Medicine urapidil composition tablet for treating hypertension
A technology of urapidil and composition, which is applied in the field of drug urapidil composition tablet for treating hypertension, can solve the problems of toxicity to patients, increase the risk of drug use for patients, poor stability, etc., and achieves high bioavailability, suitable for In clinical application, the effect of fluidity improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
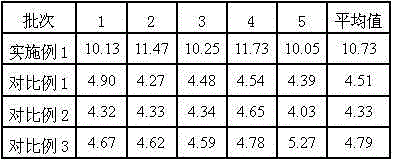
Examples
Embodiment 1
[0029] Example 1: Preparation of urapidil crystals
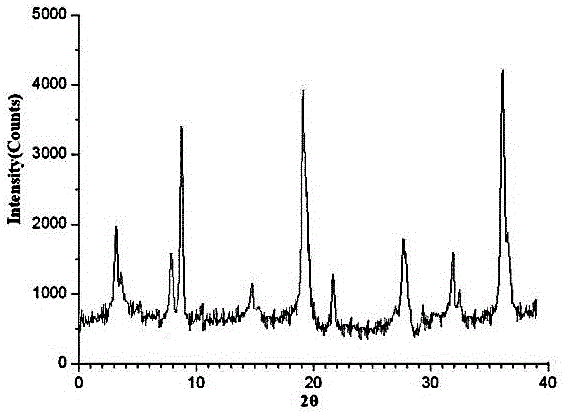
[0030] Prepare urapidil crude product saturated acetone solution, then add the mixed solvent of isobutanol and sherwood oil whose volume is 8 times of the volume of saturated acetone solution, the volume ratio of described isobutanol and sherwood oil is 1:3, after stirring , stirring while cooling down, the cooling rate is 10°C / hour, the stirring speed is 105 rpm, and at the same time, add diethyl ether whose volume is 3 times the volume of the mixed solvent of isobutanol and petroleum ether, stop stirring after cooling down to 0°C, and let it stand After growing the crystal for 3 hours, filtering, and drying under reduced pressure, the crystal compound of urapidil was obtained.
[0031] The X-ray powder diffraction pattern obtained by measuring the obtained urapidil crystal using Cu-Kα ray is as follows: figure 1 shown.
Embodiment 2
[0032] Example 2: Preparation of Urapidil Tablets
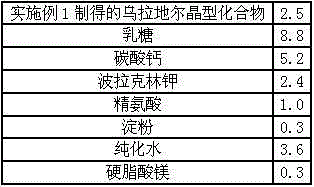
[0033] Prescription: in parts by weight
[0034]
[0035] Preparation:
[0036] (1) Processing of raw and auxiliary materials: crush urapidil through a 100-mesh sieve, and sieve arginine through a 80-mesh sieve;
[0037] (2) Weighing: Weighing according to the process prescription;
[0038] (3) Adhesive preparation: prepare starch slurry with the prescribed amount of starch and purified water by cooking slurry method, and cool to room temperature for use;
[0039] (4) Mixing and granulation: Add urapidil, lactose, calcium carbonate, polacrilin potassium, and arginine to the wet mixing granulator, turn on the stirring motor and dry mix for 10 minutes; add the prepared binder Wet mixing and cutting, using 18-mesh sieve to make soft materials;
[0040] (5) Drying: Add the wet granules obtained from granulation into a fluidized bed dryer, set the temperature at 65-70°C, and dry for 2-3 hours. After drying, the material i...
Embodiment 3
[0044] Example 3: Preparation of Urapidil Tablets
[0045] Prescription: in parts by weight
[0046]
[0047] Preparation:
[0048] (1) Processing of raw and auxiliary materials: crush urapidil through a 100-mesh sieve, and sieve arginine through a 80-mesh sieve;
[0049] (2) Weighing: Weighing according to the process prescription;
[0050] (3) Adhesive preparation: prepare starch slurry with the prescribed amount of starch and purified water by cooking slurry method, and cool to room temperature for use;
[0051] (4) Mixing and granulation: Add urapidil, lactose, calcium carbonate, polacrilin potassium, and arginine to the wet mixing granulator, turn on the stirring motor and dry mix for 10 minutes; add the prepared binder Wet mixing and cutting, using 18-mesh sieve to make soft materials;
[0052] (5) Drying: Add the wet granules obtained from granulation into a fluidized bed dryer, set the temperature at 65-70°C, and dry for 2-3 hours. After drying, the material i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com