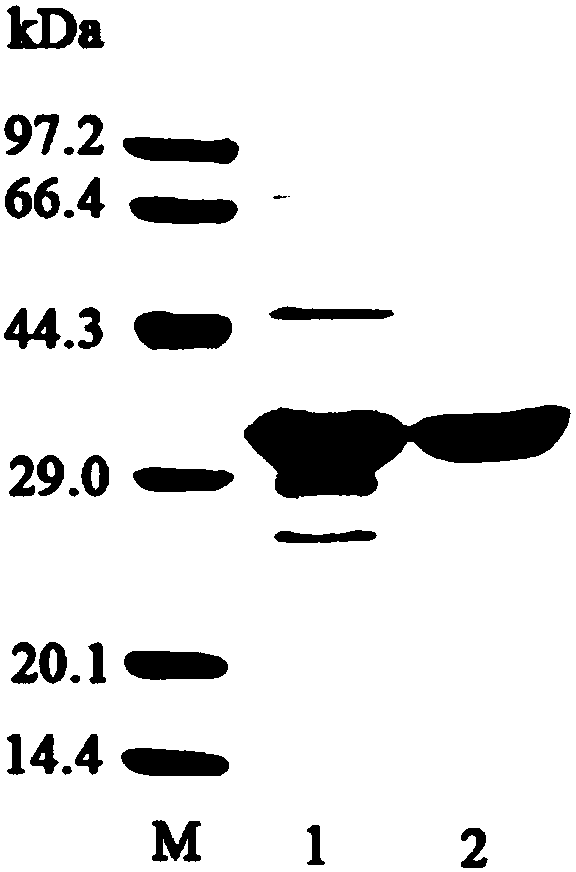Application of protein AxMan113A as beta-mannase
A technology of mannanase and mannan, applied in the field of microorganisms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Embodiment 1, the preparation of recombinant β-mannanase
[0074] 1. Construction of recombinant plasmid pET28a-AxMan113A
[0075] 1. Extract the genomic DNA of xylan facultative bacillus NBRC15112 and use it as a template, and use the artificially synthesized upstream primer: 5'-CCG GAATTC ATGGAATTTATTAAGGGTTTTACAT-3' (the underline is the cutting site of the restriction endonuclease EcoRI) and downstream primer: 5'-ATAAGAAT GCGGCCGC TTAACGTGATTGGTAATAAGCTTTA-3' (the underline is the restriction endonuclease NotI restriction endonuclease cutting site) is primer, carries out PCR amplification, obtains PCR amplification product.
[0076] The reaction program was: pre-denaturation at 95°C for 5 min; denaturation at 94°C for 30 s, annealing at 55°C for 30 s, extension at 72°C for 1 min, 30 cycles; extension at 72°C for 10 min.
[0077] 2. Take the PCR amplification product obtained in step 1, perform agarose gel electrophoresis, and then recover a DNA fragment of about...
Embodiment 2
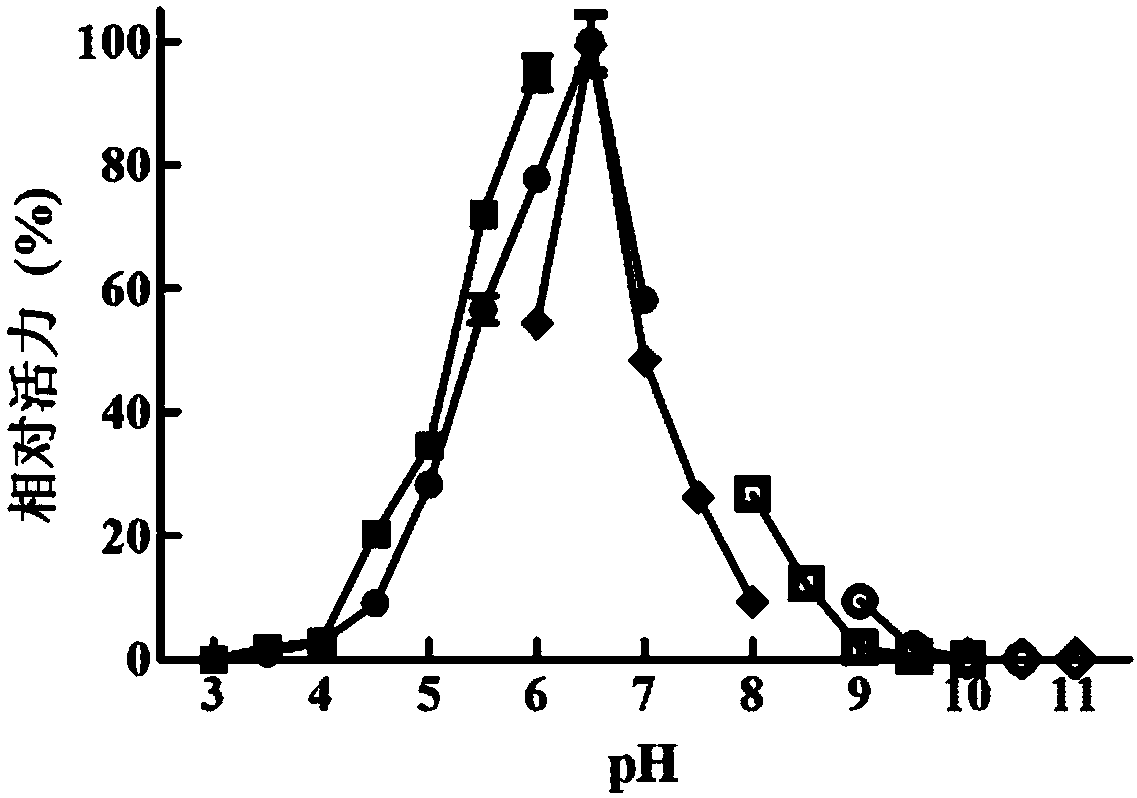
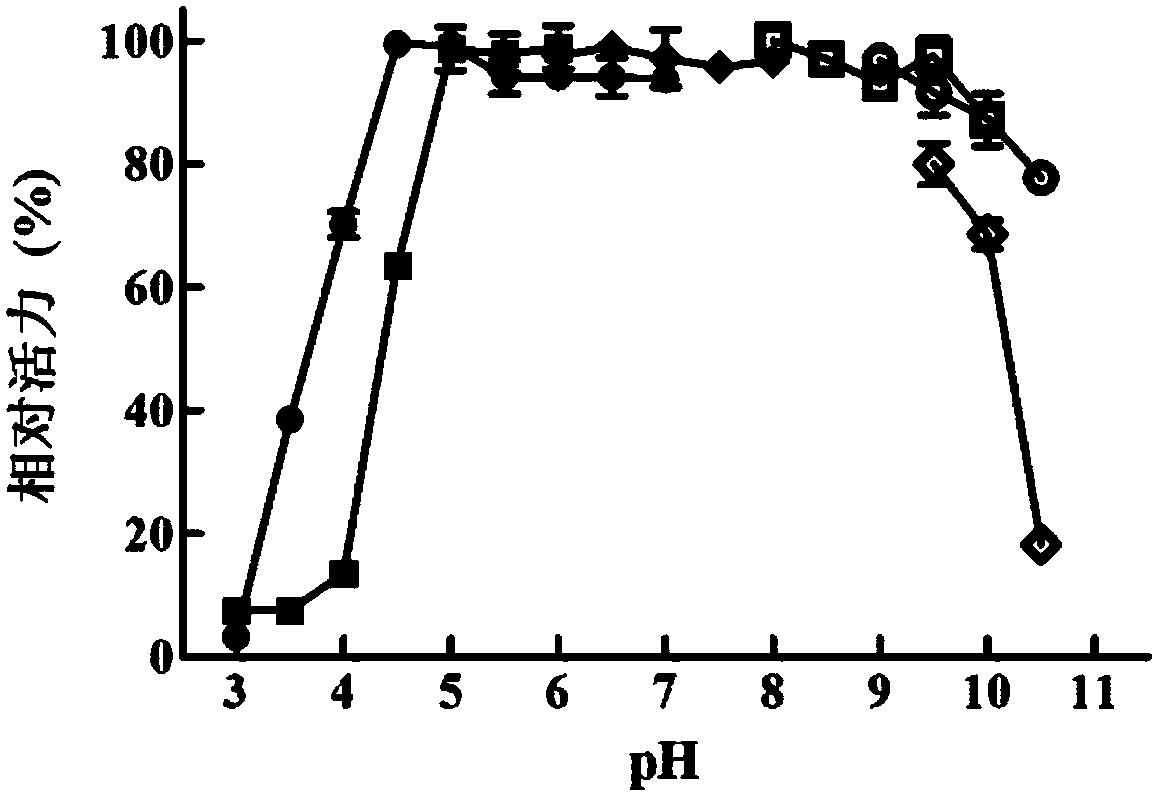
[0095] Embodiment 2, the determination of the enzymatic property of recombinant β-mannanase
[0096] Buffer to be tested: pH3.0 citrate-phosphate buffer, pH3.5 citrate-phosphate buffer, pH4.0 citrate-phosphate buffer, pH4.5 citrate-phosphate buffer, pH5.0 lemon Acid phosphate buffer, pH5.5 citrate phosphate buffer, pH6.0 citrate phosphate buffer, pH6.5 citrate phosphate buffer, pH7.0 citrate phosphate buffer, pH3.0 lemon Acid buffer, pH3.5 citrate buffer, pH4.0 citrate buffer, pH4.5 citrate buffer, pH5.0 citrate buffer, pH5.5 citrate buffer, pH6 .0 citrate buffer, pH6.0 phosphate buffer, pH6.5 phosphate buffer, pH7.0 phosphate buffer, pH7.5 phosphate buffer, pH8.0 phosphate buffer, pH8. 0 CHES buffer, pH 8.5 CHES buffer, pH 9.0 CHES buffer, pH 9.5 CHES buffer, pH 10.0 CHES buffer, pH 9.0 glycine-sodium hydroxide buffer, Glycine-NaOH buffer at pH 9.5, Glycine-NaOH buffer at pH 10, Glycine-NaOH buffer at pH 10.5, CAPS buffer at pH 10.0, CAPS buffer at pH 10.5 or CAPS buffer a...
Embodiment 3
[0115] Example 3. Recombinant β-mannanase hydrolyzes locust bean gum to produce mannan oligosaccharides
[0116] Thin-layer chromatography analysis: Spot 2 μL of the sample to be tested on the TLC analysis plate, add the developing agent dropwise for two times, then completely wet it with the color developing solution and dry it, and develop the color at 100°C. The spreading agent is composed of 2 parts by volume of n-butanol, 1 part by volume of ethanol and 1 part by volume of water. The developer consists of 95 parts by volume of methanol and 5 parts by volume of sulfuric acid. The mixture of standard products of mannose, mannobiose, mannotriose, mannotetraose, mannopentaose and mannohexaose was used as the standard control.
[0117] Reaction system (total volume: 1 mL): pH 6.5, 50 mM citrate phosphate buffer, locust bean gum and the recombinant β-mannanase solution prepared in Example 1 were mixed to obtain a reaction system. In the reaction system, the initial concentrat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com