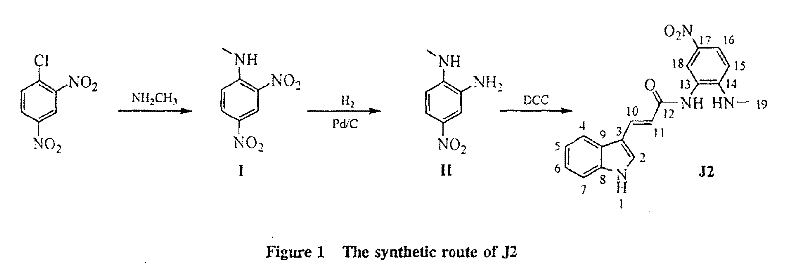
A novel j2-sodium alginate microsphere slow-release immunosuppressant for anti-graft immune rejection, preparation method and application
An immunosuppressant and sodium alginate technology, applied in the field of immunosuppressants, can solve the problems such as the inability of relatively uniform sustained release of drugs, the great individual differences in the degree of drug absorption, and the inability to effectively suppress immune rejection. Allergic reactions and various inconveniences, the effect of improving the level of treatment, and promoting development
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] The method for preparing novel J2-sodium alginate microsphere slow-release immunosuppressant against graft immune rejection:
[0049] (1) Handling of glassware:
[0050] Air-dry the cleaned glassware, put it in a high-temperature oven and bake at 280 degrees Celsius for 3 hours (sterilize and remove heat source);
[0051] (2) Weigh the immunosuppressant J2, dissolve it with absolute ethanol or dimethyl sulfoxide at a concentration of 0.1-8.8% (mass volume percentage) of the drug, and obtain a drug solution;
[0052] (3) Weigh the sodium alginate, dissolve it with water for injection or physiological saline, and make it into a concentration of 1-6% (mass volume percentage) to obtain a sodium alginate solution;
[0053] (4) Divalent metal cation calcium is weighed, and described cation calcium is selected from calcium lactate, calcium chloride or barium chloride, is mixed with the solution of 1~15% (mass volume percent) concentration, obtains solidification liquid; Then ...
Embodiment 2
[0067] The method for preparing novel J2-sodium alginate microsphere slow-release immunosuppressant against graft immune rejection:
[0068] (1) Handling of glassware:
[0069] Air-dry the cleaned glassware, put it in a high-temperature oven and bake at 280 degrees Celsius for 3 hours (sterilize and remove heat source);
[0070] (2) Weigh the immunosuppressant J2, and dissolve it with absolute ethanol or dimethyl sulfoxide at a concentration of 0.3-8.8% (mass volume percentage) of the drug to obtain a drug solution;
[0071] (3) weigh sodium alginate, dissolve it with water for injection or physiological saline, and make it into a concentration of 1.5-6% (mass volume percentage) to obtain sodium alginate solution;
[0072] (4) Divalent metal cation calcium is weighed, and described cation calcium is selected from calcium lactate, calcium chloride or barium chloride, is mixed with the solution of 1.5~15% (mass volume percentage) concentration, obtains solidification liquid; Th...
Embodiment 3
[0084] The method for preparing novel J2-sodium alginate microsphere slow-release immunosuppressant against graft immune rejection:
[0085] (1) Handling of glassware:
[0086] Air-dry the cleaned glassware, put it in a high-temperature oven and bake at 280 degrees Celsius for 3 hours (sterilize and remove heat source);
[0087] (2) Weigh the immunosuppressant J2, dissolve it with absolute ethanol or dimethyl sulfoxide at a concentration of 0.5-8.8% (mass volume percentage) of the drug, and obtain a drug solution;
[0088] (3) Weigh the sodium alginate, dissolve it with water for injection or physiological saline, and make it into a concentration of 1.7-6% (mass volume percentage) to obtain a sodium alginate solution;
[0089] (4) Divalent metal cation calcium is weighed, and described cation calcium is selected from calcium lactate, calcium chloride or barium chloride, is mixed with the solution of 2~15% (mass volume percent) concentration, obtains solidification liquid; The...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com