Preparation method and application of Aeromonas salmonicida live vaccine preparation and freeze-dried vaccine product
A technology of Aeromonas salmonicida and live vaccines, applied in the direction of microorganism-based methods, biochemical equipment and methods, bacteria, etc., which can solve the problems of restoring virulence, potential harm of exogenous gene fragment environment, and easy reversion of mutations, etc. problems, to achieve the effect of easy differentiation, possibility of eliminating virulent pathogens, and easy monitoring
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0036] Experimental example 1: Verification and preparation of vaccine strains
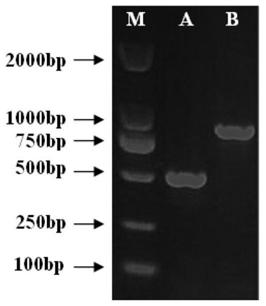
[0037] The present invention selects a wild virulent strain of Aeromonas salmonicida (a highly pathogenic strain isolated from the focus of a farmed Atlantic salmon suffering from scabies in Yantai, Shandong, my country Aeromonas salmonicida SDy0701 is the starting strain for vaccine construction, with its housekeeping gene aroC Genes and coding genes in the T3SS secretion system acr1 , aopN , ascN Is the deletion target gene, where aroC The deletion of the gene blocks the synthesis of aromatic amino acids and their folate, acr1 , aopN Is a regulatory element of the secretion system, ascN Because ATP phosphatase participates in the assembly of type III secretion system, their deletion will lead to the destruction of T3SS secretion system function and effectors. Large fragments of double deletion of key coding genes involving the colonization ability of Aeromonas salmonicida and important virul...
experiment example 2
[0059] Experimental example 2: The half-lethal dose (LD 50 ) Determination.
[0060] Breeding of experimental fish: The experimental fish (zebrafish) purchased from Jiading, Shanghai. The experimental fish were first placed in the SPF (Special Pathogen Free) laboratory to adapt to breeding for 1 week to pick out abnormal individuals. Before the infection test, the SPF test fish were stocked in a 10L infection test tank in the infection laboratory and continued to be fed for 1 week, stocking 10 fish per tank (average body length 2-3cm). The test tank uses sterile water to replace 2 / 3 of the aquaculture water every day, and the water temperature is 28.5°C with a fluctuation of 2°C.
[0061] Evaluation of virulence: randomly divide the test fish into groups, and each group will be tested in parallel with 3 tanks.
[0062] In the infection test, each group of test fish used a certain gradient dose of the virus strain: the wild strain of Aeromonas salmonicida Aeromonas salmonicida SDy07...
experiment example 3
[0071] Experimental example 3: Immunity protection test of injection administration with zebrafish as the test animal.
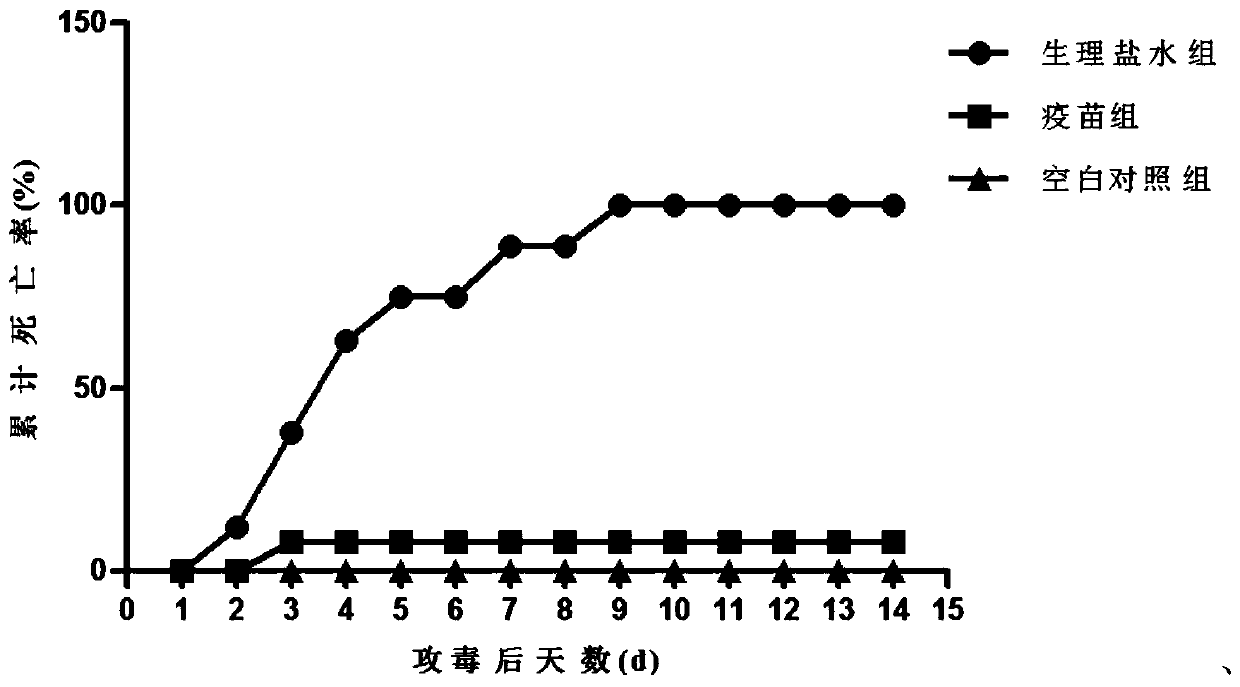
[0072] Evaluation of immune efficacy: test zebrafish are randomly divided into groups, each with 3 parallel tanks, 10 fish per tank. The prepared live attenuated vaccine was immunized by intraperitoneal injection. The injection dose of the immunization group is 2×10 5 CFU / tail. The control group was injected with the same volume of sterile saline. After 4 weeks of immunization, each group of zebrafish immunized with live bacteria of the wild strain of Aeromonas salmonicida (1×10 intraperitoneal injection) 4 CFU / tail) for artificial infection and challenge. Observe and count the number of deaths in the control group and the immunized group within 15 days, the statistical results are as follows image 3 Shown.
[0073] Use the following formula to calculate the relative immune protection rate:
[0074] Relative immune protection rate (RPS)% = (control group mor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com