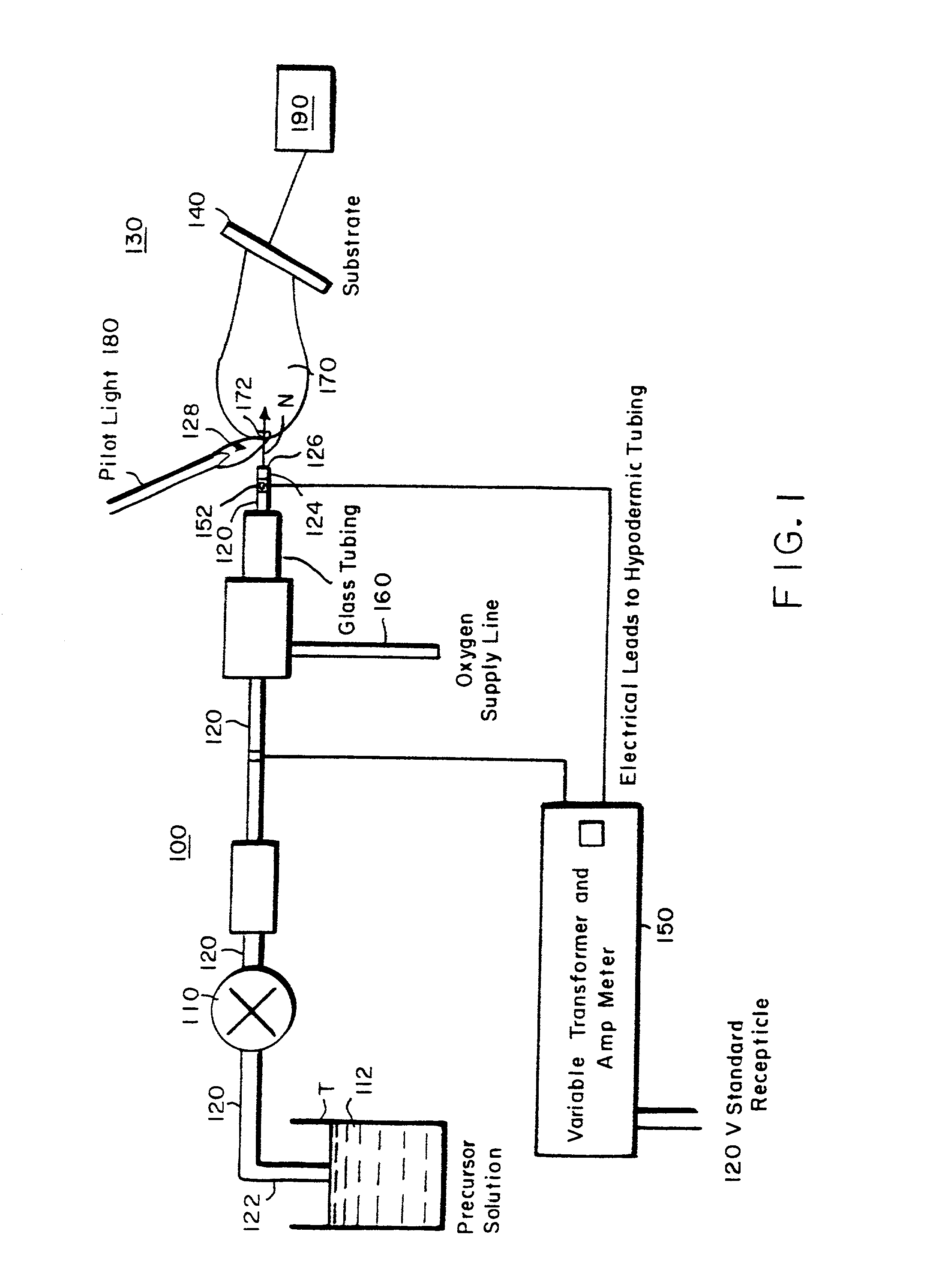
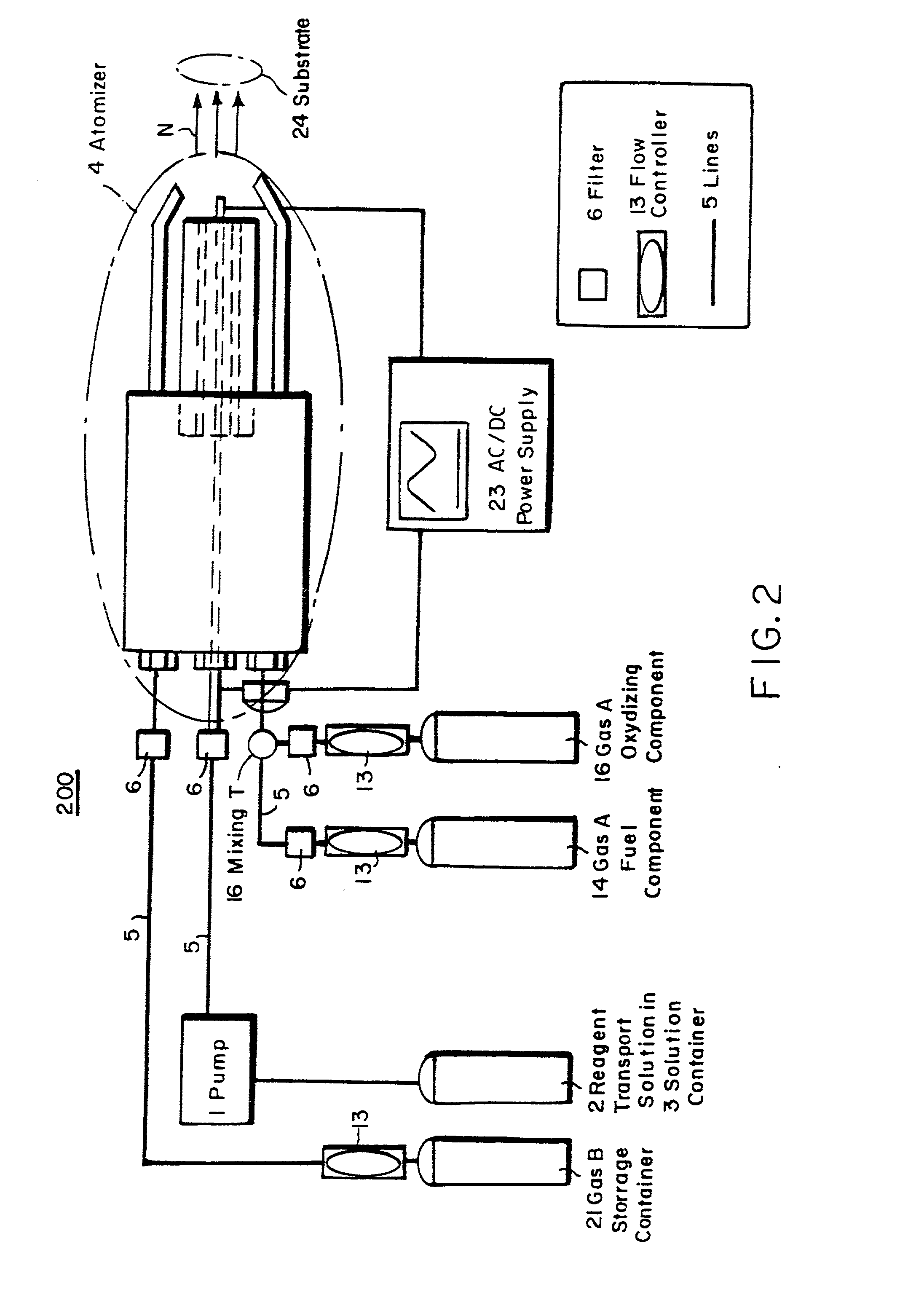
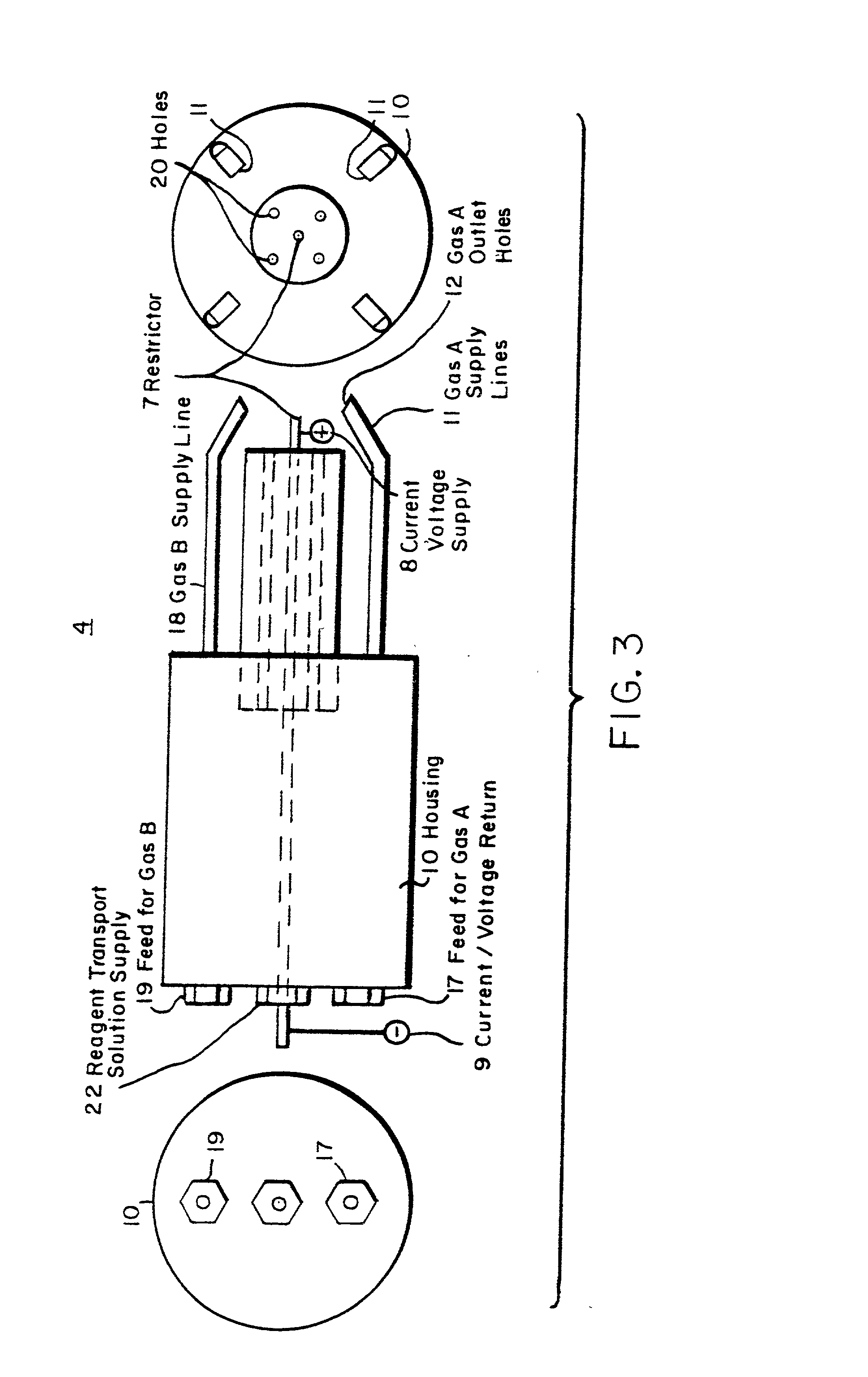
[0106] To illustrate the coating deposition capability of the process of the present invention, simple oxide coatings were formed on a metal substrate. SiO.sub.2 was deposited onto water cooled aluminum foil from a solution of tetraethoxysilane [Si(OC.sub.2H.sub.5).sub.4] dissolved in isopropanol to 2.1 wt % Si, additional isopropanol (3.2 ml) and propane (51 ml) were added for an overall silicon concentration of 0.06 M. The gas temperature for deposition was 1190.degree. C. The needle used to nebulize the precursor, as seen in FIG. 3, was 304 stainless steel with OD=0.012 inches and ID=0.004 inches. The resistance over the electrical flow length of the needle was about 1.6 W. Small pilot flames formed from combusted ethane and oxygen were used throughout the deposition to maintain the flame. The solution was pumped to the needle at 3 ml / min and nebulized by controlling the amount of current through the needle. In this example, the current was 2.65 A. The solution pressure from pump...
example ii
[0108] In addition to coatings formed on metal substrates, such as the oxide deposited on aluminum in Example I, coatings have also been formed on plastic substrates. Platinum was deposited onto Teflon at a gas temperature of 200 to 260.degree. C. from a 0.005M solution of platinum-acetylacetonate [Pt(CH.sub.3COCHCOCH.sub.3)..sub.2], toluene and methanol. The deposition apparatus used was similar to that used for Example I, except two separate pilot lights were used and the oxygen was supplied via a coaxial tube surrounding the reagent solution. The solution flow rate was 2 ml / min with a pressure of 1500 psi and a needle current of approximately 3.3 A. The oxygen flowed at a pressure of 20 psi and a rate of 4750 ml / min. The resulting adherent film was smooth, dense and uniform. X-ray diffraction ("XRD") confirmed the formation of platinum with a (111) preferred growth direction.
[0109] This example also illustrates that the coatings produced by the process of the present invention ar...
example iii
[0110] The coatings developed by the present invention are not limited to formation on planar substrates. Films have been deposited on ceramic fiber tows using the apparatus of the present invention. LaPO.sub.4 was deposited onto an alumina fiber tow from a solution of triethylphosphate [C.sub.2H..sub.5O.sub.3PO.sub.4] dissolved in toluene to 1.7 wt % P, lanthanum 2-ethylhexanoate dissolved in toluene to 1 wt % La, additional toluene (16 ml) and propane (273 ml). The resulting solution had concentrations of 0.0010M P and 0.0013M La. The solution flowed at a rate of 3 ml / min with a pressure of 410 psi during the deposition and was nebulized with a needle current of 2.36 A. The flow rate of oxygen to the solution flame was 4750 ml / min at a pressure of 30 psi.
[0111] The 400 fibers in the tow were coated at the same time. Each fiber was approximately 12 mm in diameter. The tow was slowly moved through the deposition zone of the flame two times. Only two passes through the flame (where t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Pressure | aaaaa | aaaaa |
| Energy | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com