Multi-alkyl-substituted diamine compound containing polycyclic aromatic hydrocarbon structure and preparation method and application thereof
A technology for amine compounds and fused-ring aromatics, which is applied in the field of diamine compounds substituted by polyalkylenes containing fused-ring aromatics and its preparation, can solve the problem of single substitution positions of alkyl groups, and achieve large number of alkyl groups and tight packing Small, CTC effect inhibition effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
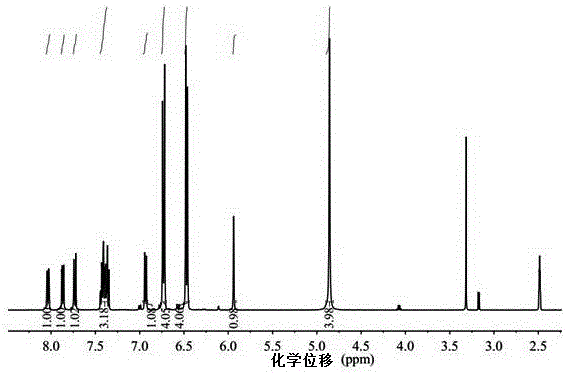
Embodiment 1
[0038] The diamine structural formula that present embodiment synthesizes is as follows:
[0039]
[0040] The synthesis steps of the above-mentioned diamine are as follows: put 16.63g of aniline in a 100mL three-necked flask, stir, blow nitrogen to replace the air in the bottle, and after heating to 130°C, use a dropping funnel to mix 7.87g of 1-naphthaldehyde and 4.2mL of concentrated The hydrochloric acid mixed solution was dropped into the reaction system, and the temperature was maintained for 18 hours. The reaction system became thick and dark brown. After the reaction, the temperature of the system was lowered to 60° C., and 10 g of 20 wt % sodium hydroxide aqueous solution was added dropwise with stirring, and then dropped into 100 mL of methanol to precipitate a solid. After filtration, the solid was washed with methanol and water for several times, and then recrystallized with methanol to precipitate brown-yellow crystals, which were collected by filtration and dr...
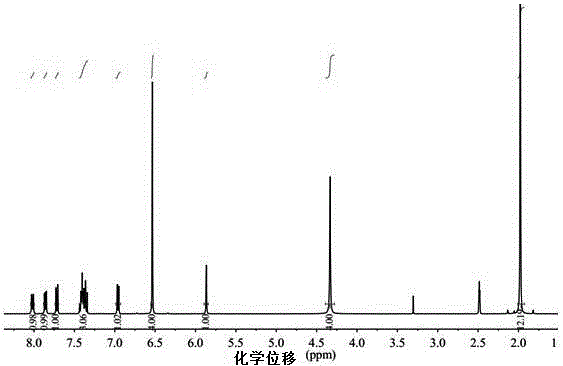
Embodiment 2
[0046] The diamine structural formula that present embodiment synthesizes is as follows:
[0047]
[0048] The synthesis steps of the above-mentioned diamine are as follows: 15.59 g of 2,6-dimethylaniline was placed in a 100 mL three-necked flask, stirred, the air in the flask was replaced by nitrogen gas, and heated to 80°C. Mix 4.29g of 1-naphthaldehyde with 2.5mL of concentrated hydrochloric acid to activate it, and drop the activated 1-naphthaldehyde solution into the reaction system within 1.5h. Then heated to 120 ° C, reflux reaction 10h. As the reaction progressed, the reaction system changed from a brown solution to a blue one, and finally turned into a blue viscous liquid. After the system dropped to 60° C., 7.5 g of 20 wt % sodium hydroxide aqueous solution was added. The temperature was then lowered to room temperature, and the reaction system was added into 200 mL of methanol under stirring, and a pale yellow solid was precipitated. After filtering, washing t...
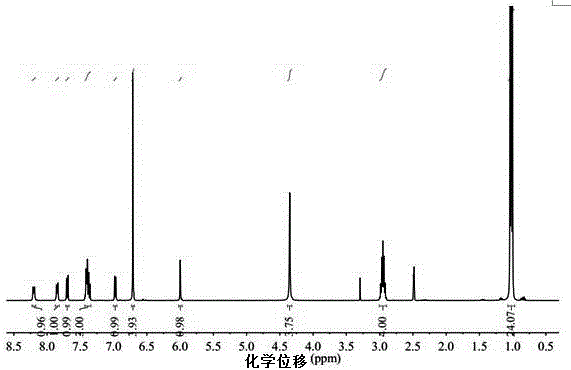
Embodiment 3
[0054] The diamine structural formula that present embodiment synthesizes is as follows:
[0055]
[0056] The synthesis steps of the above-mentioned diamine are as follows: sequentially add 22.33g of 2,6-diisopropylaniline, 15mL of water, and 30mL of ethylene glycol methyl ether into a 100mL three-necked bottle, replace the air in the bottle with nitrogen, and heat to 90°C. Slowly drop 7.5mL of concentrated sulfuric acid into the bottle to obtain a colorless and transparent solution, then drop in 10.40g of 1-naphthaldehyde, raise the temperature to 100°C, and react for 14h. A yellow precipitate appeared in the reaction system. After the reaction, cool down to room temperature, add the reaction system to 800 mL of water, adjust the pH to 9-10, stir for 4 hours, obtain a solid by filtration, and wash the solid repeatedly with a large amount of water. Dissolve the solid in dichloromethane, extract it with water three times, remove the inorganic salt, remove the dichlorometha...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com