Synthesizing method for helicobacter pylori O:6 serotype O-antigen sugar chains
A technology of Helicobacter pylori and serotypes, applied in the field of carbohydrate chemistry, can solve problems such as constraints, insufficient specificity of lipopolysaccharide structure, and research interference.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
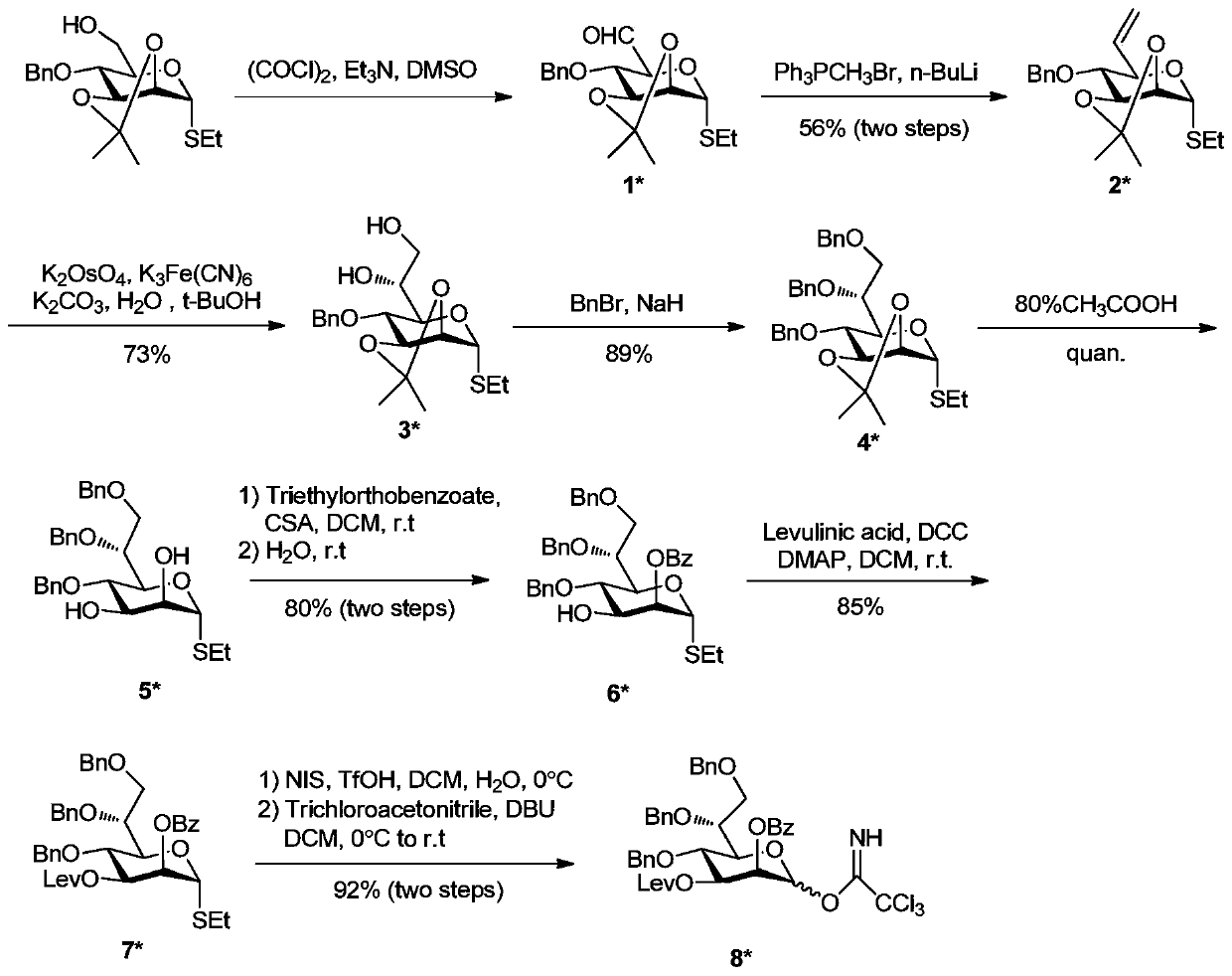
[0086] Synthesis of Example 1 Sugar Block 8*:
[0087] Synthetic route such as image 3 shown.
[0088] Using 2,3-O-propylidene-4-O-benzylmannothioside as the starting material, after swern oxidation, the 6-position hydroxyl was oxidized to aldehyde to obtain compound 1*. Then, the carbon chain at the 6-position was extended by wittig reaction to obtain the 6-position deoxygenated alkene compound 2*. Olefin compounds in potassium osmate (K 2 OSo 4 ), potassium ferricyanate (K 3 Fe(CN) 6 ) and potassium carbonate (K 2 CO 3 ) under the combined action of dihydroxylation to obtain 6,7-di-hydroxyl compound 3*. Bn protection of 6,7-di-hydroxyl under the action of sodium hydride (NaH) gave compound 4*. After removing the propylidene group under the action of 80% acetic acid, the compound 5* was obtained, and then under the action of D(+)-10-camphorsulfonic acid (CSA), the 2,3-position hydroxyl group was ring-formed and protected. The ring was opened under weak acid conditi...
Embodiment 2
[0098] Synthesis of Example 2 Sugar Block 13*:
[0099] Synthetic route such as Figure 4 shown.
[0100] Such as figure 2 , starting from compound 3*, using dibutyltin oxide (Bu 2 SnO) selective 7-OH Bn protection to obtain compound 9*, and then 6-OH protection with Lev to obtain compound 10*. After removing the propylidene group of compound 10* under the action of 80% acetic acid, the 2,3-OH was protected with acetyl group to obtain the sugar block 11*.
[0101] The synthesis of sugar building blocks 13*, firstly, using the previously prepared intermediate compound 3,4-position starting materials, in dibutyltin oxide (Bu 2 Under the action of SnO), the 3-OH of compound 5* was selectively protected by Bn to obtain compound 12*, and finally the 2-OH was protected by acetyl to obtain the heptose block 13*.
[0102] Specific test operation and steps:
[0103] Compound 9*: Compound 3* (0.77g, 2mmol) and Bu 2 SnO (0.75g, 3mmol) was dissolved in dry toluene (10mL), and the ...
Embodiment 3
[0108] The synthesis of embodiment 3 reducing end trisaccharides:
[0109] The synthetic route of the reducing end trisaccharide is as follows Figure 5 shown.
[0110] 3.1 pair Figure 5 The conditions of the glycosylation reaction in the trisaccharides at the reducing end were optimized (Table 1), and the conditions for determining the optimal glycosylation reaction were as follows: the glycosyl donor and the acceptor were co-distilled three times in toluene; adding anhydrous DCM , the reaction concentration is 0.1M, the activated or Molecular sieves; after the mixture was cooled to -10°C and stirred for 15 minutes, the activation reagents TMSOTf (0.12eq) and NIS (1.2eq) were added, and the reaction time was 3h. After the reaction, triethylamine (Et 3 N) terminate the reaction. The reaction solution was filtered, diluted with DCM and then diluted with saturated NaHCO 3 Wash, anhydrous Na 2 SO 4 After drying and concentration, it was separated and purified by silic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com