A whole-cell catalytic method for preparing tagatose
A whole-cell, tagatose technology, applied in biochemical equipment and methods, microorganism-based methods, microorganisms, etc., can solve problems such as inability to be widely used, high production cost of tagatose, and impact on the final price of tagatose , to achieve the effect of low pollution, high yield and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
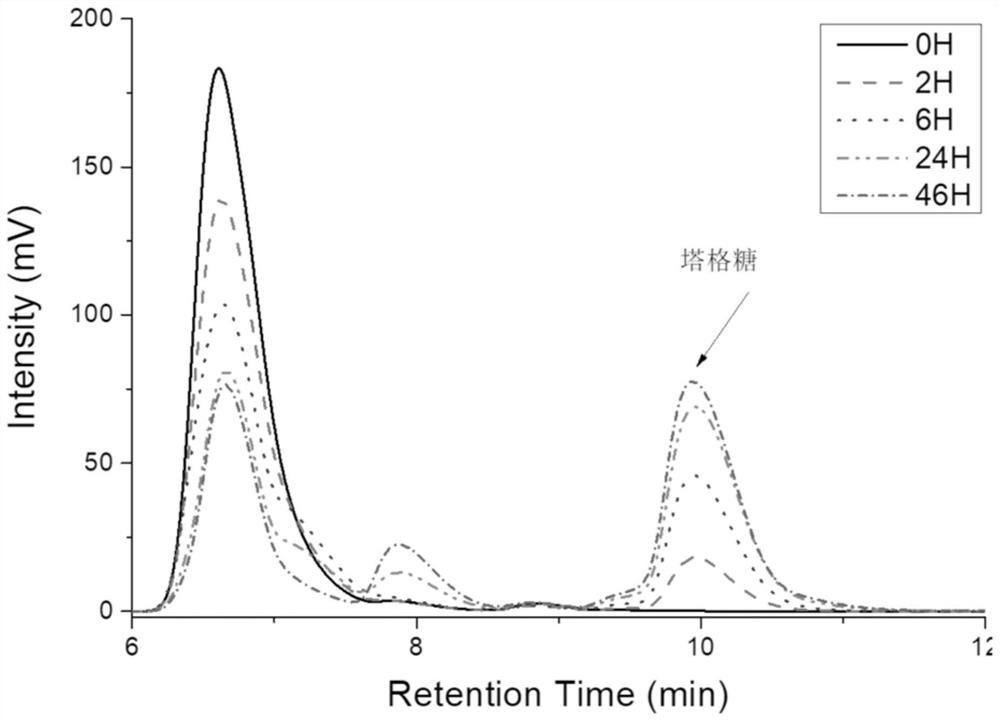
Image
Examples
Embodiment 1
[0041] Embodiment 1 prepares tagatose from starch
[0042] (1) Constructed genes containing α-glucan phosphorylase gene, glucose phosphate mutase gene, glucose phosphate isomerase gene, 6-phosphate tagatose epimerase gene, 6-phosphate tagatose phosphate The prokaryotic expression plasmid of the enzyme gene; the constructed prokaryotic expression plasmid was transferred into Escherichia coli engineering bacteria to obtain recombinant strains; the recombinant strains were induced to express and produce enzymes and then permeabilized:
[0043] In this example, α-glucan phosphorylase is derived from Thermotoga maritima, and the gene number on KEGG is TM1168; glucose phosphomutase is also derived from Thermotoga maritima, and the gene number on KEGG is TM0769; The isomerase is derived from Clostridium thermocellum, and the gene number on KEGG is Cthe0217; the 6-phosphate tagatose epimerase is from Thermoanaerobacter indiensis, and the enzyme coded by the gene is numbered WP_0199072...
Embodiment 2
[0049] Embodiment 2 prepares tagatose from starch
[0050] (1) Constructed genes containing α-glucan phosphorylase gene, glucose phosphate mutase gene, glucose phosphate isomerase gene, 6-phosphate tagatose epimerase gene, 6-phosphate tagatose phosphate Prokaryotic expression plasmids of enzyme genes and starch debranching enzymes; the constructed prokaryotic expression plasmids were transformed into Escherichia coli engineering bacteria to obtain recombinant strains; the recombinant strains were induced to express and produce enzymes and then permeabilized:
[0051] In this embodiment, the starch debranching enzyme is derived from Sulfolobus tokodaii, and the number of the gene on KEGG is ST0928. Genomic DNA can be obtained from the official website of ATCC (www.atcc.org). The gene was obtained by PCR and cloned into the pET20b vector to obtain the corresponding expression vector pET20b-StIA. The plasmid was transformed into Escherichia coli expression strain BL21(DE3), and...
Embodiment 3
[0054] Embodiment 3 prepares tagatose from starch
[0055] (1) Constructed genes containing α-glucan phosphorylase gene, glucose phosphate mutase gene, glucose phosphate isomerase gene, 6-phosphate tagatose epimerase gene, 6-phosphate tagatose phosphate Prokaryotic expression plasmids of enzyme genes, starch debranching enzyme, glucan transferase, and polyphosphoglucokinase; the constructed prokaryotic expression plasmids were transformed into E. Personalization:
[0056]In this embodiment, polyphosphoglucokinase is derived from Thermobifida fusca, and the number of the gene on KEGG is Tfu1811. Glucanotransferase is derived from Thermococcus litoralis, and the gene number on KEGG is OCC_10078. Genomic DNA can be obtained from the official website of ATCC (www.atcc.org). These two genes were obtained from the corresponding genomic DNA by PCR with different primers, and cloned into the pET20b vector to obtain the corresponding expression vectors pET20b-TfuPPGK and pET20b-Tl4G...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com