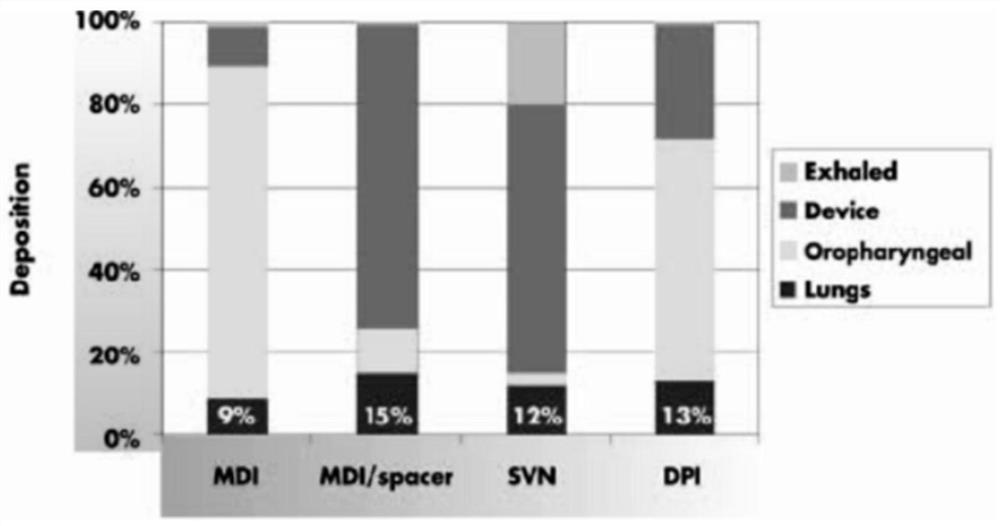
Solution used for Ribavirin aerosol inhalation, and preparation method of solution
A technology of atomized inhalation and ribavirin, applied in the field of pharmacy, can solve problems such as hidden dangers, waste of remaining drugs, inconvenience and safety, achieve excellent and effective deposition effects, and solve safety problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
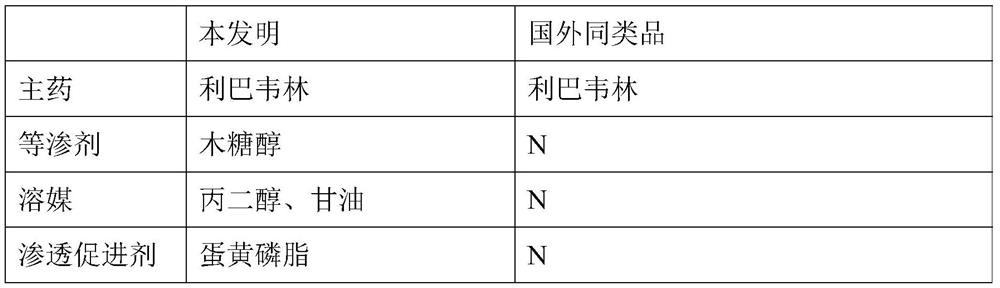
[0049] This embodiment provides a single-dose ribavirin nebulized inhalation solution in single-dose independent packaging and prescription. In the prior art, similar products from foreign manufacturers are powders, which are reconstituted and diluted before use, and are inconvenient to use due to the large packaging of 6g / bottle.
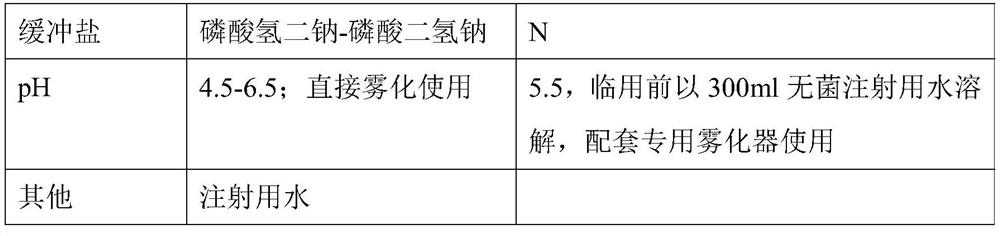
[0050] The comparison between the two is shown in Table 1:
[0051] 【Table 1】
[0052]
[0053]
Embodiment 2
[0054] Phospholipid-free ribavirin atomized inhalation solution in the prescription of embodiment 2
[0055] The prescription mix is shown in Table 2:
[0056] 【Table 2】
[0057] prescription Specification: 1ml Specifications: 5ml Specifications: 20ml Ribavirin 200mg 1g 4g Sodium chloride 0.2mg 1mg 4mg Propylene Glycol 0.2mg 1mg 4mg glycerin 0.1mg 0.5mg 2mg egg yolk phospholipids 0mg 0mg 0mg Cyclodextrin 0mg 0mg 0mg Disodium phosphate 0.25mg 1.25mg 5mg Sodium dihydrogen phosphate 0.3mg 1.5mg 6mg 4.5-6.5 4.5-6.5 4.5-6.5 4.5-6.5 Water for Injection Dilute to 1ml Dilute to 5ml Dilute to 20ml
[0058] Preparation process:
[0059] (1) Weigh ribavirin and sodium chloride respectively according to the prescription amount, add them to water for injection with a total volume of 60%, and stir evenly to obtain solution 1:
[0060] (2) In addition, take 30% of t...
Embodiment 3
[0070] Example 3 Ribavirin atomized inhalation solution containing egg yolk phospholipid
[0071] The prescription mix is shown in Table 4:
[0072] 【Table 4】
[0073] prescription Specification: 1ml Specifications: 5ml Specifications: 20ml Ribavirin 200mg 1g 4g Xylitol 0.35mg 1.75mg 7mg Propylene Glycol 0.2mg 1mg 4mg glycerin 0.1mg 0.5mg 2mg egg yolk phospholipids 0.2mg 1mg 4mg Cyclodextrin 0mg 0mg 0mg Disodium phosphate 0.25mg 1.25mg 5mg Sodium dihydrogen phosphate 0.3mg 1.5mg 6mg 4.5-6.5 4.5-6.5 4.5-6.5 4.5-6.5 Water for Injection Dilute to 1ml Dilute to 5ml Dilute to 20ml
[0074] Preparation process:
[0075] 1) Weigh ribavirin and xylitol respectively according to the prescription amount, add them to water for injection with a total volume of 60%, and stir evenly to obtain solution 1:
[0076] 2) In addition, take 30% of the prescribed amount of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com