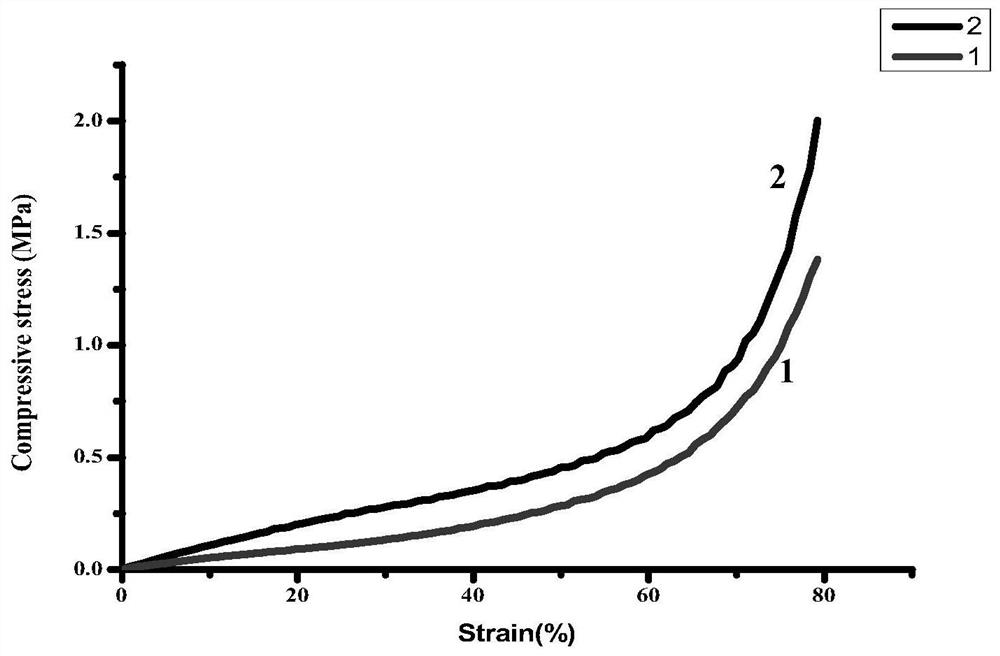
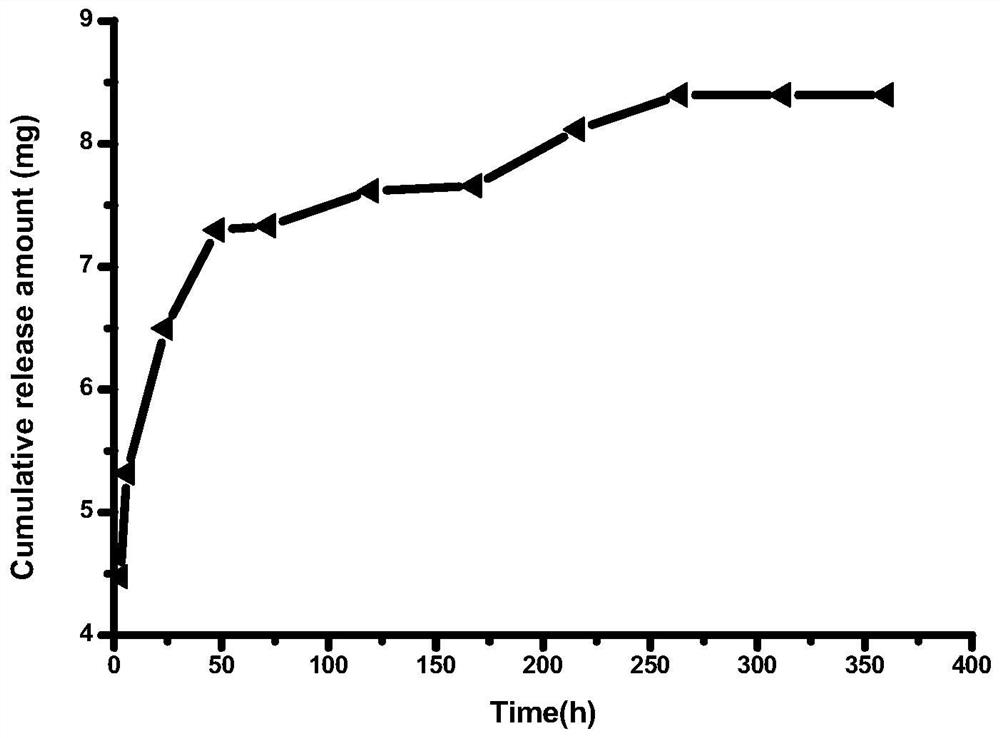
Method for preparing multifunctional sodium alginate stent embedded with drug-loaded microspheres by using 3D printing technology based on in-situ emulsification
A sodium alginate and 3D printing technology, which is applied in the fields of pharmaceutical formula, medical science, microcapsules, etc., can solve problems such as low osteogenic activity, poor mechanical properties of scaffolds, and surgical failures
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] 1) Preparation of amino-modified polylactic acid: Dissolve poly-L-lactic acid particles in 1,4-dioxane solvent in a 60°C water bath to prepare a homogeneous solution with a concentration of 1 wt%. A 1.2 wt% ethylenediamine aqueous solution was added to the phase solution, reacted for 30 min, cooled to room temperature, and then placed in a freeze dryer at -80 °C to freeze-dry to obtain aminated polylactic acid.
[0022] 2) Preparation of printing paste: Weigh a certain amount of lecithin and sodium alginate and dissolve them in 4 wt% ethanol aqueous solution to obtain a lecithin-dissolved sodium alginate solution, and lecithin-dissolved sodium alginate solution The concentration of lecithin was 1 wt%. Dissolving the amino-modified polylactic acid and ibuprofen drug prepared in step 1) in 1,4-dioxane solvent to obtain a modified polylactic acid solution dissolved with ibuprofen drug, wherein the concentration of the modified polylactic acid is is 2 wt%, and the concentr...
Embodiment 2
[0025] 1) Preparation of amino-modified polylactic acid: Dissolve poly-L-lactic acid particles in 1,4-dioxane solvent in a 60°C water bath to prepare a homogeneous solution with a concentration of 1 wt%. A 1.2 wt% ethylenediamine aqueous solution was added to the phase solution, reacted for 30 min, cooled to room temperature, and then placed in a freeze dryer at -80 °C to freeze-dry to obtain aminated polylactic acid.
[0026] 2) Preparation of printing paste: Weigh a certain amount of lecithin and sodium alginate and dissolve them in 4 wt% ethanol aqueous solution to obtain a lecithin-dissolved sodium alginate solution, and lecithin-dissolved sodium alginate solution The concentration of lecithin was 3 wt%. Dissolving the amino-modified polylactic acid and minocycline drug prepared in step 1) in 1,4-dioxane solvent to obtain a modified polylactic acid solution in which minocycline drug is dissolved, wherein the modified polylactic acid The concentration of lactic acid was 3 ...
Embodiment 3
[0029] 1) Preparation of amino-modified polylactic acid: Dissolve poly-L-lactic acid particles in 1,4-dioxane solvent in a 60°C water bath to prepare a homogeneous solution with a concentration of 1 wt%. A 1.2 wt% ethylenediamine aqueous solution was added to the phase solution, reacted for 30 min, cooled to room temperature, and then placed in a freeze dryer at -80 °C to freeze-dry to obtain aminated polylactic acid.
[0030] 2) Preparation of printing paste: Weigh a certain amount of lecithin and sodium alginate and dissolve them in 4 wt% ethanol aqueous solution to obtain a lecithin-dissolved sodium alginate solution, and lecithin-dissolved sodium alginate solution The concentration of lecithin is 2 wt%. Dissolving the amino-modified polylactic acid, loxoprofen sodium and vancomycin drug prepared in step 1) in 1,4-dioxane solvent to obtain loxoprofen sodium and vancomycin drug dissolved Modified polylactic acid solution, wherein the concentration of modified polylactic aci...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com