Conjugated polymer with main chain containing osmapentalyne as well as preparation method and application of conjugated polymer
A technology of conjugated polymers and osmium pentapentyl, which is applied in the field of polymer chemistry and can solve problems such as hindering performance application research and polymer synthesis difficulties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0089] The preparation of monomer bis-osmopentyne M1: under nitrogen atmosphere, under magnetic stirring, the polyyne carbon chain organic compound L1 (1.50g, 3.55mmol), triphenylphosphine (9.32g, 35.5mmol) and triphenylphosphine (9.32g, 35.5mmol) and (Triphenylphosphine) osmium dichloride complex OsCl 2 (PPh 3 ) 3 (11.18 g, 10.65 mmol) was dissolved in 80 mL of dichloromethane. After reacting at room temperature for 6 hours, the reaction solution was concentrated to 15 mL, diethyl ether (200 mL) was added to precipitate the product, filtered, and the solid product was washed several times with diethyl ether (3×150 mL). The product was further separated and purified through a silica gel column (washing solution with dichloromethane / methanol=10:1), and 5.73 g of a tan solid product was obtained with a yield of 65%. The structural characterization data of monomer bis-osmopentyne M1 are as follows: 1 H NMR plus 1 H- 13 C HSQC (600.1MHz, CD 2 Cl 2 ): δ=13.09(s, 2H, C 7 H)...
Embodiment 1
[0095]
[0096] [Os] in the above formula is OsCl(PPh 3 ) 2 . Among them, HCl·Et 2 O is diethyl ether hydrochloride (purchased from Sarn Chemical Technology (Shanghai) Co., Ltd., the trade name is A01W8101821000), 1,4-diethynylbenzene E1 is purchased from Bailingwei Technology Co., Ltd., the trade name is 009135), and the DCM used is dichloro Solvent redistilled with methane.
[0097] The preparation of the above-mentioned main chain containing osmium pentyne conjugated polymer: under nitrogen atmosphere, under magnetic stirring, the monomer bis osmium pentyne compound M1 (0.08mmol) and 1,4-diethynylbenzene (0.081mmol) was dissolved in 10mL of dichloromethane, quickly added 1mL of 2mol / L ether hydrochloride solvent, reacted at room temperature for 5 hours, added a large amount of ether, a blue solid compound was precipitated, filtered, and repeated several times with ether The solid product was washed and dried to obtain 188 mg of the main chain-containing ospentyne con...
Embodiment 2
[0101]
[0102] [Os] in the above formula is OsCl(PPh 3 ) 2 . Among them, HCl·Et 2 O is diethyl ether hydrochloride (purchased from Sarn Chemical Technology (Shanghai) Co., Ltd., trade name is A01W8101821000), diacetylene E2 is synthesized according to the method reported in document Organometallics, 2001, 20 (11), 2262-2269, and the DCM used is dichloromethane Solvent redistilled.
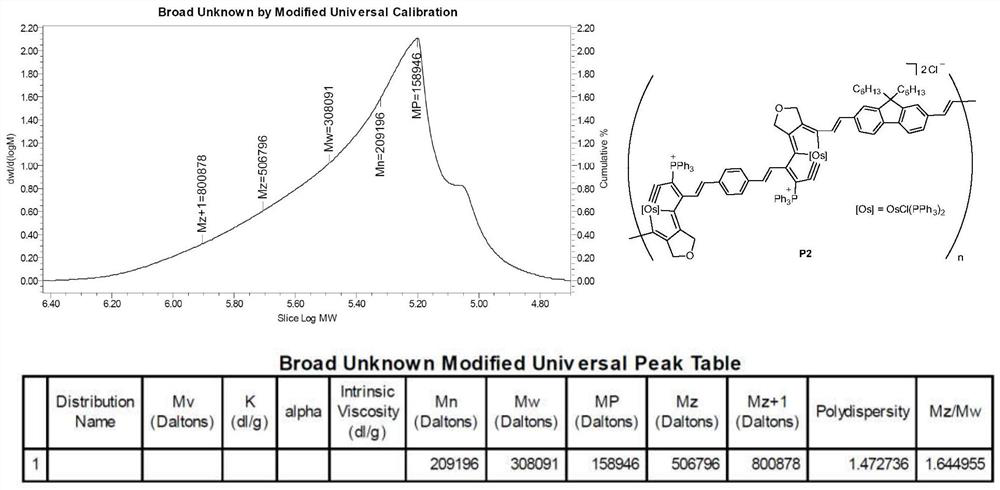
[0103] The preparation of the above-mentioned main chain containing osmium pentyne conjugated polymer P2: under nitrogen atmosphere, under magnetic stirring, the monomer bis osmium pentyne compound M1 (0.10mmol) and diyne E2 (0.101mmol) Dissolve in 12 mL of dichloromethane, quickly add 1.2 mL of 2 mol / L diethyl ether hydrochloride solvent, add a large amount of diethyl ether, a purple solid compound precipitates, filter, wash the solid product with diethyl ether repeatedly, and dry to obtain 252 mg such as figure 1 The backbone shown contains the osmalpentyne conjugated polymer P2.
[0104...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com