Preparation method for k-carrageenan oligosaccharide with low polymerization degree
A technology of carrageenan oligosaccharides and low degree of polymerization, which is applied in the field of microorganism and enzyme engineering, and can solve the problems of poor solubility and absorbability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1: Thin-layer chromatography (TLC) detection of κ-carrageenan oligosaccharides
[0024] Mix TLC silica gel powder and sodium carboxymethyl cellulose in a ratio of 1:3, grind thoroughly, spread evenly on a glass plate, activate at 100°C for 2 hours, and store in a desiccator for later use. Use a capillary pipette to spot the sample on the prepared silica gel chromatography plate. The distance between the point of sample point and the bottom of the plate is 1cm, and the distance between the point of point of sample point is 1cm. Use a hair dryer to slowly blow over the point of sample point to make the solvent evaporate quickly. Put the chromatographic plate in the chromatographic tank and develop it at room temperature, wait until the developing agent (n-butanol: glacial acetic acid: water is 2:1:1) is developed to a distance of 1 cm from the top of the plate, take it out and dry it, and use the chromogenic agent ( Aniline-diphenylamine) for color development, an...
Embodiment 2
[0026] Embodiment 2: HPLC and ESI-TOF-MS detection carrageenan enzymolysis product
[0027] Agilent1100 high-performance liquid chromatography was used to separate the preliminary purified samples. Separation conditions: mobile phase A: ACN (acetonitrile), mobile phase B: 100 mM ammonium formate (pH 3.0), gradient: 80% to 60% A, time 0 to 20 min. The molecular weight of κ-carrageenan oligosaccharides was detected by ESI-TOF-MS in positive ion mode (G1969A, Agilent, UN).
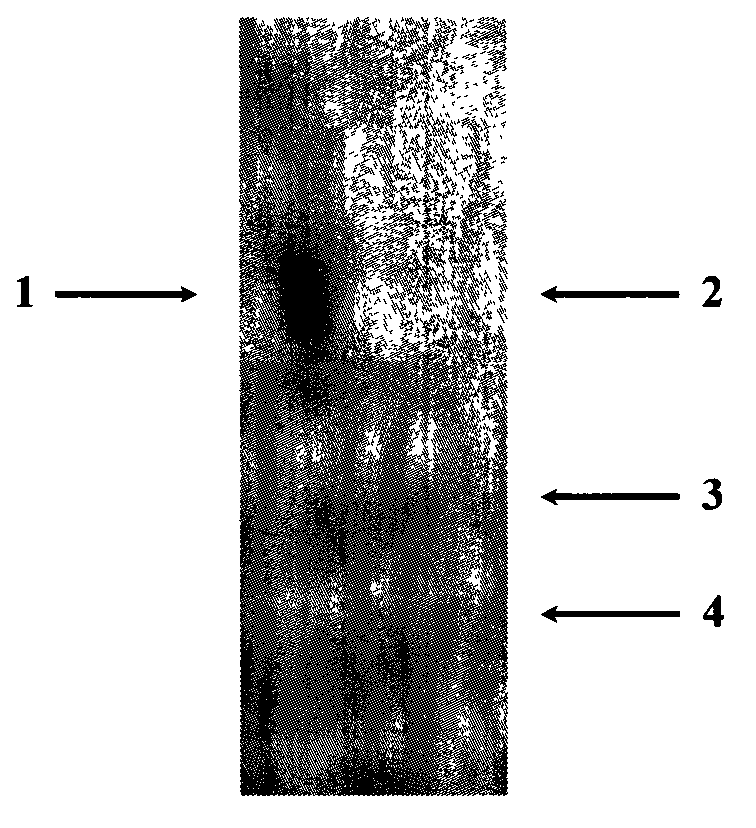
[0028] Results: After preliminary separation and purification of enzymatically hydrolyzed κ-carrageenan oligosaccharides, the results obtained by high performance liquid chromatography were as follows: figure 2 , as shown in the figure, three sample peaks b, c, and d are obtained, indicating that the degradation products of κ-carrageenan degrading enzyme are three components, and peak a is the solvent peak.
[0029] The ESI-TOF-MS spectrum shows that the basic unit of κ-carrageenan oligosaccharide is disac...
Embodiment 3
[0030] Example 3: 13 C-NMR chromatography to detect the structure of oligosaccharides
[0031] Use German Bruker AVANCE500MHz NMR instrument to carry out oligosaccharide 13 C-NMR spectrum analysis, 10mg sample was dissolved in 0.5mL 2 h 2 In O, the detection process was carried out at 30°C.
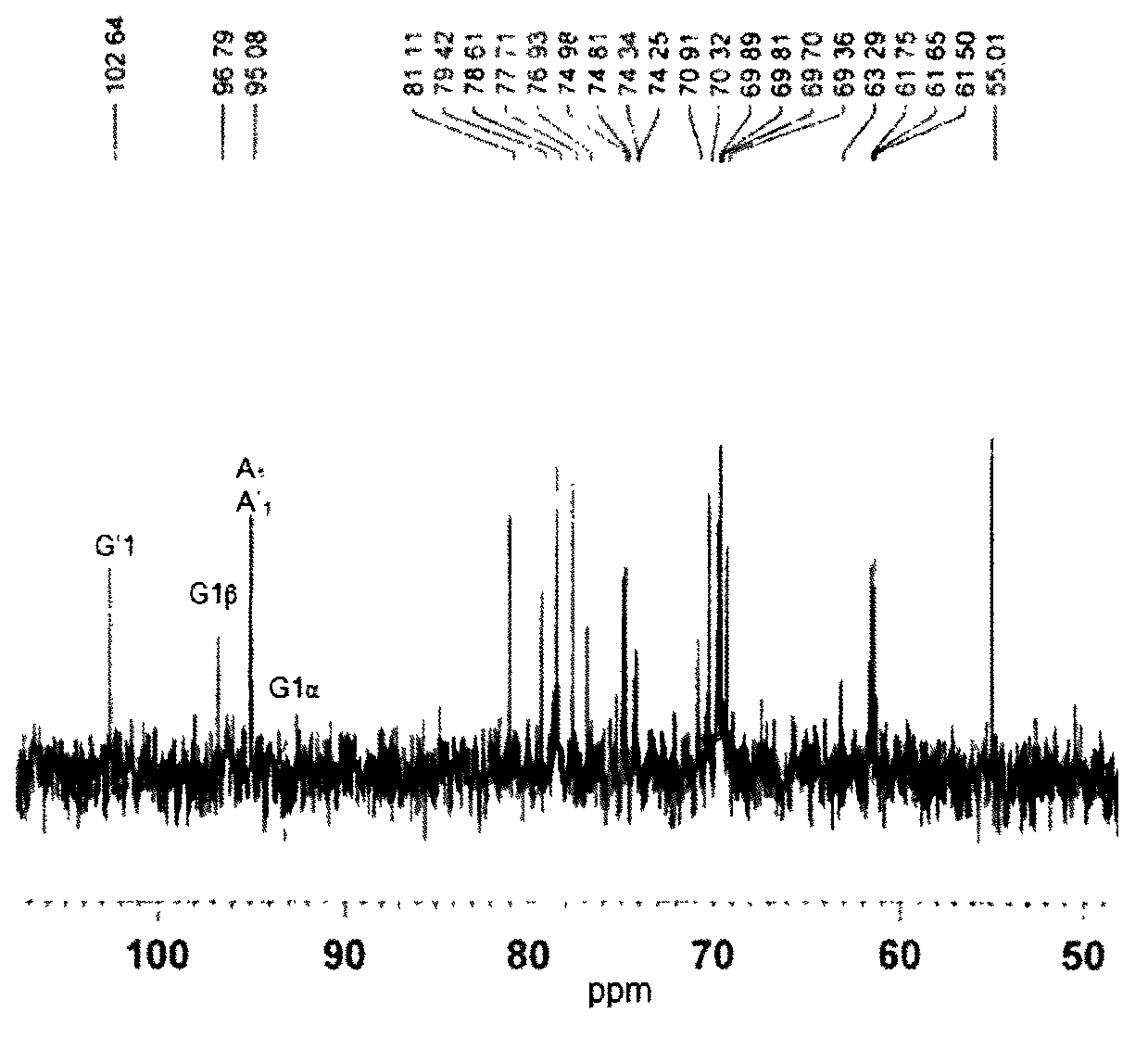
[0032] result: 13 C-NMR chromatography is used to detect the structure of the enzymatic hydrolysis product, such as image 3 , only one characteristic signal was detected at 102.64ppm, and the carbon reducing end of the A-terminus of the oligosaccharide was detected at 96.79ppm and 95.08ppm.
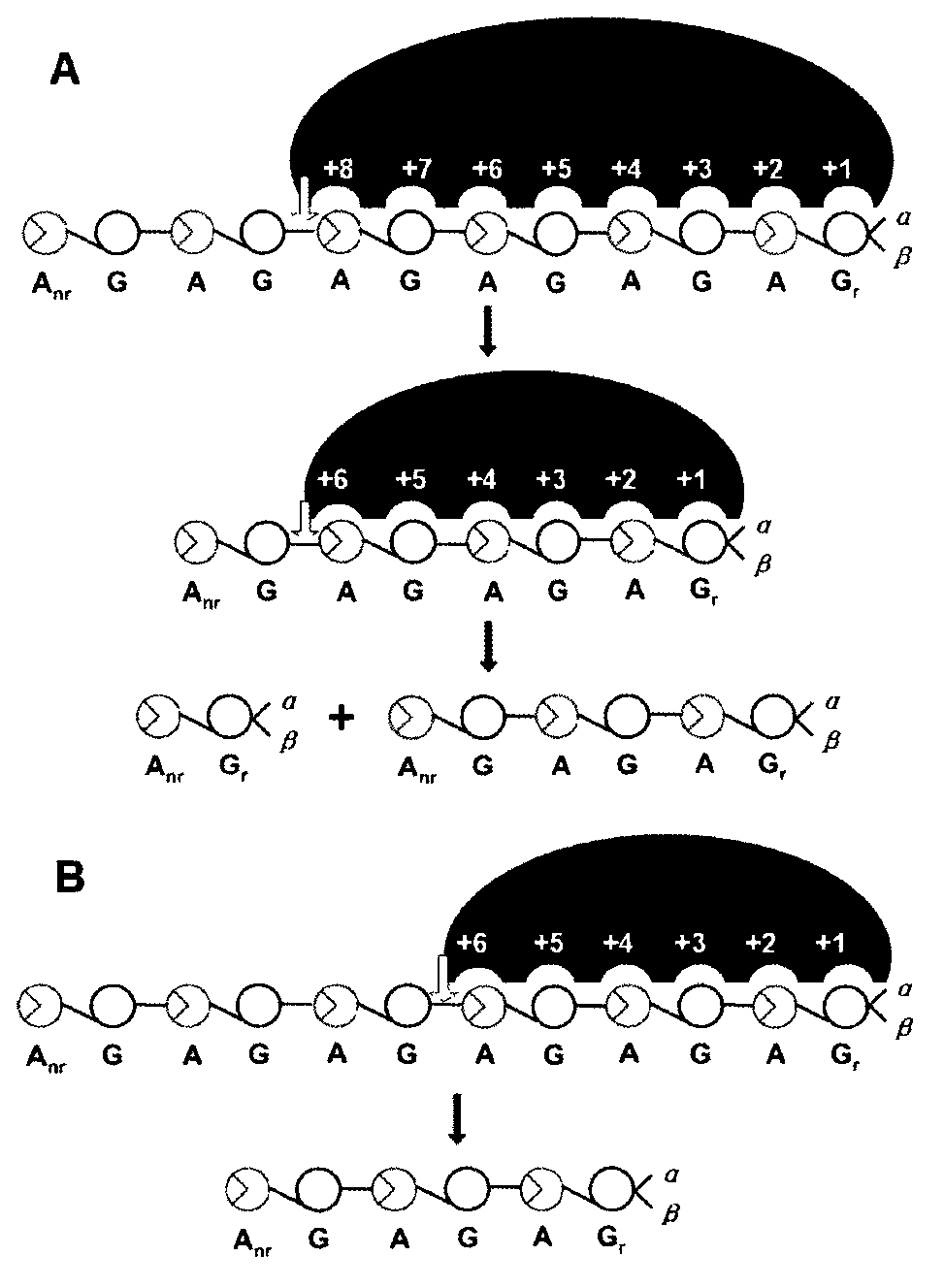
[0033] NMR spectroscopy can provide valuable information for the structural analysis of polysaccharides, especially polysaccharide molecules composed of the same or similar block units. pass 13 C-NMR analysis can determine the action site and enzymatic hydrolysis mode of κ-carrageenan degrading enzyme cleavage. Such as image 3The spectrum of κ-carahexaose sulfate shown shows two characteri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com