A sort of 18 Use of f-sfb-cml and method for detecting atherosclerosis
An atherosclerosis and purpose-based technology, applied in the field of nuclear medicine, can solve the problems of inability to effectively monitor plaque composition and vulnerability, the display of multiple vascular lesions is not as good as nuclear medicine, and the specificity and sensitivity of F-FDG is poor. Beneficial for rapid imaging analysis, not easy to degrade, and easy for high-resolution imaging
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1: 19 Synthesis and quality control of F-SFB-CML standard reference substance
[0040] (1) 19 Synthetic steps of F-SFB-CML standard reference substance
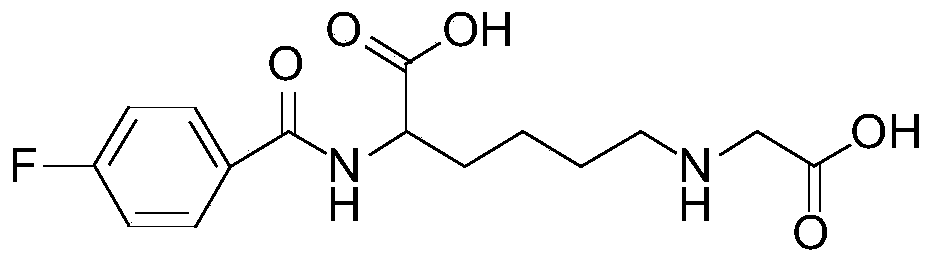
[0041] Take 25 μL of carbonic acid buffer solution (pH=9) of 1 mg / mL CML (carboxymethyllysine complex) dissolved in advance to the reaction tube, and then add [ 19 F]-SFB (N-succinimide-4-fluorobenzoate) solution (1mg / mL) 25μL, then put the reaction tube in an oil bath, react at 65°C for 1h, and use a thin-layer chromatography plate during the reaction After monitoring the reaction, take out the reaction solution and add it to semi-preparative high-performance liquid chromatography (HPLC) for separation and purification, and collect the target product 19 F-SFB-CML. The structure was confirmed by mass spectrometry and NMR, and its chemical purity was detected by analytical high performance liquid chromatography (HPLC).
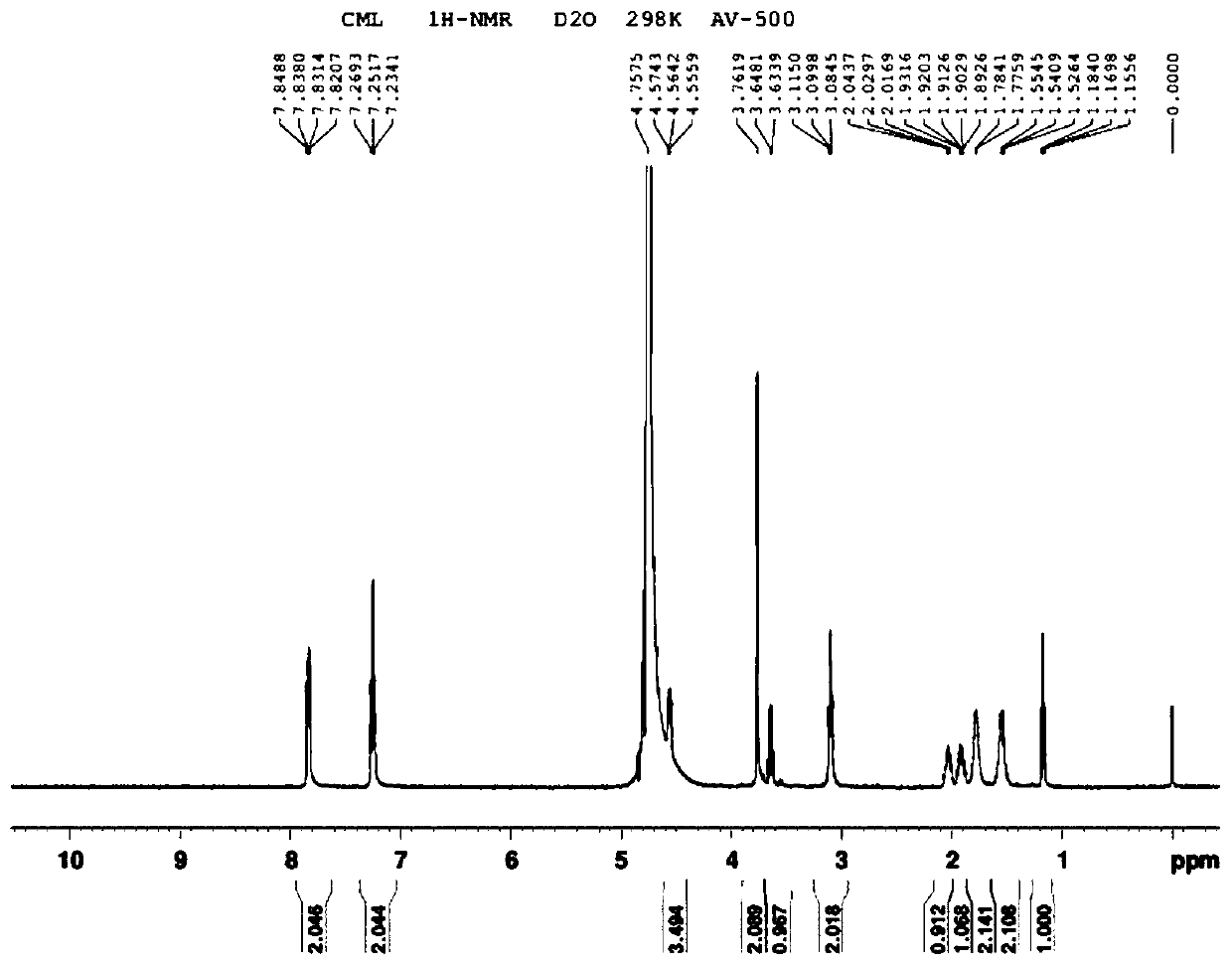
[0042] (2) 19 NMR and MS structure confirmation of F-SFB-CML standard
[0043] obtain...
Embodiment 2
[0046] Example 2: 18 F-SFB-CML labeling and isolation and purification
[0047] (1) 18 Radiolabeled synthetic steps of F-SFB-CML
[0048] Produced by bombarding heavy oxygen water with an accelerator 18 F ions first pass through the QMA column (use 10mL 0.5M NaHCO 3 Rinse, and then rinse with 20mL of sterile water for injection) to capture, after enrichment, use K222 / K 2 CO 3 Eluted to reaction tube No. 1, dried to remove water and cooled to room temperature, then added a certain amount of anhydrous acetonitrile to dry and remove water again, and cooled to room temperature.
[0049] Add SFB (10mg SFB, dissolved in 1mL acetonitrile) to react at 90°C for 7min, and cool to room temperature after the reaction. Then add 6 mL of 0.1 M HCl, ventilate and stir for 1 min, and the reaction solution passes through a C18 column (washed with a mixture of 7.5 mL of 0.1 M HCl and 2.5 mL of acetonitrile before use) to a waste liquid bottle. Add 3 mL of acetonitrile to flow through the ...
Embodiment 3
[0071] Example 3: ApoE - / - mouse micro PET scan
[0072] (1) Construction of animal model of diabetic atherosclerosis
[0073] Male apoE used in this experiment - / -The mice were fed in the SPF-grade mouse room of the Experimental Animal Center of Jiangsu University. Feeding conditions: temperature 22±2°C, humidity 40-60%, 12-hour cycle lighting, normal diet, free intake of food and water. All items entering the SPF room must be sterilized by high temperature and high pressure, and strict aseptic operation is implemented. At the age of 6 weeks, the mice in the experimental group were given intraperitoneal injection of streptozotocin (STZ, dissolved in 0.05 mol / L citrate buffer at pH 4.5) 40 mg / kg / day for 5 consecutive days. After 2 weeks, the mice with blood glucose level > 300 mg / dL were included in the research object of this example, and changed from normal diet to semi-synthetic high-fat diet (high-fat diet, HFD) (21% fat, 0.15% cholesterol, other ingredients same diet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com