Nucleic acids and polypeptides useful for diagnosing and treating complications of pregnancy
A technology of polypeptides and nucleic acid molecules, which is applied in the fields of diagnosis, disease diagnosis, diagnostic recording/measurement, etc., and can solve problems such as the unidentified role of proteins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0235] Antibody preparation
[0236]Monoclonal antibodies that specifically bind any polypeptide of the invention can be produced by methods known in the art. These methods include Kohler and Milstein (Nature, 256:495-497, 1975) and Campbell ("Monoclonal Antibody Technology, The Production and Characterization of Rodent and Human hybrid omas", Burdon et al., Eds., Laboratory technique in Biochemistry and Molecular Biology, Volume 13, Elsevier Science Publishers, Amsterdam, 1985), and the recombinant DNA method described by Huse et al. (Science, 246, 1275-1281, 1989).
[0237] Monoclonal antibodies can be used in the supernatant of cultured hybridoma cells or ascites fluid induced by intraperitoneal inoculation of hybridoma cells into mice. The hybridoma technique, originally described by Kohler and Milstein (Eur. J. Immunol, 6, 511-519, 1976), has been widely used to generate hybrid cell lines that secrete high levels of monoclonal antibodies directed against any specific ant...
Embodiment 1
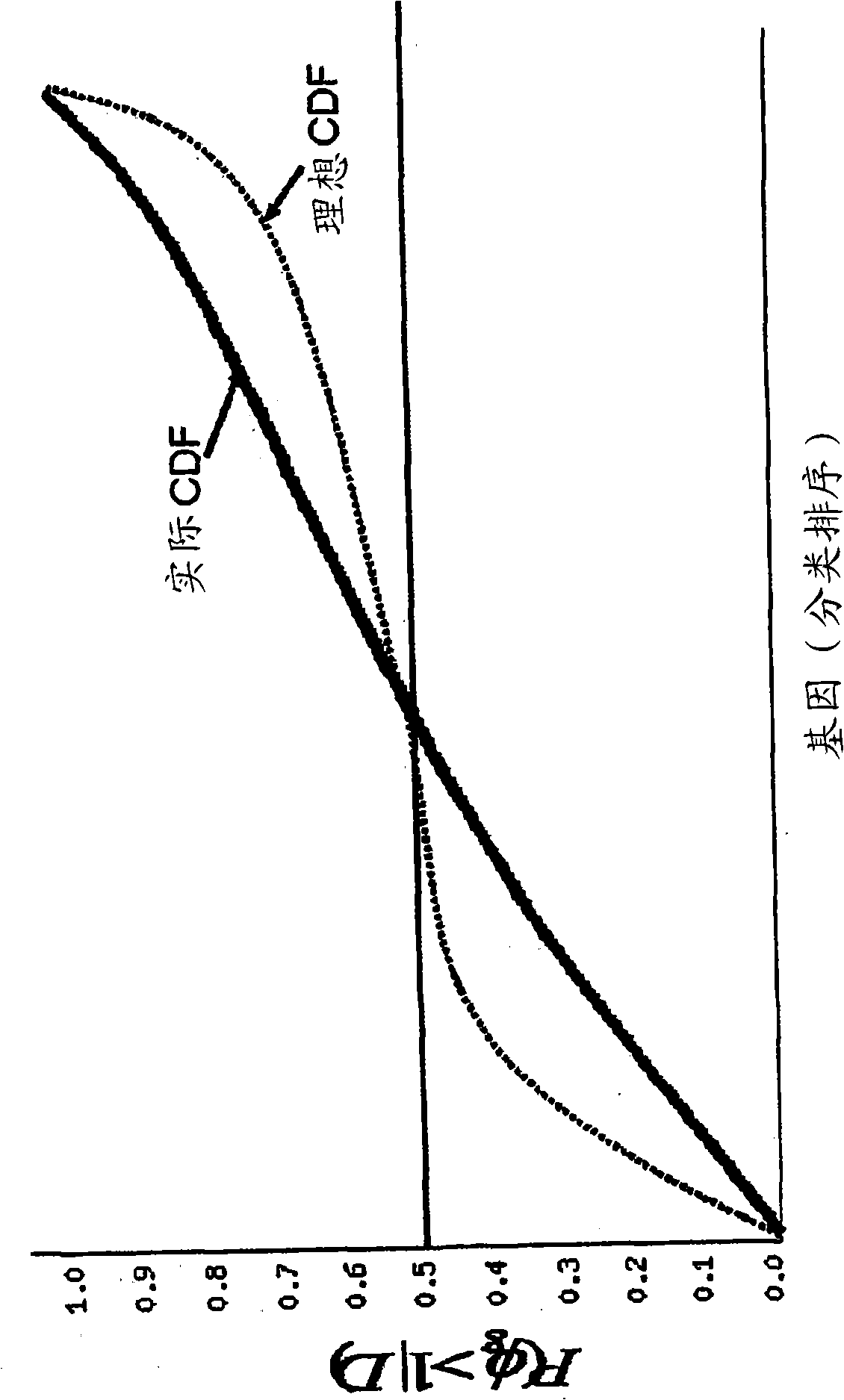
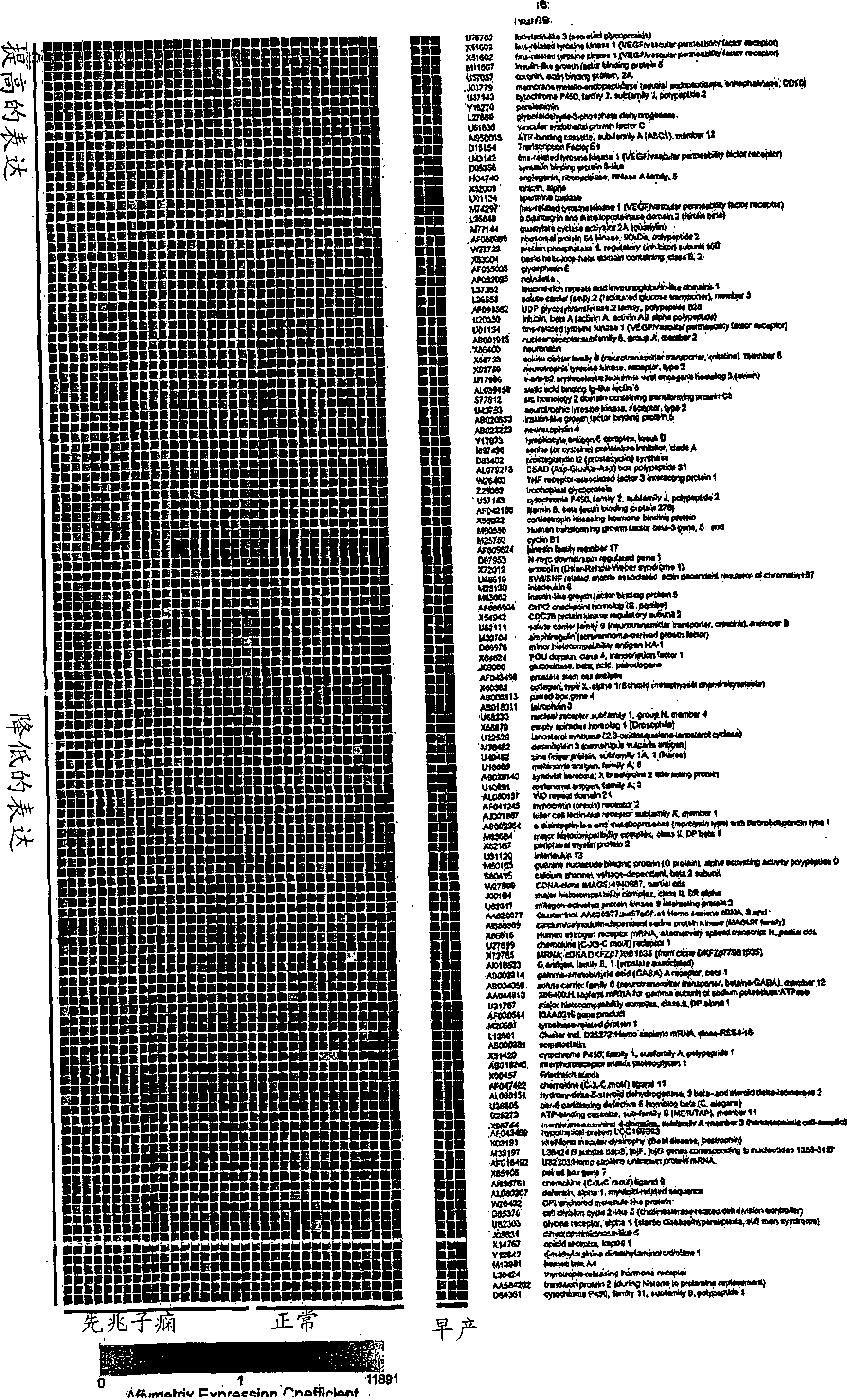
[0284] Example 1. Gene expression patterns in placental tissue from preeclamptic and normotensive women
[0285] To identify novel secreted factors involved in the pathogenesis of preeclampsia, we analyzed gene expression patterns in placental tissues from 19 women with preeclampsia and 15 normotensive women using Affymetrix U95A microarrays (see Table 1 ).
[0286] Table 1: Clinical characteristics of study patients
[0287] Normal (n=15)
Preeclampsia (n=19)
Mother's age (years)
35.2
31.9
Pregnancy (weeks)
39.0
31.1 *
Primiparous (%)
19
81 *
Systolic BP(mm Hg)
107
167.2 **
Diastolic BP(mm Hg)
83
101.8 **
Proteinuria (g protein / g creat)
<0.3
5.2 **
Serum uric acid (mg / dl)
NA
6.8
Hematocrit (%)
35.7
33.9
Platelet count (K / μl)
217
198
Serum creatinine (mg / dl)
0.5
0.6
...
Embodiment 2
[0303] Example 2. mRNA Expression of FIt-1 and sFlt-1 in Preeclampsia
[0304] Since the above clustering identified sFlt1 as well as other genes validated in the literature, we chose to utilize the sFlt1 validation array for its ability to identify predictive markers for pre-eclampsia. For these experiments, placental sFlt-1 mRNA expression from 3 patients with preeclampsia (P1, P2, P3) and 3 normotensive pregnant women (N1, N2, N3) was determined by Northern blot analysis ( Figure 4 ). The upper band (7.5 kb) is the full length Flt-1 mRNA, while the lower more abundant band (3.4 kb) is the alternatively spliced sFlt-1 mRNA. Actin is included as a control and 28S is shown as an arrow. These results show increased sFlt-1 gene expression for preeclampsia patients and confirm the set of predictive genes identified using the array as markers of preeclampsia or convulsions or a propensity to develop preeclampsia or convulsions.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com