Biological sample safety protective agent and application thereof
A technology for biological samples and safety protection, applied in the fields of biology and medicine, can solve problems such as chemical pollution, protein disintegration and inactivation, and huge impact on test results, and achieve the effects of fast reproduction, low nutritional requirements, and enhanced bactericidal effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] The specific steps of the experimental research method of this application are:
[0051] 1. Bactericidal test method
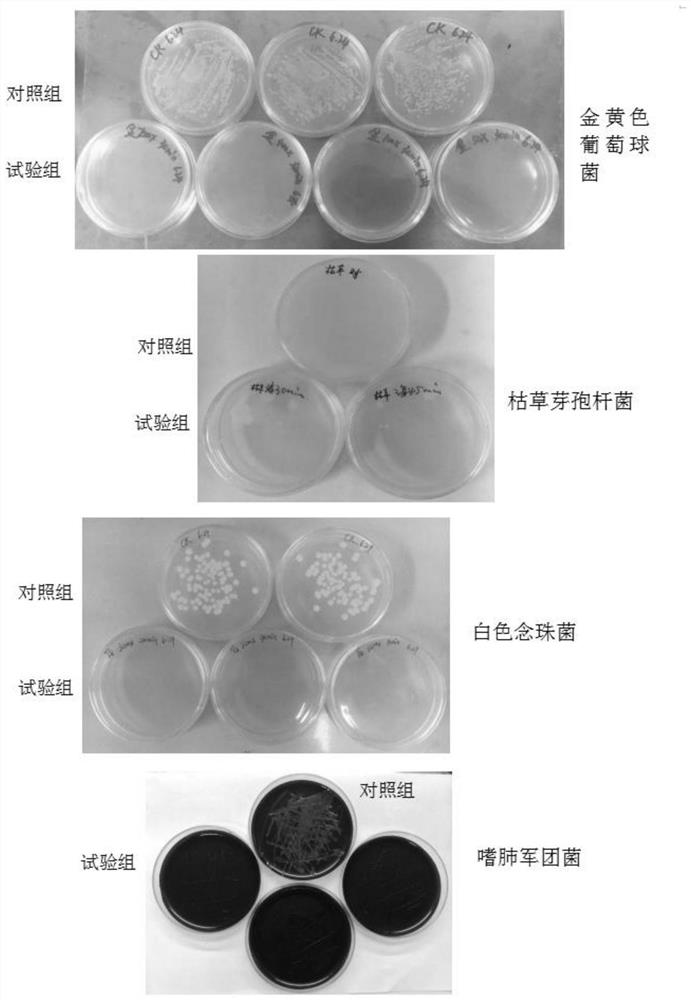
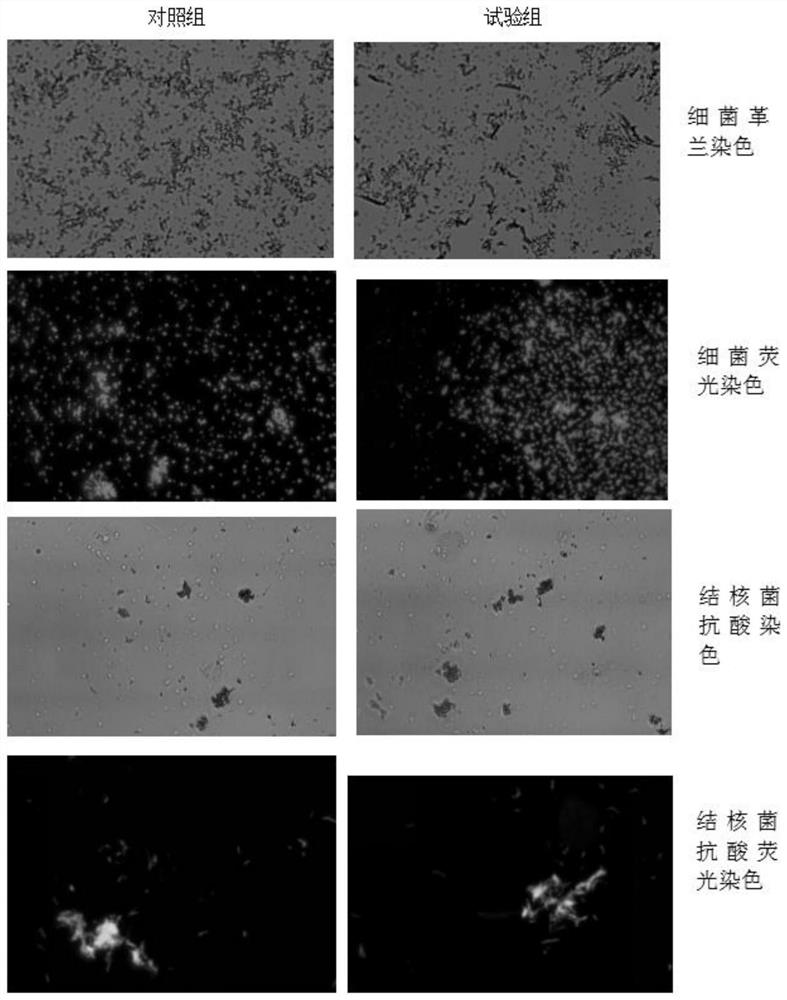
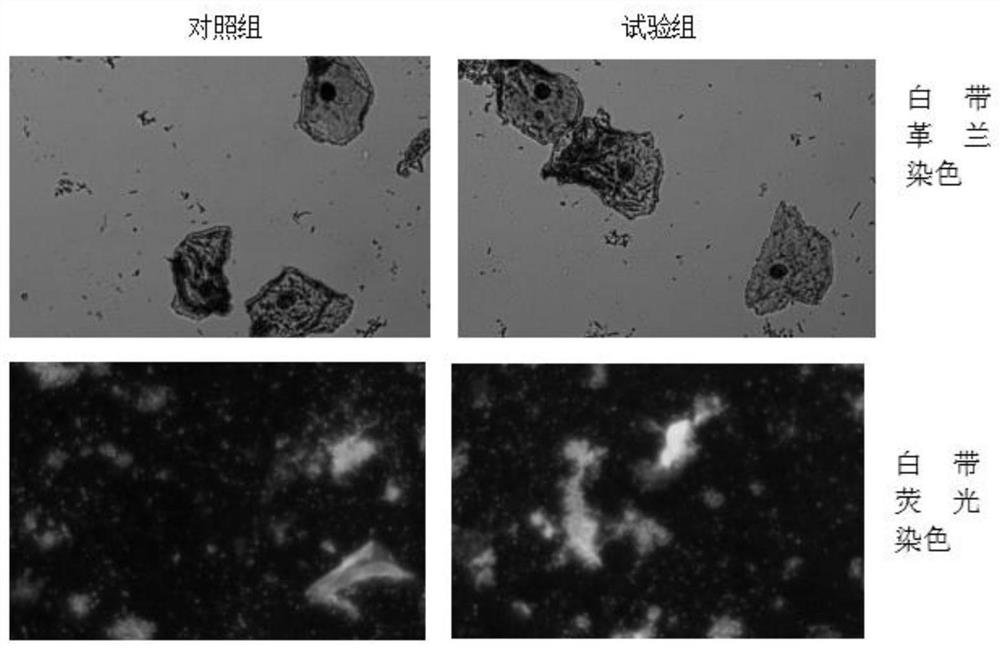
[0052] 1. Preparation of test bacterial solution: select Staphylococcus aureus, Bacillus subtilis, Legionella pneumophila, and Candida albicans solid plate cultures in the logarithmic growth phase, and scrape the colonies into sterile saline respectively. Adjust the bacterial concentration to 0.5 McFarland turbidity, and then make a 1:1000 dilution to make the bacterial concentration about 5*10 5 CFU / ml, made into 4 kinds of test bacterial solutions;
[0053] 2. Divide each bacterial solution into two parts, one part is used for bactericidal test, and the other part is used for control test;
[0054] 3. Sterilization: Add an equal volume of the bactericide to be tested to the test tube / well, and add an equal volume of sterile saline to the control tube / well;
[0055] 4. Inoculation: 10min, 20min, 30min, and 45min after mixing, pipette the liquid in t...
Embodiment 2
[0074] Embodiment 2 minimum inhibitory concentration test
[0075] 1. Minimum inhibitory concentration test of hydrogen peroxide system
[0076] Hydrogen peroxide is produced by oxidase-catalyzed decomposition of substrate. When the amount of substrate is large enough, the production of hydrogen peroxide is controlled by the amount of enzyme.
[0077] Preparation of different concentrations of hydrogen peroxide sterilizing solution: the mass percent concentration of the prepared glucose aqueous solution is 5%, the concentration of the glucose oxidase stock solution is 12800u / ml, after the glucose oxidase contacts with the glucose, hydrogen peroxide can be continuously produced to form a sterilizing solution. The sterilizing solutions with different concentrations were prepared by doubling dilution method in a sterile plastic microwell plate. Wells 1-8 contained hydrogen peroxide, and well 9 was a control without hydrogen peroxide, as shown in Table 2.
[0078] Table 2
[007...
Embodiment 3
[0108] One, the preparation of the safety protective agent of the present invention, concrete proportioning is as shown in table 11:
[0109] Table 11
[0110]
[0111] Bisdecyldimethylammonium chloride solution, MIT solution, and CMIT solution were all prepared with deionized water.
[0112] Prepare component A and component B according to the above ratio, and then use 0.22um microporous membrane to filter and sterilize, and set aside. Component A and component B are added into the biological sample to be tested in equal volumes during use, and the sum of the volumes of component A and component B is the same as the volume of the biological sample to be tested.
[0113] 2. The disinfecting effect of safety protective agent on pathogenic bacteria
[0114] (1) Carry out with reference to the aforementioned "one. Bactericidal test method", get the bacterium liquid of each kind of test bacteria, and the amount of substances added to the test tube and the control tube is as s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com