Fusion protein with antibacterial and repairing function and production method and application thereof
A fusion protein and fusion gene technology, applied in the field of genetic engineering, can solve the problems of degradation, safety half-life extension related fusion proteins, failure to promote wound healing, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
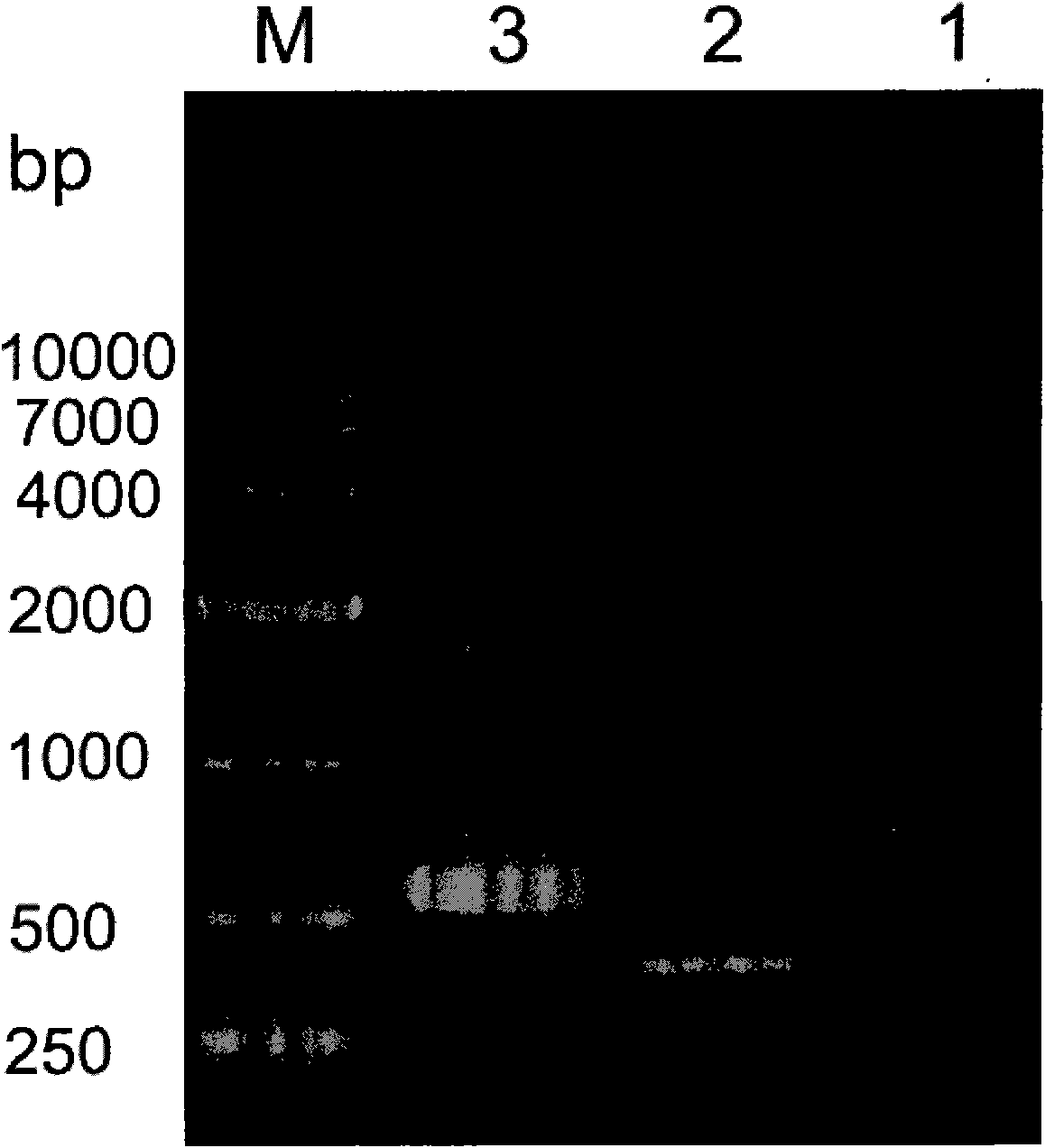
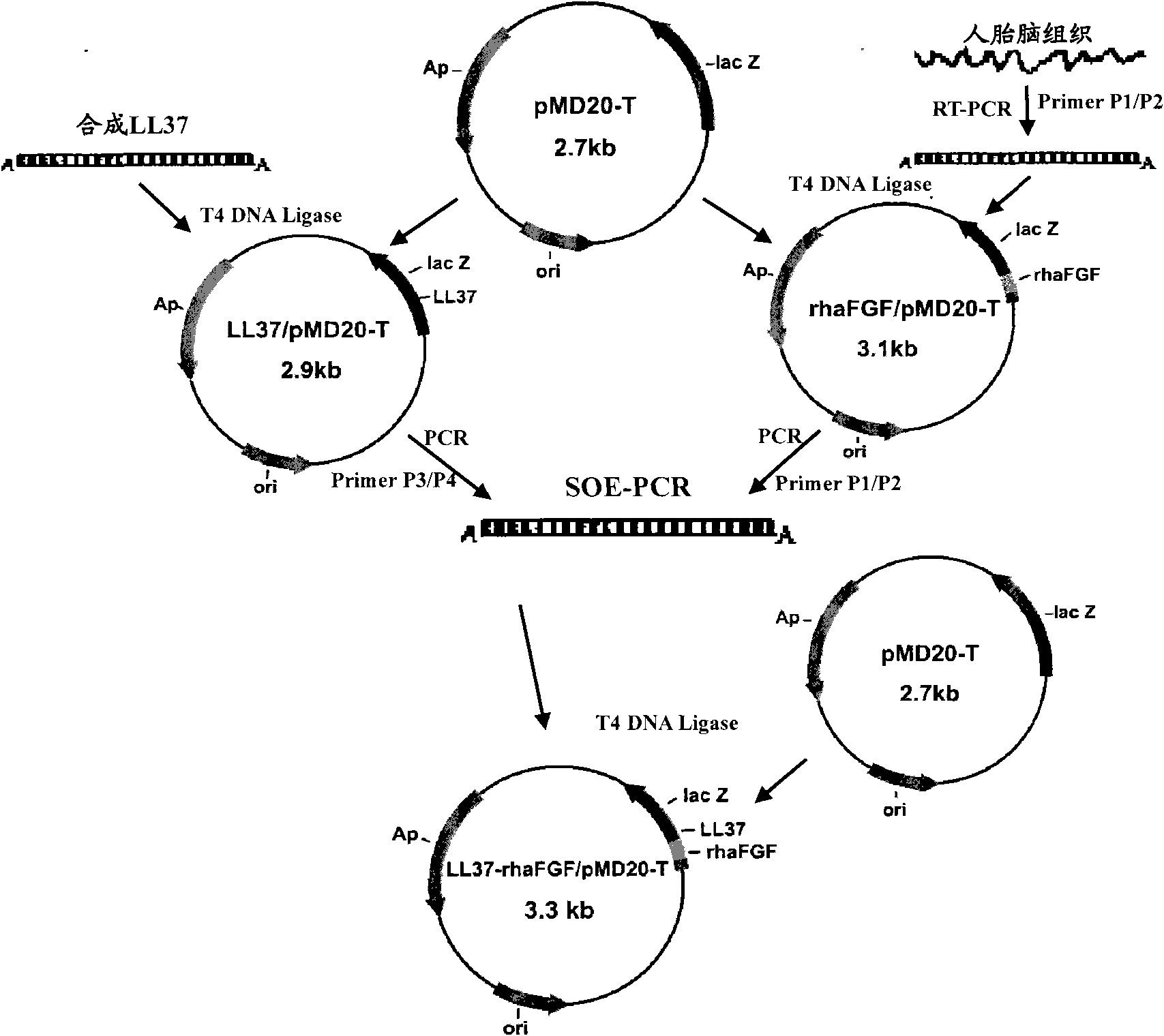
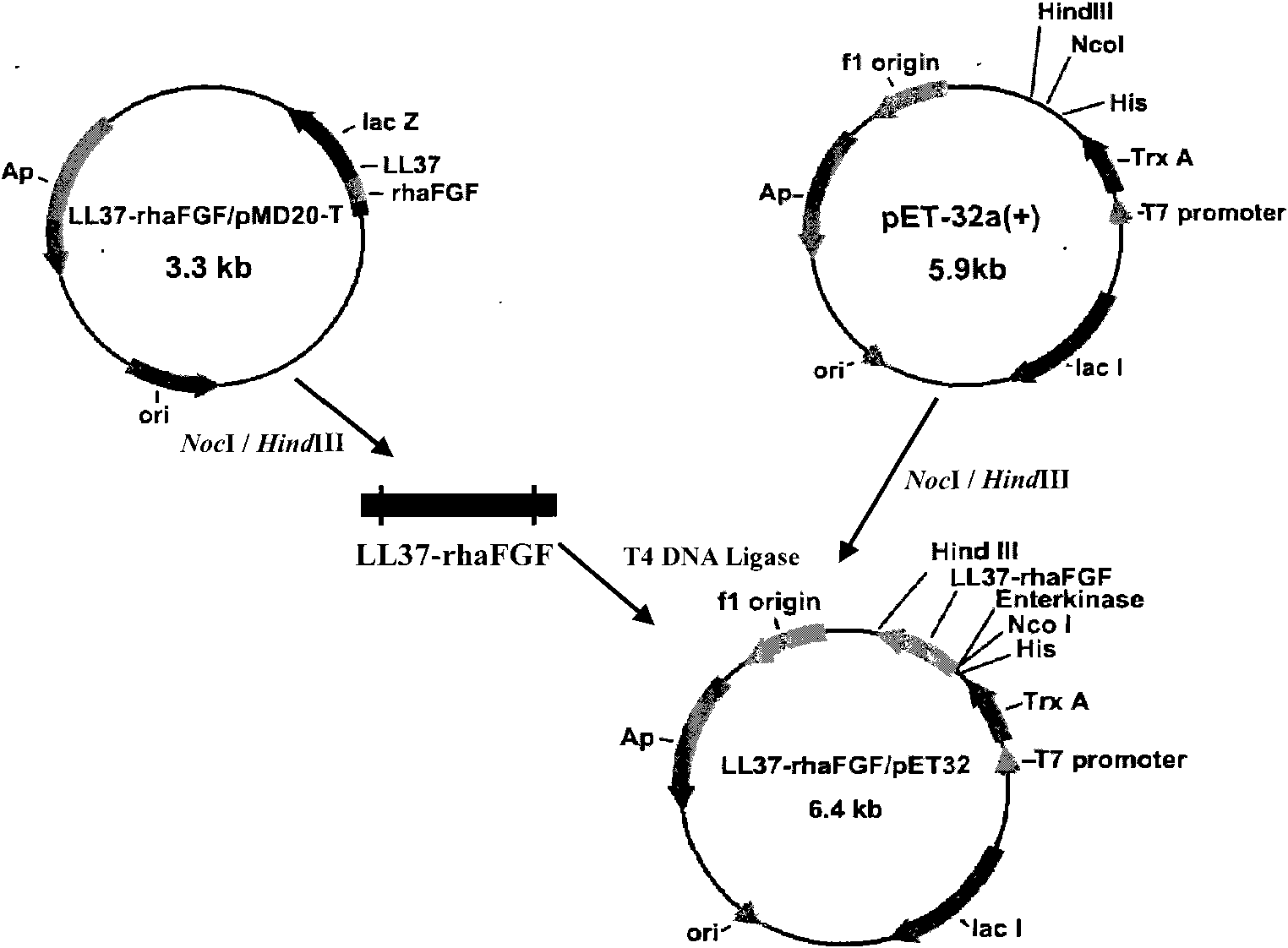
[0052] Example 1 Transformation of LL37 and haFGF genes and construction of fusion gene LL37-rhaFGF and preparation of fusion protein LL37-rhaFGF
[0053] In this example, the fusion gene LL37-rhaFGF was expressed by using the pET expression system of Novagen, the pET32a(+) plasmid was used as the expression vector, and the host cell was Escherichia coli.
[0054] Synthesize the human antimicrobial peptide LL37-Linker gene according to the preferred codons of Escherichia coli, introduce the NcoI restriction site and the enterokinase protease restriction site at the 5′ end of the gene in sequence, remove the stop codon at the 3′ end of the LL37 gene, and add Optimized to encode a hydrophobic polypeptide of 15 amino acids (Gly 4 Ser) 3 The DNA sequence; its nucleotide sequence is shown in SEQ ID NO:3.
[0055] According to the instructions of Trizol Reagent, total RNA was extracted from human fetal brain tissue. According to the cDNA sequence of haFGF (Genebank accession numbe...
Embodiment 2
[0083] Example 2 Pharmacodynamic study of the fusion protein LL37-rhaFGF of the present invention
[0084] (1) In vitro experiment:
[0085] (1) The inhibitory effect of the fusion protein on Staphylococcus aureus, Pseudomonas aeruginosa and other bacteria was detected by agar hole infiltration method.
[0086] The purified lyophilized fusion protein was dissolved into a 2.5g / L solution with physiological saline, and added to the agar wells containing Pseudomonas aeruginosa and Staphylococcus aureus respectively, with 2.5g / L Ampicillin as Positive controls, sterile water and normal saline were used as negative controls. Place them in an incubator at 37°C for 12-16 hours, measure the size of the inhibition zone, and take pictures to record the results. Sterile distilled water and normal saline control have no inhibition zone, but Ampicillin has an inhibition zone in the two bacterial plates with a diameter of about 2.5cm; the fusion protein LL-37-rhaFGF has obvious inhibition...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com