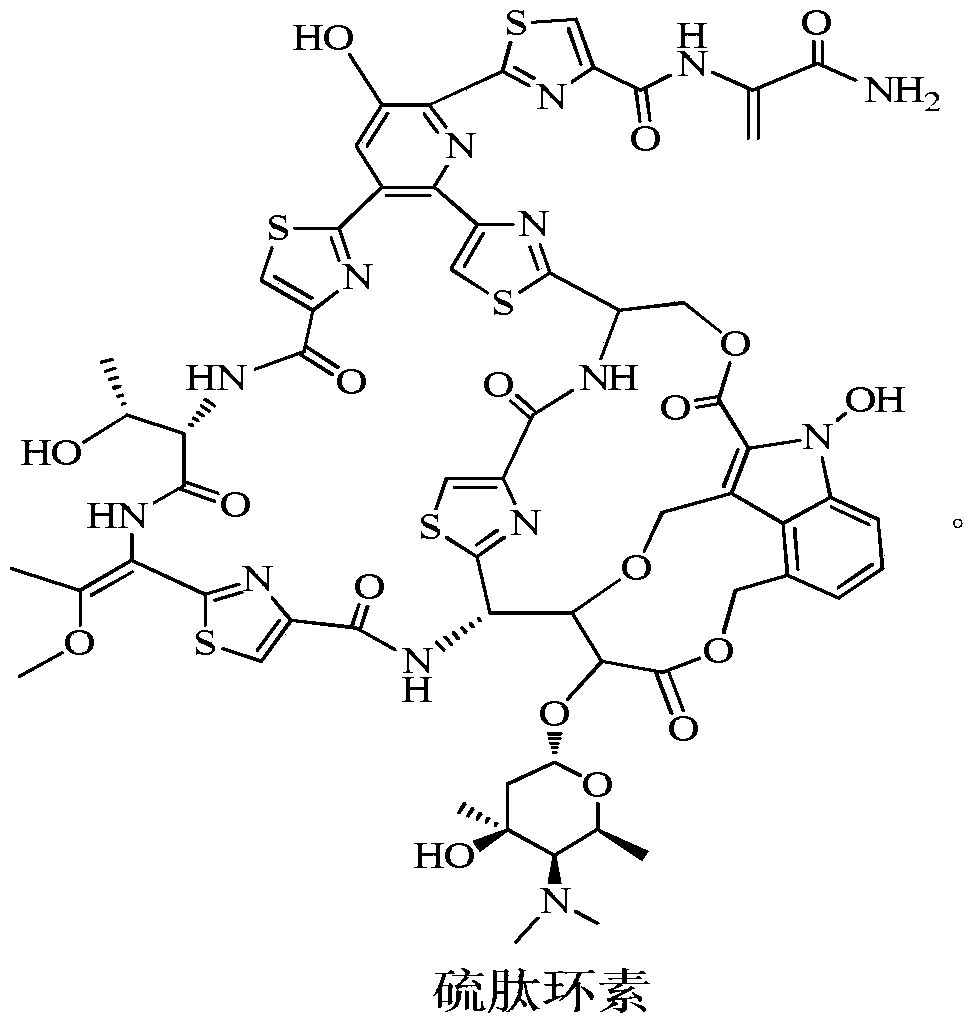
A new application of Thiopeptidecycline
A technology of thiopeptidecycline and antibiotics, which is applied in the direction of medical preparations of non-active ingredients, cyclic peptide components, dispersion liquid delivery, etc., can solve the problems of anti-anaerobic bacteria, Clostridium difficile and other problems that have not been seen. Achieve super antibacterial activity, strong antibacterial activity, and small safety hazards
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1 In vitro anti-Clostridium difficile and its drug-resistant strain activity of thiopeptcycline
[0028] The in vitro antibacterial activity of Thiopeptidecycline was tested against Clostridium difficile ATCC 9689, a clinically isolated strain of Clostridium difficile LCN 001, and a clinically isolated drug-resistant strain, and compared with vancomycin. According to the broth dilution method recommended by CLSI M11-A7, the minimum inhibitory concentration (MIC) of Thiopeptidecycline was determined. According to the measurement needs, the Brooke's broth supplemented with Hemin (5 mg / ml), vitamin K1 (1 μg / ml), lysed horse blood (5%) and oxidase (1:25v / v) was divided into different measurement groups, and then Thiopeptidecycline or vancomycin in DMSO was added. The concentration range of the test drug was 0.025 μg / ml-128 μg / ml. Check the growth of Clostridium difficile in each culture medium, the minimum antibacterial drug concentration at which the experimental...
Embodiment 2
[0031] Example 2 Thiopeptidecycline LC-MS / MS detection method
[0032] Add 90 μl of methanol containing 5 ng / ml verapamil to 30 μl of blood or urine sample, vortex for 3 minutes, then centrifuge at 14,000 rpm for 5 minutes, take 80 μl of the supernatant, and inject it into LC-MS / MS for analysis.
[0033] High performance liquid chromatography: Chromatographic column: Waters Symmetry 300 C18 3.5μm 2.1*100mm;
[0034] Mobile phase A: 10mM ammonium acetate + 0.02% ammonia solution; mobile phase B: methanol;
[0035] Analysis time: 5.2min Injection volume: 5μL Column temperature: 25°C;
[0036] Gradient elution:
[0037] time (min) Flow rate (mL / min) B% 0.00 0.4 67 2.40 0.4 67 2.45 0.4 90 3.80 0.4 90 3.85 0.4 67 5.20 0.4 67
[0038] Mass spectrometry: positive ion mode (Agilent6460B)
[0039] Detection ion pair: Thiopeptidecycline: 1437.3→172.0, fragmentor=135, CE=22;
[0040] Verapamil (internal standard): 455.2→165.1, frag...
Embodiment 3
[0042] Example 3 Pharmacokinetic Study of Oral Thiopeptidecycline
[0043] Preparation method of Thiopeptidecycline medicinal solution: Take a certain amount of Thiopeptidecycline and place it in a mixed solvent containing 10% PEG400, 1% Tween 80, and 5 mg / ml citric acid at pH 5.0, and shake until clear to obtain Thiopeptidecycline Gastrointestinal solution.
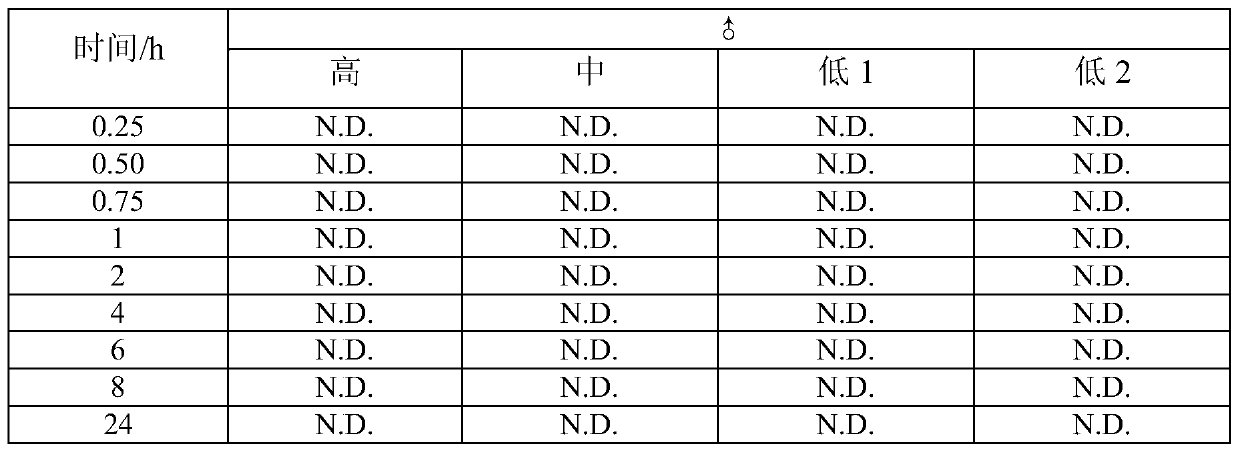
[0044] Male rats were divided into high, medium and low dose groups according to the purpose of the experiment. Among them, the high dose group is 50 mg / Kg, the middle dose group is 25 mg / Kg, the low 1 dose group is 5 mg / Kg, and the low 2 dose group is 1 mg / Kg. About 0.3 ml of blood samples were collected at each time point of about 15 minutes, 30 minutes, 45 minutes, 1, 2, 4, 6, 8 and 24 hours after the administration for the determination of bioavailability. See Example 2 for the method.
[0045] Table 2 Concentration-time data (ng / ml) of thiopeptcycline in plasma after oral administration of thiopeptetcycline in rat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com