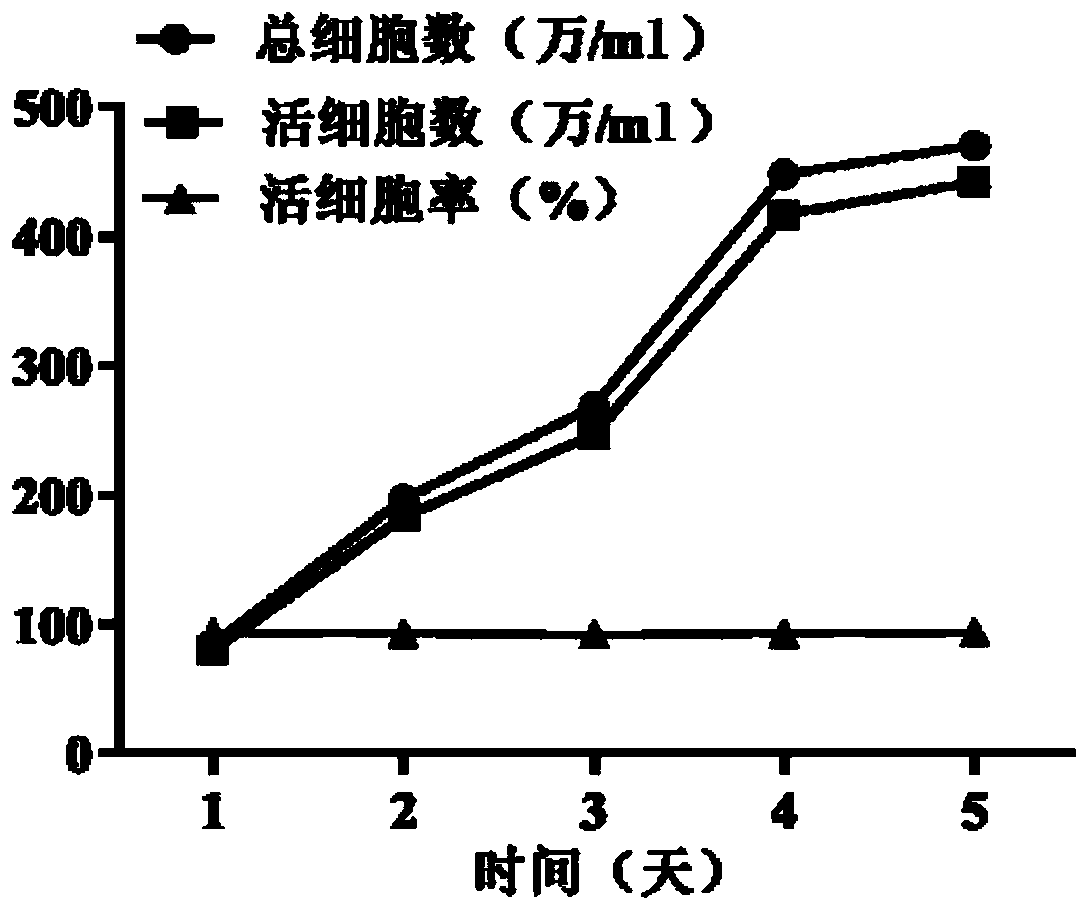
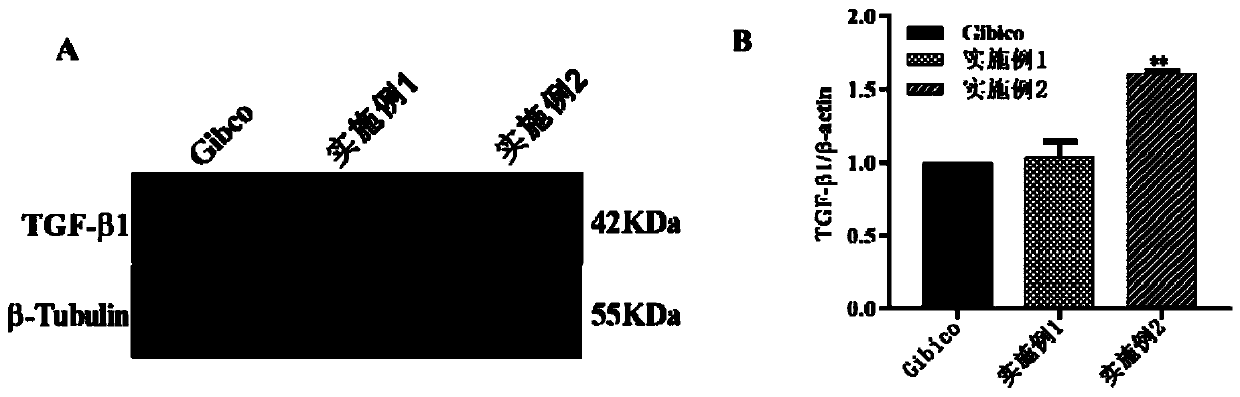
Serum-free protein-free culture medium supporting HEK293 cell suspension culture and preparation method and application thereof
A protein-free medium, cell suspension technology, applied in the field of cell engineering, can solve the problems of cell clumping, affecting cell activity, culture pollution, etc., to achieve the effect of promoting expression and promoting metabolism
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] In this embodiment, the serum-free and protein-free medium supporting the suspension culture of HEK293 cells is composed as follows (the unit of concentration of each substance is mg / L):
[0051] Amino acid composition:
[0052] L-histidine hydrochloride monohydrate 94.44;
[0053] L-isoleucine 163.41;
[0054] L-Leucine 177.15;
[0055] L-Lysine 273.75;
[0056] L-Methionine 50;
[0057] L-phenylalanine 106.44;
[0058] L-threonine 160.35;
[0059] L-tryptophan 51.1;
[0060] L-valine 158.55;
[0061] Glycine 56.25;
[0062] L-alanine 13.35;
[0063] L-Glutamine 1626;
[0064] L-arginine hydrochloride 442.5;
[0065] L-asparagine 200;
[0066] L-Aspartic Acid 39.95;
[0067] L-cysteine hydrochloride monohydrate 52.68;
[0068] L-cystine hydrochloride 62.58;
[0069] L-glutamic acid 22.05;
[0070] L-proline 115;
[0071] L-serine 72;
[0072] L-tyrosine disodium salt dihydrate 167.37;
[0073] Trace element composition:
[0074] Ammonium metavanad...
Embodiment 2
[0135] In this example, the serum-free and protein-free medium that supports the suspension culture of HEK293 cells contains, in addition to the amino acids, trace elements, vitamins, lipids, inorganic salts, additives and anti-agglomeration substances in Example 1, it also contains autophagy Inhibitor 3-methyladenine. Before adding autophagy inhibitors, dissolve 500mM 3-methyladenine stock solution in 0.5M HCl, and add 3-methyladenine to a final concentration of 10mM one day after the cell concentration reaches its peak, and then use 7.5% w / vNaHCO 3 The solution adjusted the pH of the culture to 7.0.
[0136] The preparation method of the serum-free and protein-free medium in this example is the same as that in Example 1.
Embodiment 3
[0138] In this embodiment, the serum-free and protein-free medium supporting the suspension culture of HEK293 cells is composed as follows:
[0139] Amino acid components: L-histidine hydrochloride monohydrate 100mg / L, L-isoleucine 160mg / L, L-leucine 185mg / L, L-lysine 265mg / L, L-methionine 55mg / L, L-Phenylalanine 100mg / L, L-Threonine 165mg / L, L-Tryptophan 45mg / L, L-Valine 165mg / L, Glycine 50mg / L, L-Alanine Acid 18mg / L, L-glutamine 1600mg / L, L-arginine hydrochloride 450mg / L, L-asparagine 190mg / L, L-aspartic acid 45mg / L, L- Cysteine hydrochloride monohydrate 45mg / L, L-cystine hydrochloride 70mg / L, L-glutamic acid 18mg / L, L-proline 120mg / L, L-serine 65mg / L , L-tyrosine disodium salt dihydrate 175mg / L;
[0140] Trace element components: ammonium metavanadate 0.0012mg / L, sodium selenite 0.05mg / L, manganous chloride tetrahydrate 0.3mg / L, copper sulfate pentahydrate 0.3mg / L, zinc chloride 0.75mg / L ;
[0141] Vitamin components: vitamin H (biotin) 0.15mg / L, folic acid 8mg / L, vit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com