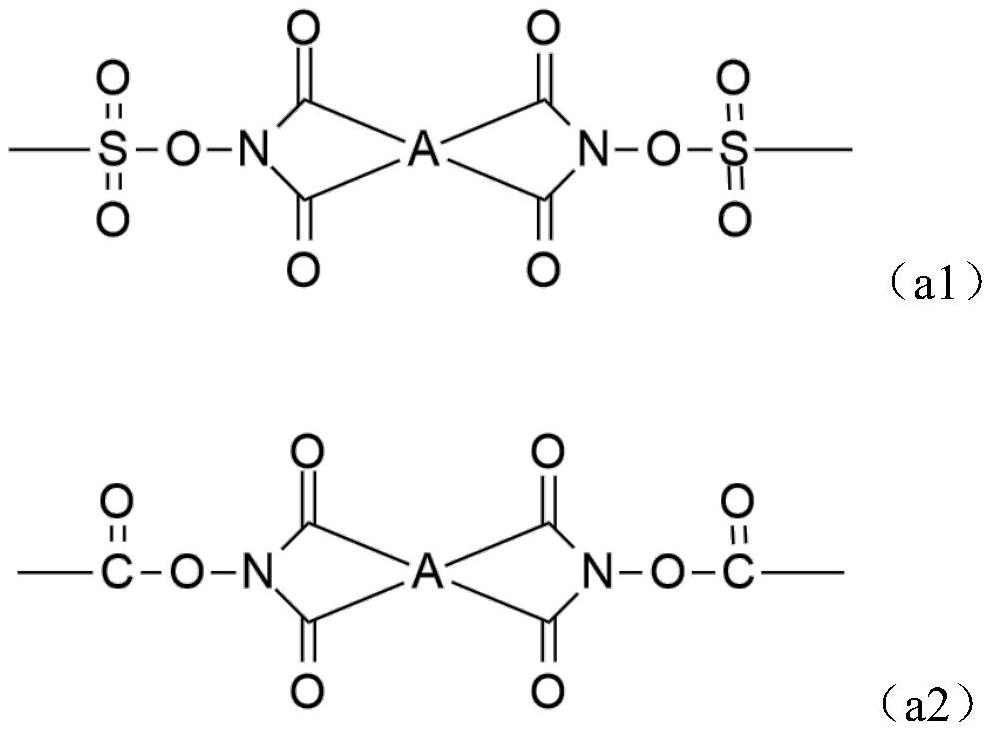
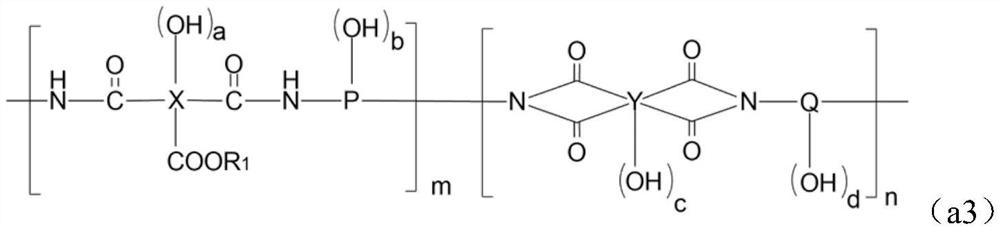
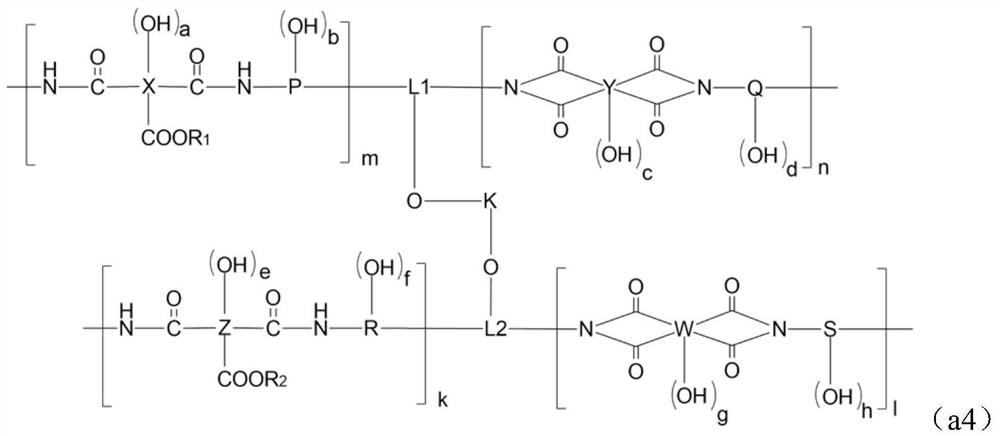
Alkali-soluble resin, positive photosensitive resin composition, cured film and display device
A technology of positive-type photosensitive resin and alkali-soluble resin, which is applied in the field of positive-type photosensitive resin composition, cured film and display device, and alkali-soluble resin, and can solve problems such as slow dissolution rate, low image resolution, and slow development, and achieve The effect of excellent thermodynamic performance and high exposure sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0108] The above and other advantages of the present invention can be better understood through the following examples, but the following examples are not intended to limit the scope of the present invention.
[0109] Description of abbreviations:
[0110] ODA: 4,4'-diaminodiphenyl ether
[0111] 6FAP: 2,2-bis(3-amino-4-hydroxyphenyl)hexafluoropropane
[0112] SiDA: 1,3-bis(3-aminopropyl)-1,1,3,3-tetramethyldisiloxane
[0113] Y1:
[0114] Y2:
[0115] 6FDA: 2,2'-bis(3,4-dicarboxylic acid phenyl)hexafluoropropane dianhydride
[0116] ODPA: 3,3’,4,4’-Diphenyl ether tetracarboxylic dianhydride
[0117] BTDA: 3,3’,4,4’-Benzophenone tetracarboxylic dianhydride
[0118] CBDA: cyclobutane tetracarboxylic dianhydride
[0119] BPDA: 3,3',4,4'-Biphenyltetracarboxylic dianhydride
[0120] X1:
Synthetic example 1
[0122] Synthesis of diamine derivative A1 of 6FDA
[0123] Dissolve 0.1mol of 2-amino-4-nitrophenol in 50ml of acetone and 0.34mol of propylene oxide, cool down to -15°C, and slowly add dropwise the A3 solution synthesized in Synthesis Example 3 (0.048mol, dissolved in 150ml of propylene glycol monomethyl ether), after the dropwise reaction was continued at -15°C for 4h, then warmed up to room temperature, filtered, the filter cake was washed several times with acetone and then vacuum-dried at 50°C for 10h to obtain the nitro intermediate.
[0124] The nitro compound was dissolved in 100ml of NMP, 3g of 5% palladium-carbon was added, hydrogen gas was introduced into the autoclave, stirring was continued at room temperature for 6h, then the reaction solution was filtered to remove the catalyst, the filtrate was poured into water, and a solid product was precipitated. Filtration and vacuum drying at 50° C. for 24 h gave the diamine derivative A1 of 6FDA.
[0125]
Synthetic example 2
[0127] Synthesis of diamine derivative A2 of 6FDA
[0128] The only difference from Synthesis Example 1 is that the raw material A3 is replaced by A4 synthesized in Synthesis Example 4, and other conditions remain unchanged, and the diamine derivative A2 of 6FDA is obtained.
[0129]
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com