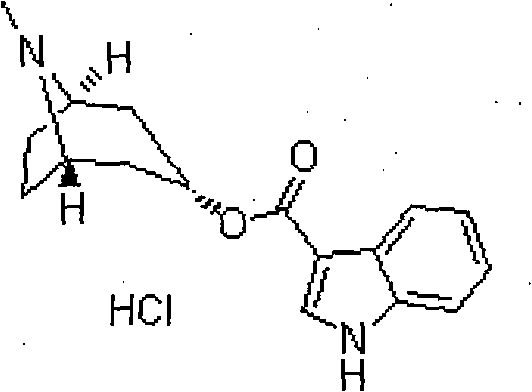
Tropisetron hydrochloride liposome injection
A technology for tropisetron hydrochloride and tropane hydrochloride, applied in the field of medicine, can solve the problems of easy separation by hydrolysis, low bioavailability, influence on drug quality, etc., and achieves improved bioavailability, simple preparation method, and increased retention time Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 Cefadroxil liposome injection
[0033] Prescription (1000 bottles):
[0034]
[0035] Preparation Process:
[0036] (1) Accurately weigh 2g Tropisetron Hydrochloride, 140g Sodium Taurocholate, 70g Cholesterol and 40g Gelatin and be dissolved in 500ml volume ratio is 3: 1 in the mixed solvent of n-butanol and chloroform, stirring and dissolving;
[0037] (2) 600ml of a phosphate buffer solution with a pH of 6.0 dissolved in 2g of ascorbic acid and 100g of trehalose was dissolved in the above solution, and short-term ultrasonic was performed until a stable W / O emulsifier was formed;
[0038] (3) The above-mentioned emulsifier is evaporated under reduced pressure to remove the organic solvent. After reaching the colloidal state, add 700ml PH of 6.0 phosphate buffer solution, rotate and vibrate until the gel on the wall of the device comes off, and continue to evaporate under pressurized conditions. Prepare an aqueous suspension, and then sonicate for a short...
Embodiment 2
[0040] Embodiment 2 cefadroxil liposome injection
[0041] Prescription (1000 bottles):
[0042]
[0043] Preparation Process:
[0044] (1) Accurately weigh 2g tropisetron hydrochloride, 120g sodium taurocholate, 40g cholesterol and 20g gelatin and dissolve in 500ml volume ratio of n-butanol and chloroform mixed solvent of 3: 1, stirring and dissolving;
[0045] (2) Dissolve 1220ml of phosphate buffer solution with 2g ascorbic acid and 120g trehalose at a pH of 6.0 into the above solution, and perform short-time ultrasonication until a stable W / O emulsifier is formed;
[0046] (3) The above-mentioned emulsifier is evaporated under reduced pressure to remove the organic solvent. After reaching the colloidal state, add 1000ml PH of 6.0 phosphate buffer solution, rotate and vibrate until the gel on the wall of the device comes off, and continue to evaporate under pressurized conditions. Prepare an aqueous suspension, and then sonicate for a short time;
[0047] (4) Centrifu...
Embodiment 3
[0048] Example 3 Preparation of Comparative Examples 1-5
[0049] The components other than the preferred components of the present invention and the combinations other than the preferred content ratio of the components of the present invention were respectively selected to carry out comparative experiments, and the preparation process was the same as that of Example 1, so as to obtain Comparative Examples 1-3. Each comparative example component is shown in the table below:
[0050]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com