Pharmaceutical composition containing IL-15 cationic liposome compound and celecoxib liposome as well as preparation method and application of pharmaceutical composition
A technology of cationic liposome and celecoxib, which is applied in the field of medicine to achieve the effects of improving tumor targeting, reducing tumor immunosuppression, and avoiding the damage of pH environment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Embodiment 1: Preparation of celecoxib liposome
[0076]Accurately weigh 64 mg of soybean lecithin, 4 mg of celecoxib, and 8 mg of cholesterol, respectively, and place them in a 25 mL round-bottomed flask, add a mixed solution of chloroform and methanol (V:V=1:1) and oscillate until completely dissolved, and evaporate in a vacuum at 37°C. After all the films were formed, add Hepes buffer, hydrate in a water bath at 60°C for 10 minutes, sonicate with a probe ultrasonic cell disruptor (200W, 3 minutes) and pass through a 0.22 μm microporous membrane to obtain celecoxib liposomes.
Embodiment 2
[0077] Embodiment 2: the mensuration of celecoxib liposome encapsulation efficiency
[0078] Select the ultraviolet absorption wavelength 252nm to detect celecoxib. The absorption intensity value A of celecoxib with different concentrations of 5, 10, 20, 50, and 100 μg / mL was measured at this wavelength, and the standard curve was drawn with the ultraviolet absorption intensity A as the ordinate and the concentration C as the abscissa. The standard curve of CEL is A=0.0242C-0.0172, R 2 = 0.9909. The results showed that CEL had a good linear relationship in the range of 5-100μg / mL.
[0079] Precisely draw the celecoxib liposome solution after ultracentrifugation, put it in a 10mL volumetric flask, then add methanol to carry out ultrasonic demulsification, measure its ultraviolet absorption intensity A with a UV spectrophotometer, and then in different drug-to-lipid ratios: Under the conditions of 1:5, 1:10, 1:15, and 1:20, the encapsulation efficiency was measured according ...
Embodiment 3
[0083] Embodiment 3: Characterization of celecoxib liposome formulation
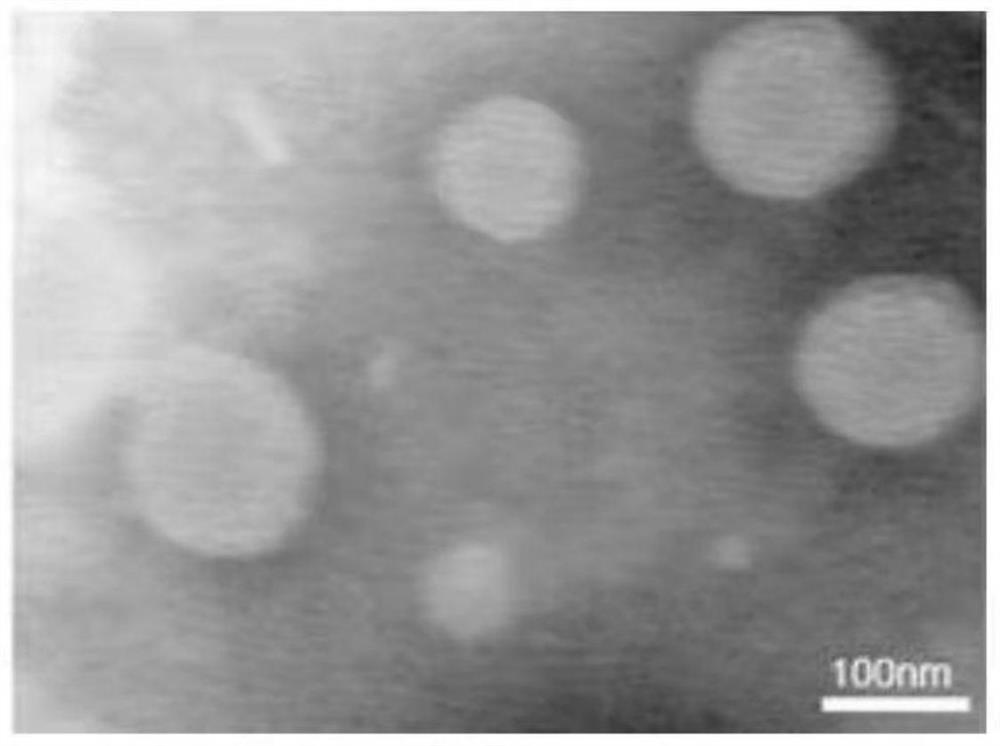
[0084] (1) Morphological observation: Take 9 μL of celecoxib liposomes prepared in Example 1 and slowly drip it onto the center of the copper mesh covered with carbon film, let it stand for 1 minute, then use filter paper to suck off the excess liquid, and absorb 9 μL of phosphotungstic acid ( 2%) negative staining for 1 minute, use the above operation to absorb excess liquid, put it into a transmission electron microscope for detection and take pictures, as figure 1 shown. Depend on figure 1 It can be observed that the shape of the complex is regular, the shape is spherical, and the distribution is uniform, and the particle size of the liposome is about 120nm.
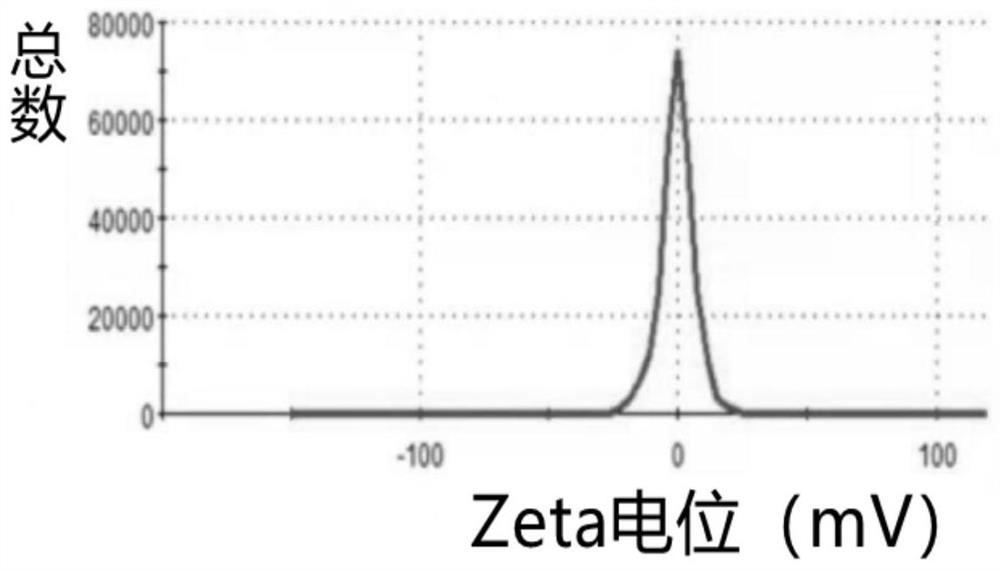
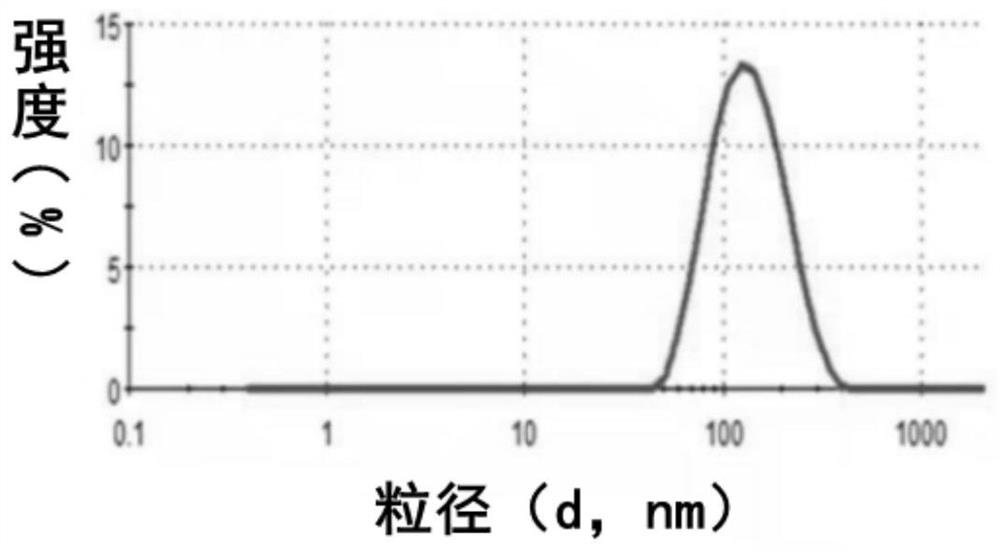
[0085] (2) Determination of particle size and potential: We used a Malvern laser particle size analyzer to characterize liposome Zeta potential. Take liposome solutions with different concentrations and carefully add them into the potentiomet...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Electric potential | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com