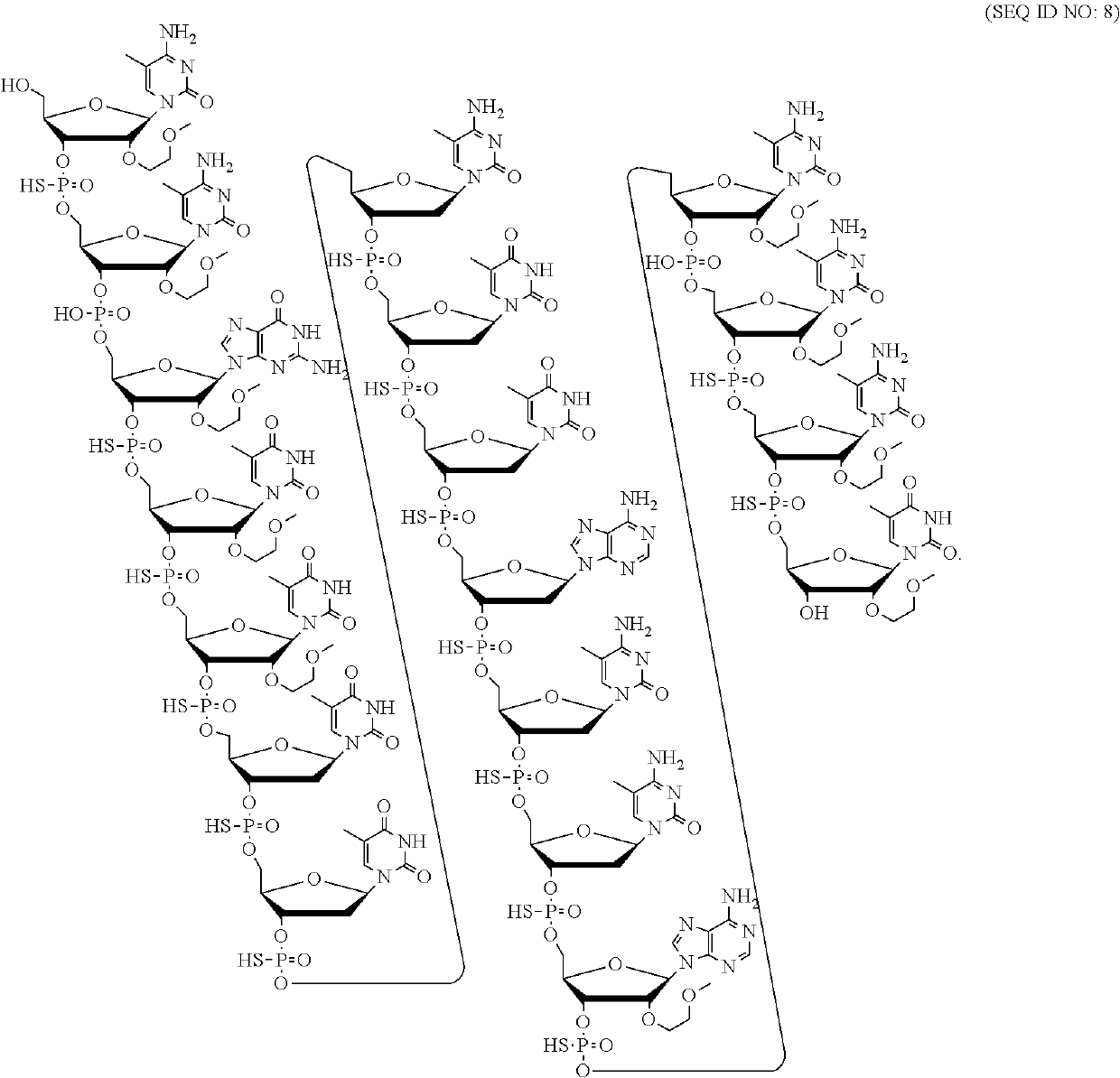
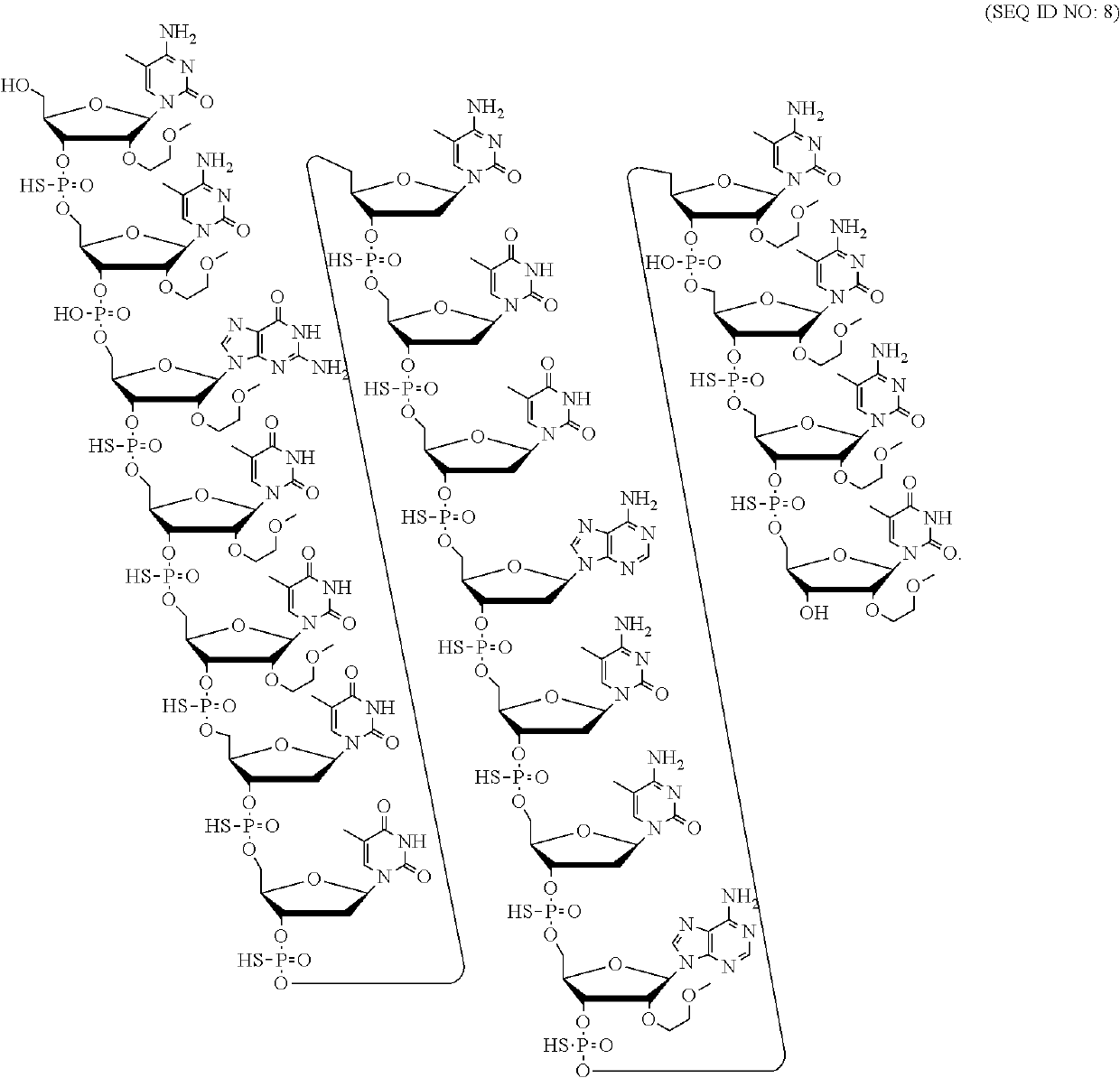
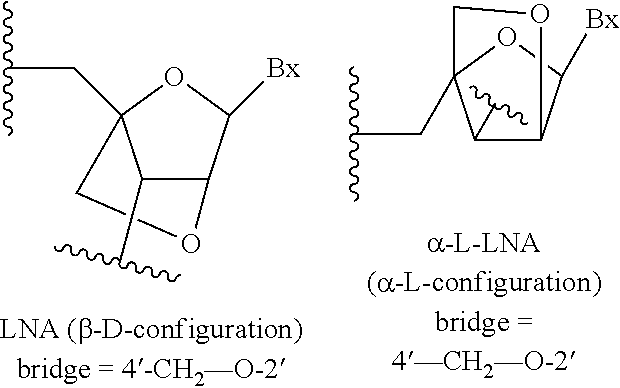
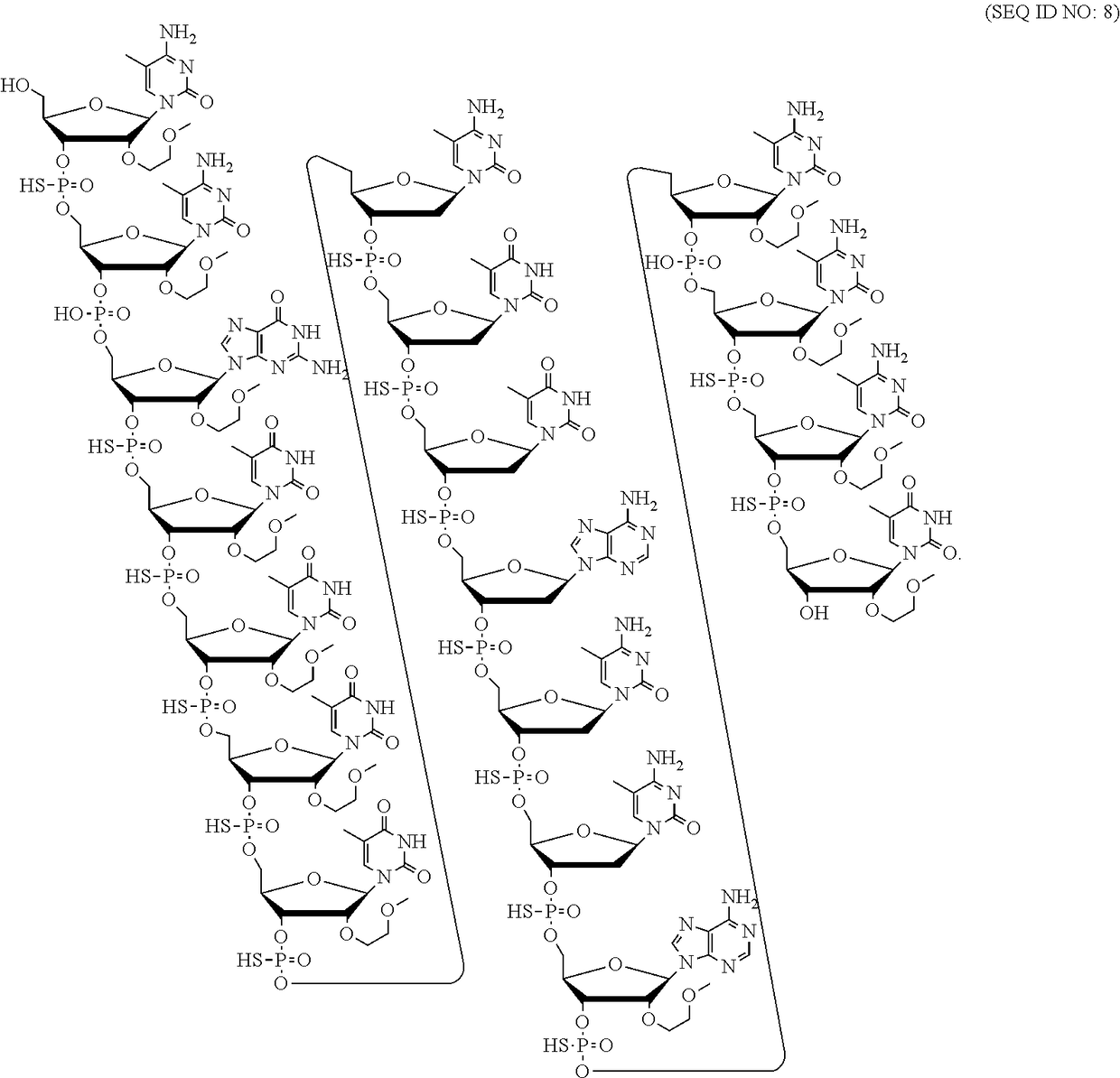
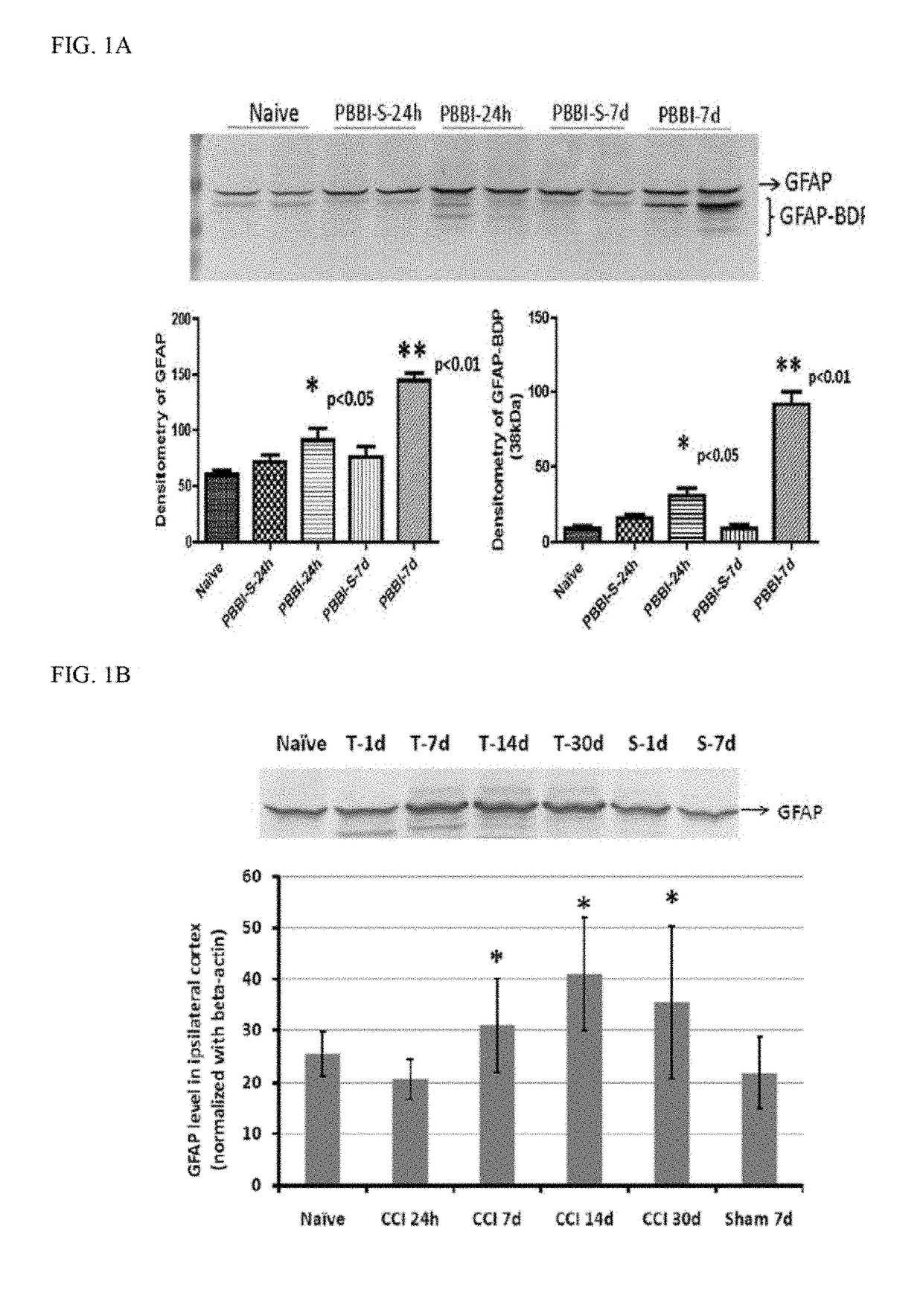
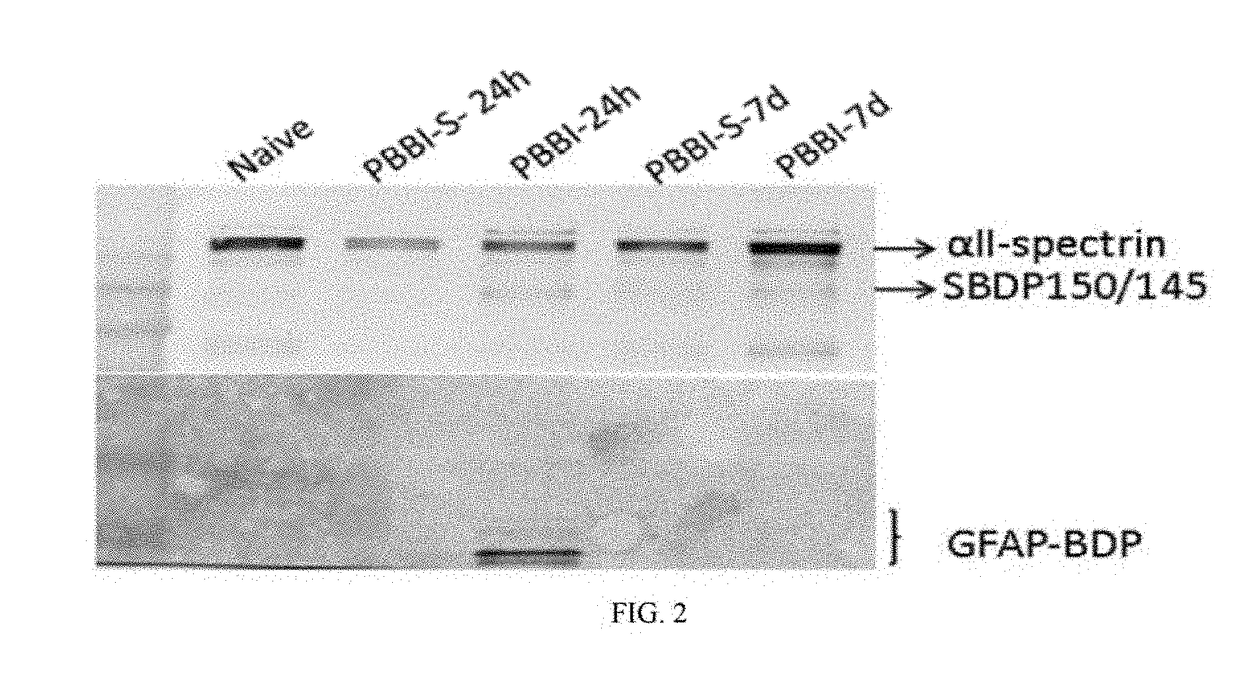
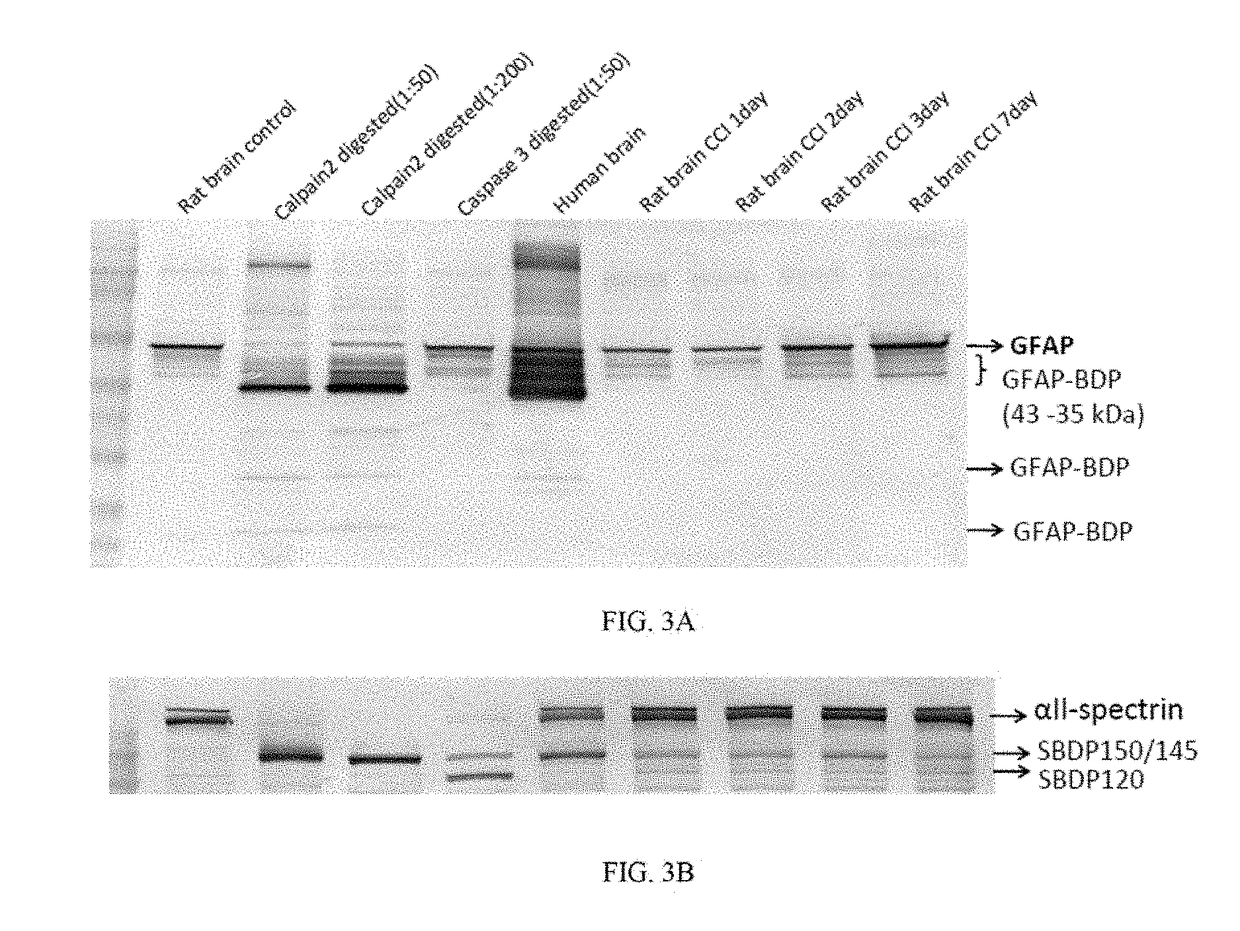
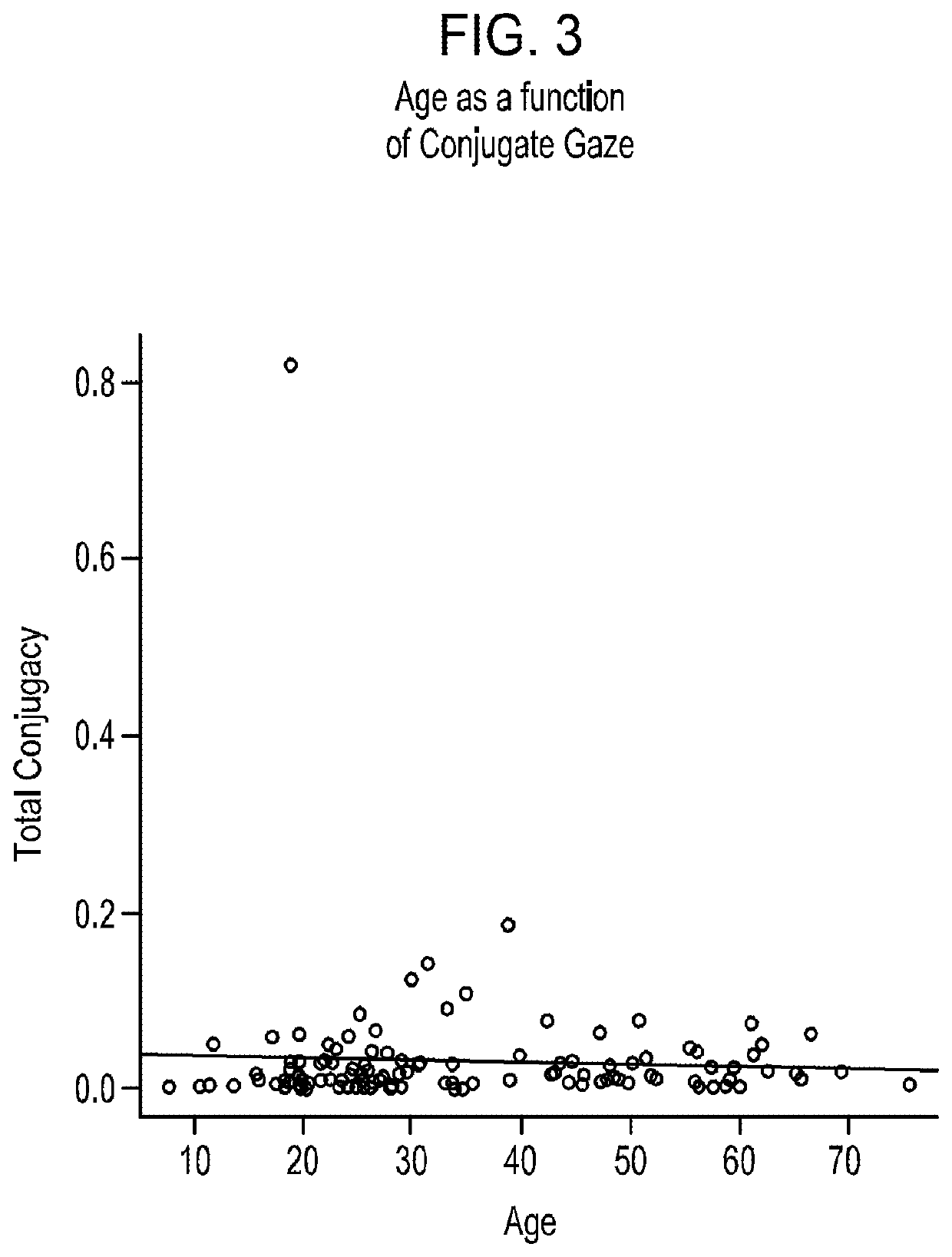
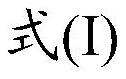Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
44 results about "Traumatic encephalopathy" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
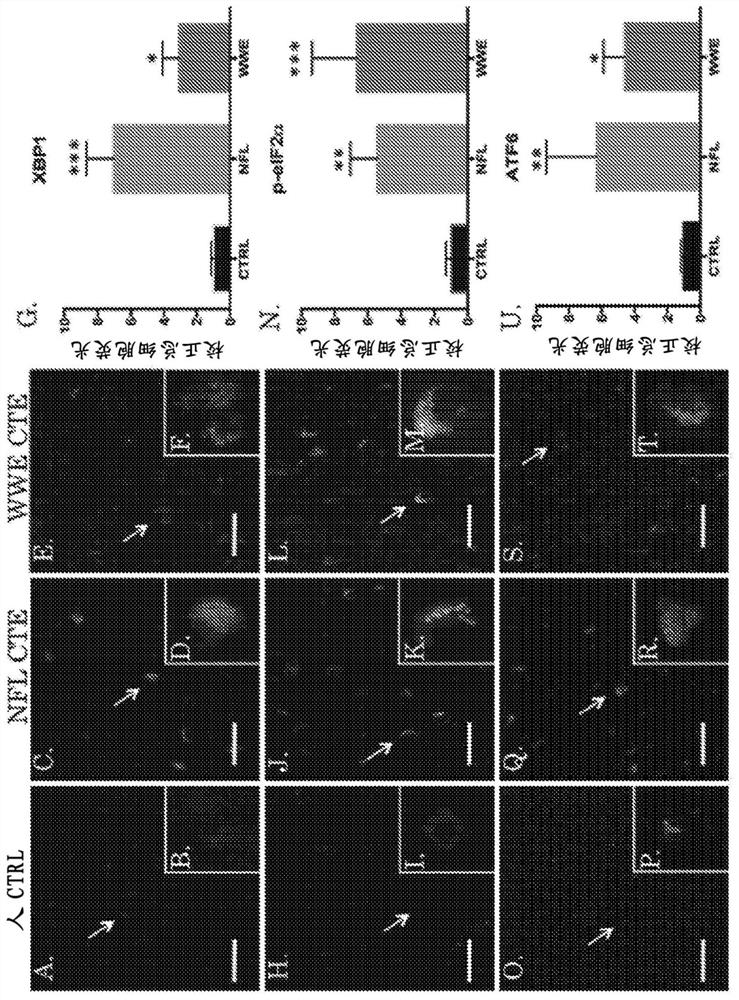
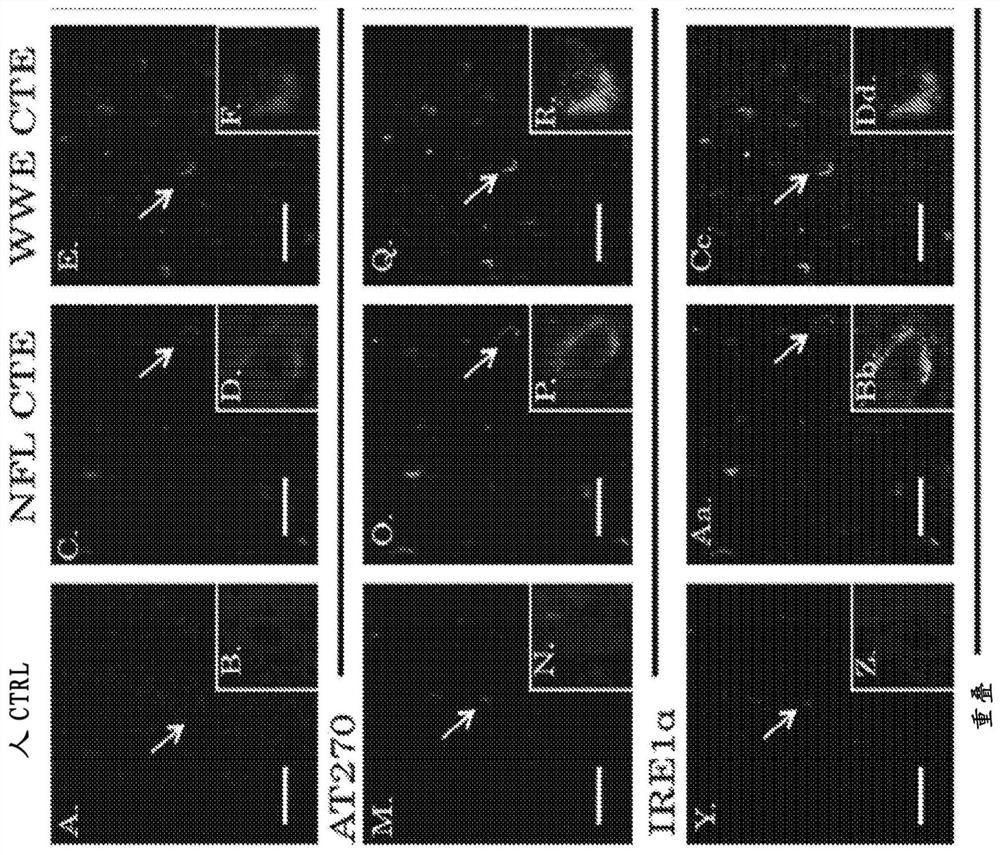
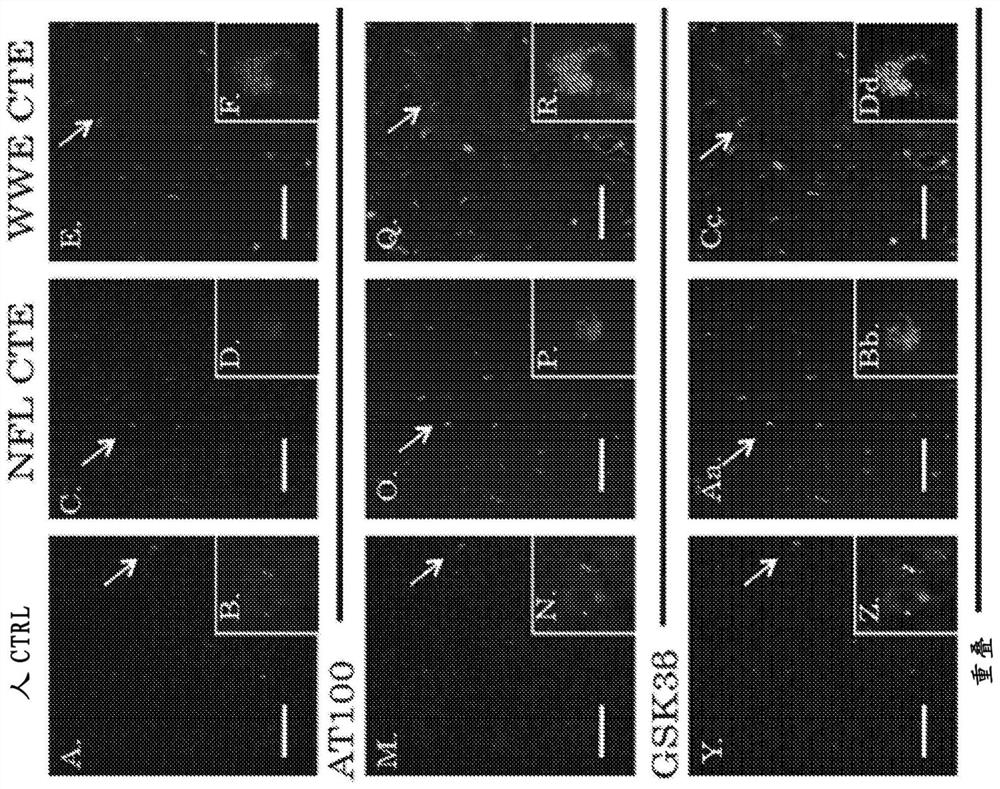
Chronic traumatic encephalopathy (CTE) is a neurodegenerative disease found in people who have had multiple head injuries. Symptoms may include behavioral problems, mood problems, and problems with thinking.
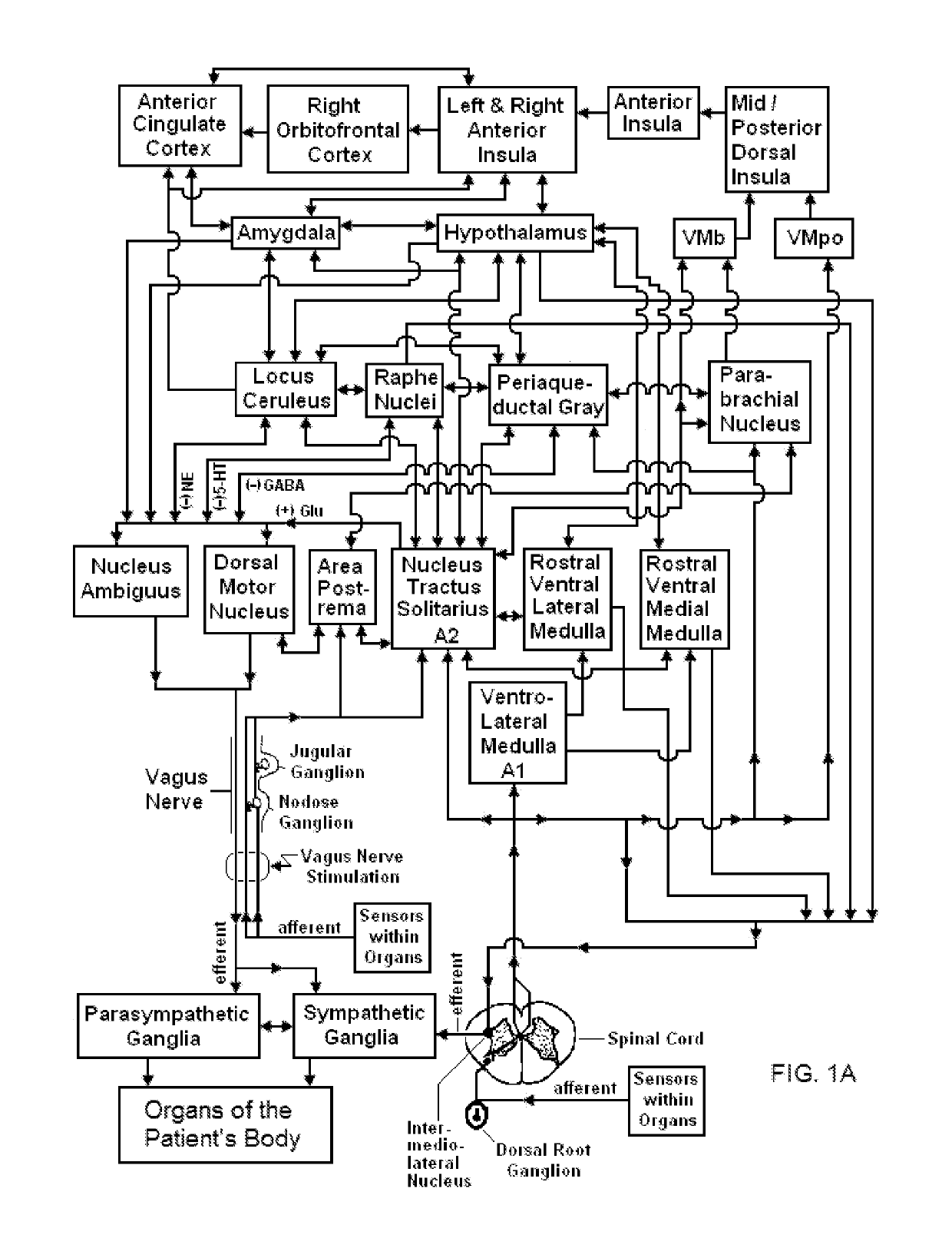
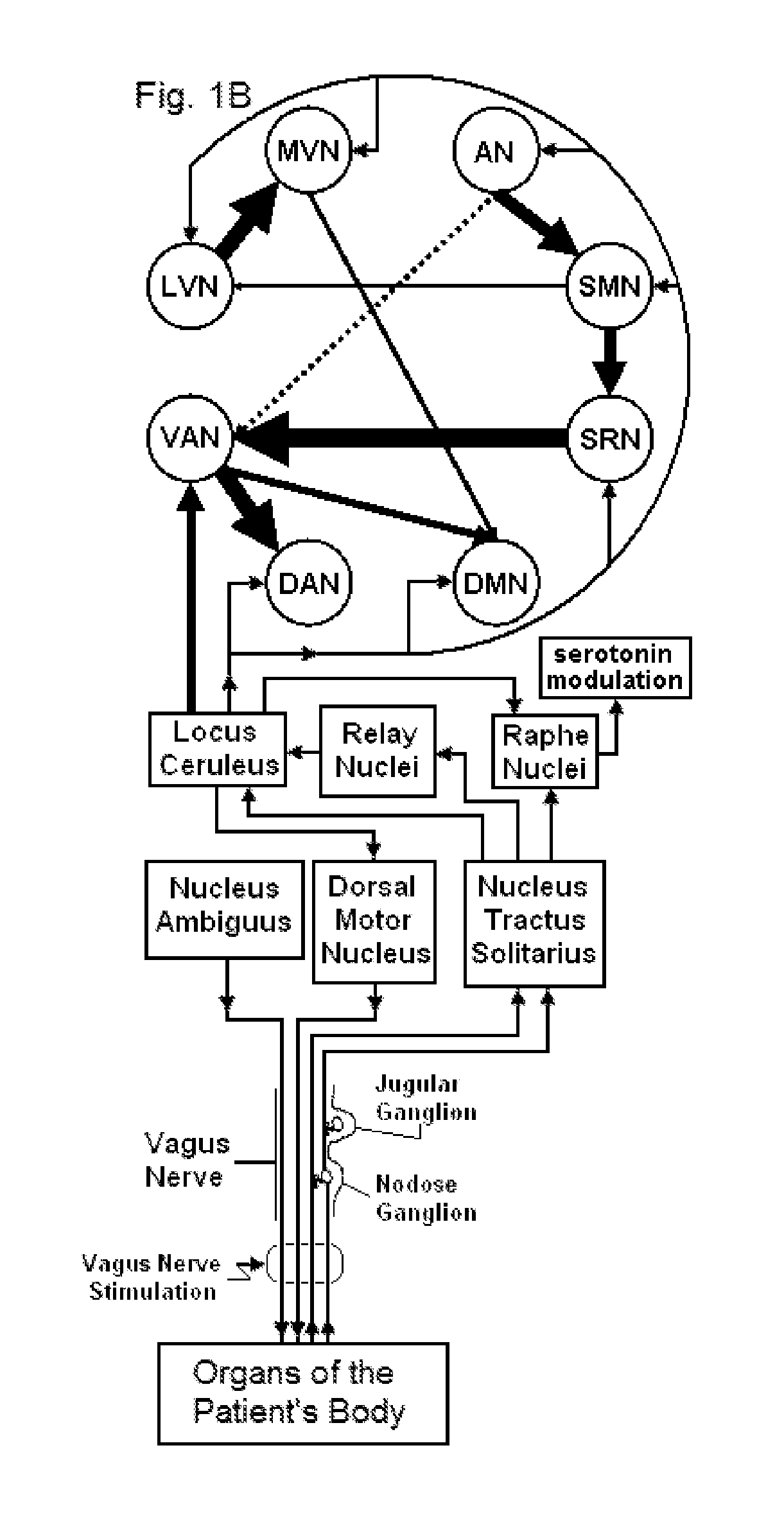
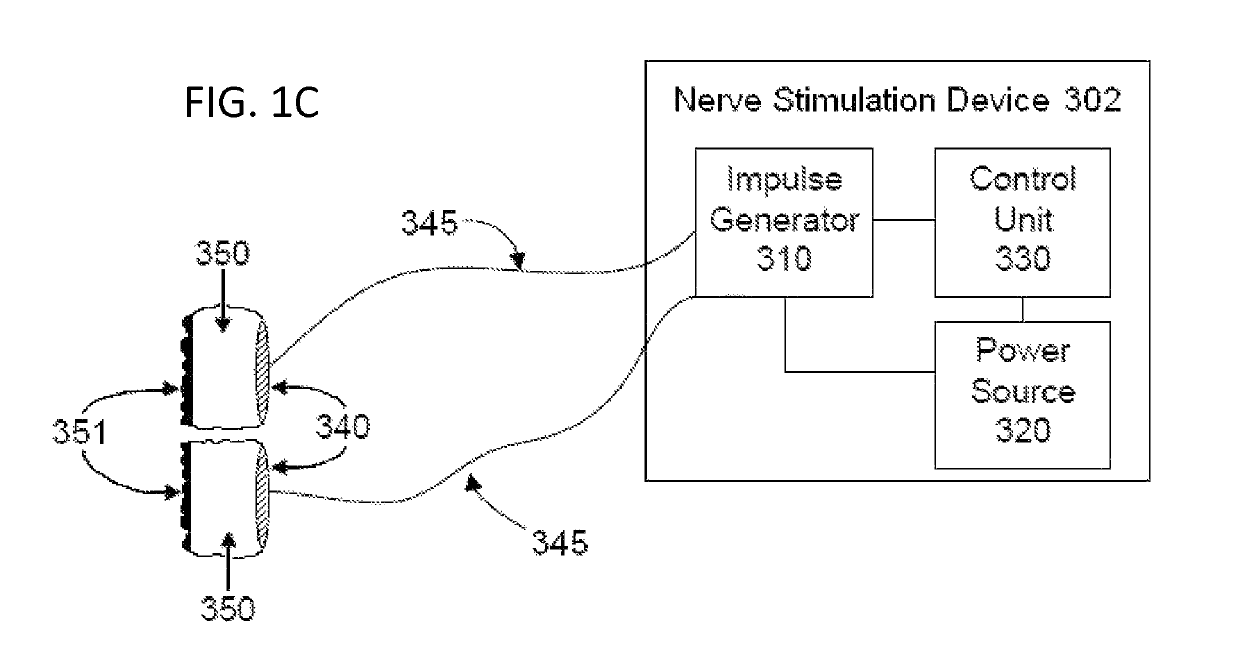
Mobile phone for treating a patient with dementia
ActiveUS20140330335A1Inhibition of excitementAvoid stimulationUltrasonic/sonic/infrasonic diagnosticsElectrotherapyDevergie's diseaseRechargeable cell
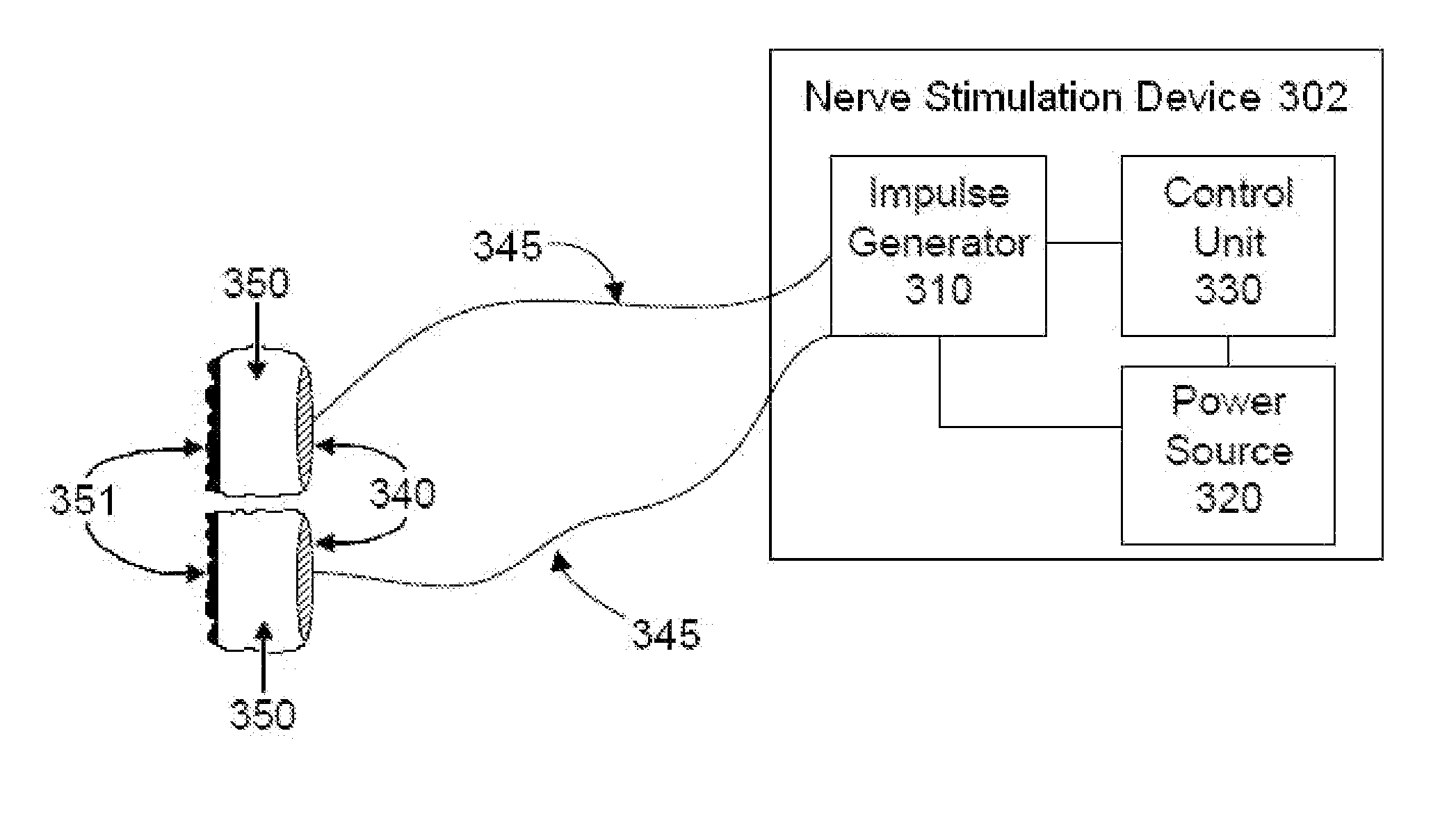
Devices, systems and methods are disclosed that allow a patient to self-treat neurodegenerative diseases, such as dementia, Alzheimer's disease, ischemic stroke, post-concussion syndrome, chronic traumatic encephalopathy and the like by electrical noninvasive stimulation of a vagus nerve. The system comprises a handheld stimulator that is applied to the surface of the patient's neck, wherein the stimulator comprises or is joined to a smartphone. A camera of the smartphone may be used to position and reposition the stimulator to a particular location on the patient's neck. The system may also comprise a base station that is used to meter the charging of a rechargeable battery within the stimulator. The base station and stimulator transmit data to one another regarding the status of a stimulation session.
Owner:ELECTROCORE
Micro-RNA, autoantibody and protein markers for diagnosis of neuronal injury
InactiveUS20130022982A1Microbiological testing/measurementImmunoglobulins against animals/humansProtein markersInjury brain
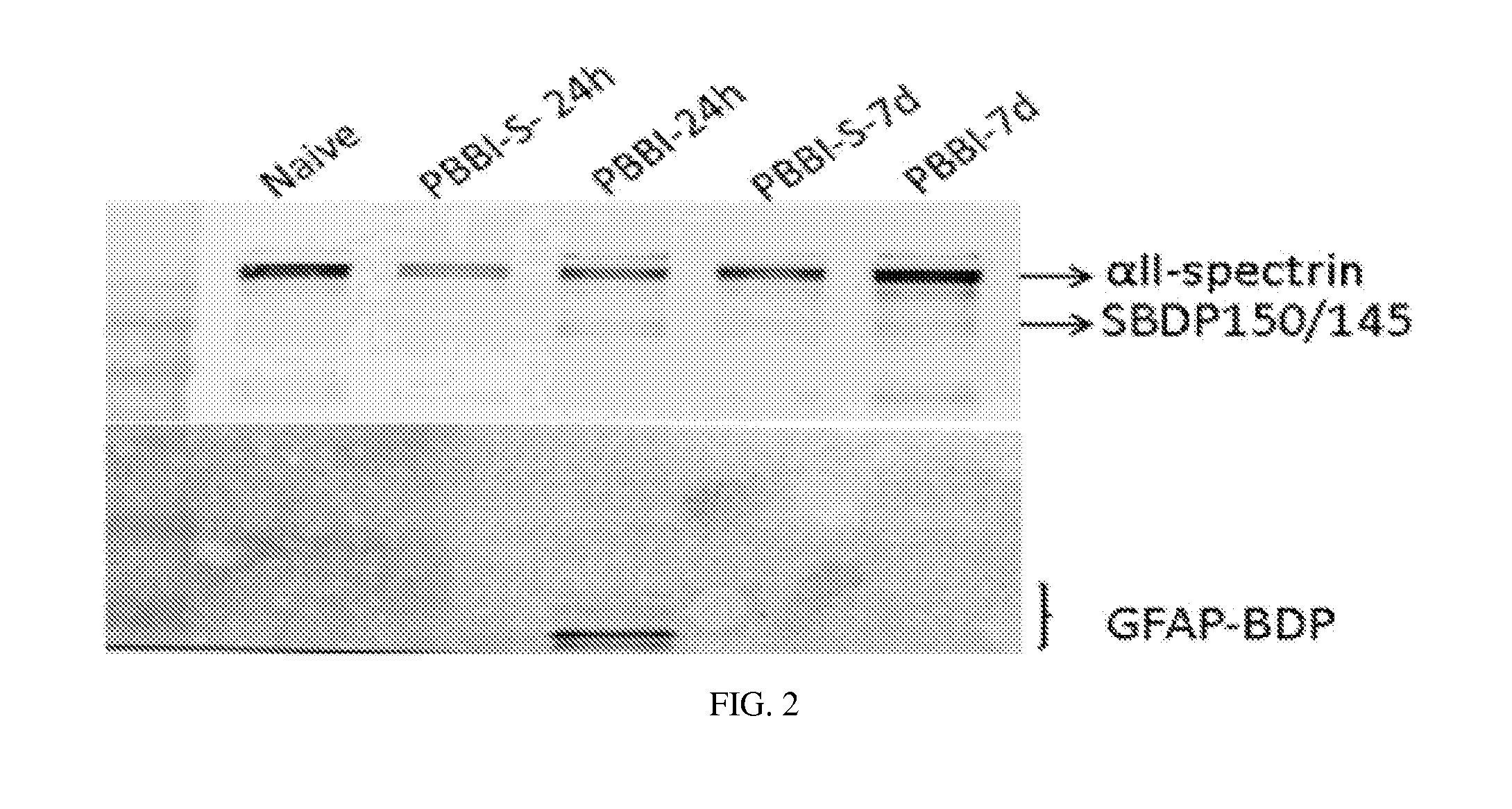
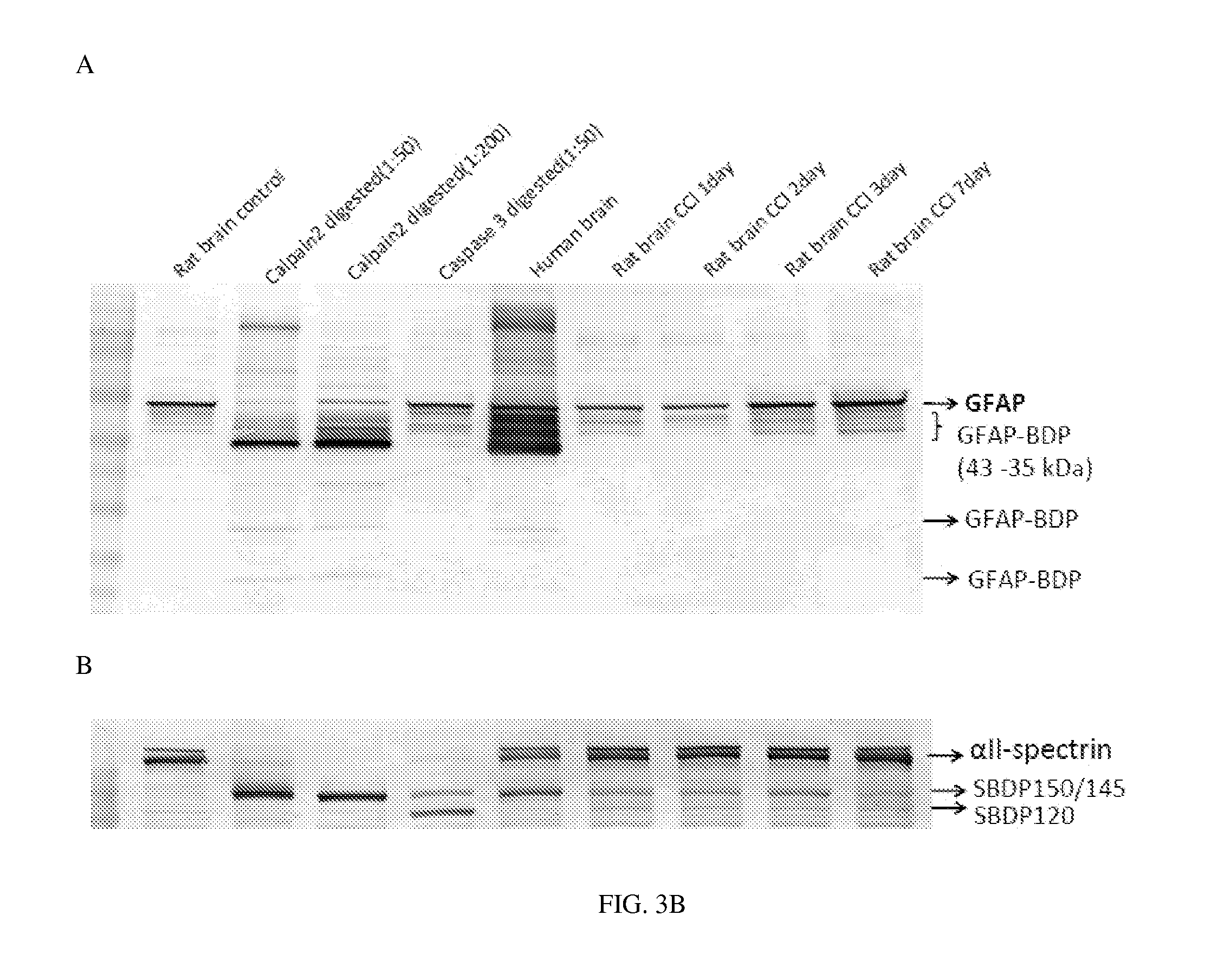
Processes and materials are provided for the detection, diagnosis, or determination of the severity of a neurological injury or condition, including traumatic brain injury, multiple-organ injury, stroke, Alzeimer's disease, Pakinson disease and Chronic Traumatic Encephalopathy (CTE). The processes and materials include biomarkers detected or measured in a biological sample such as whole blood, serum, plasma, or CSF. Such biomarkers include Tau and GFAP proteins, their proteolytic breakdown products, brain specific or enriched micro-RNA, and brain specific or enriched protein directed autoantibodies. The processes and materials are operable to detect the presence of absence of acute, subacute or chronic brain injuries and predict outcome for the brain injury.
Owner:BANYAN BIOMARKERS INC
Treatment of chronic post-traumatic encephalopathy
InactiveUS20140127171A1Inhibit host inflammatory reactionInhibit such inflammatory reactionCompounds screening/testingBiocidePost-Traumatic EncephalopathyNeural stem cell
Methods of treating chronic post-traumatic encephalopathy (PTE) using regenerative approaches is described. In one embodiment, molecules with capability of stimulating endogenous neural stem cells is provided. In another embodiment, cell therapeutics are provided capable of addressing angiogenic deficits in patients suffering from PTE. In another embodiment, cells are utilized to induce activation of endogenous progenitor cells in the central nervous system of PTE patients. Furthermore, low level laser irradiation is disclosed as a means of treatment of PTE either through direct administration to CNS tissue for stimulation of endogenous progenitor cells and reparative processes, or together with administration of exogenous stem cells, whether autologous or allogeneic. In a further embodiment exogenous stem cells are pretreated with laser prior to administration.
Owner:NOCERA ROGER +1
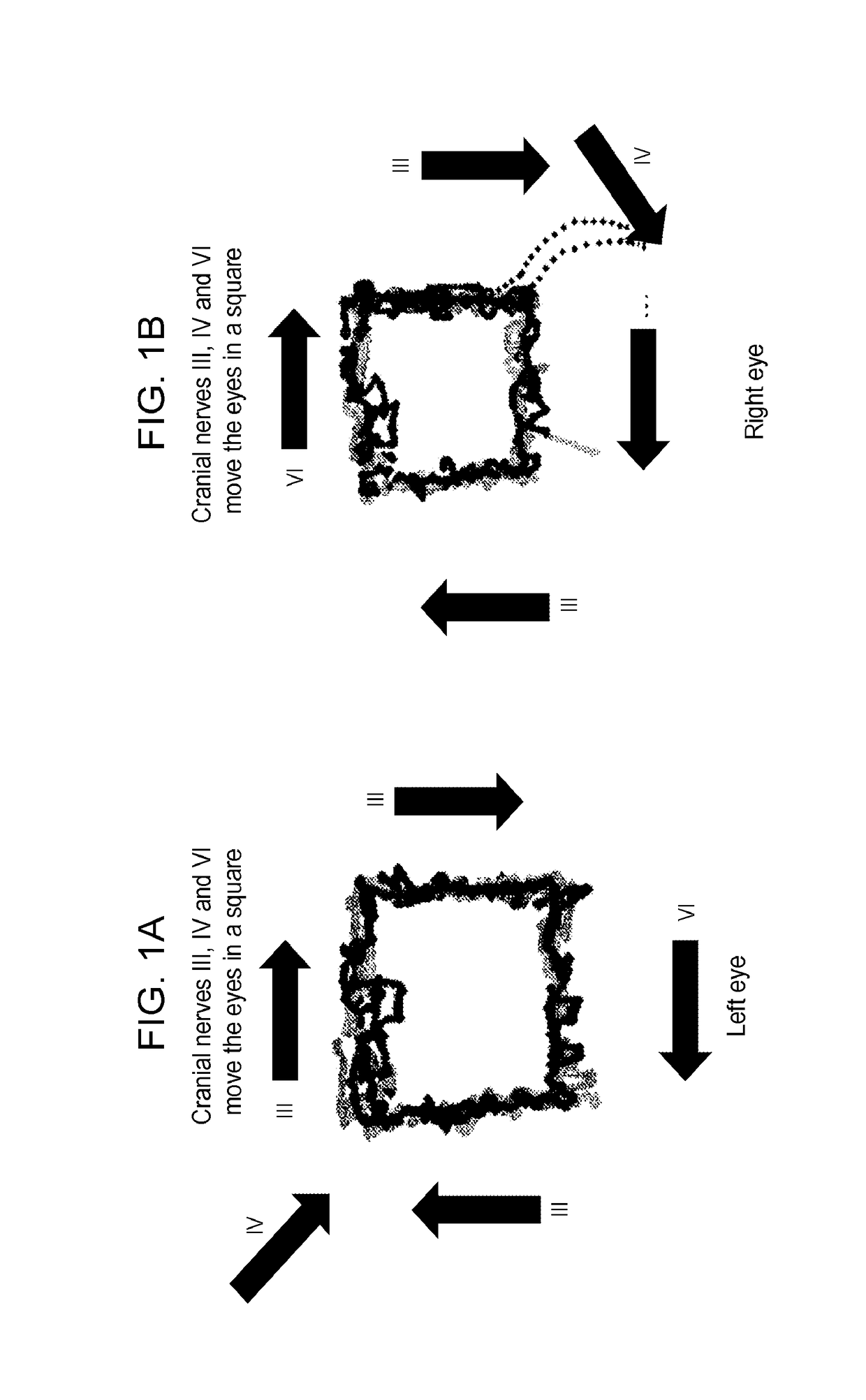
Methods and kits for diagnosing, assessing or quantitating drug use, drug abuse and narcosis, internuclear ophthalmoplegia, attention deficit hyperactivity disorder (ADHD), chronic traumatic encephalopathy, schizophrenia spectrum disorders and alcohol consumption
ActiveUS20170367633A1Slow velocityDrug and medicationsMedical automated diagnosisInjury brainAttention deficits
The invention provides methods for diagnosing, assessing or quantitating drug use, drug abuse or narcosis or for differentiating drug use, drug abuse or narcosis from brain injury in a subject by tracking eye movement of at least one eye of the subject, analyzing eye movement of at least one eye of the subject, comparing eye movement of at least one eye of the subject the normal or mean eye movement; and, optionally calculating a standard deviation or p value for eye movement of at least one eye of the subject as compared to the normal or mean eye movement.
Owner:NEW YORK UNIV
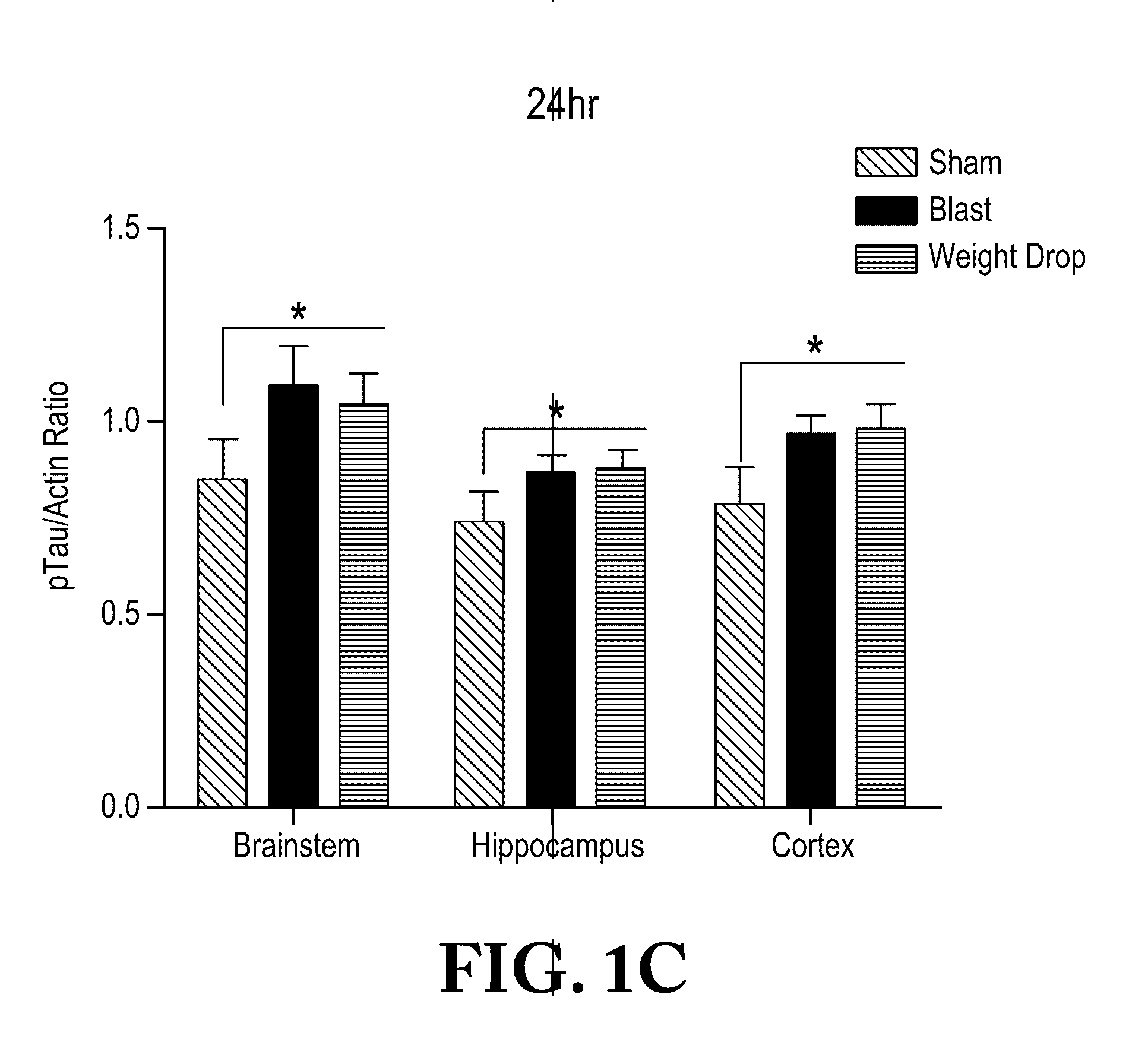
Chronic Traumatic Encephalopathy in Blast-Exposed Individuals
InactiveUS20150119273A1Delay progressIncreased riskLibrary screeningDisease diagnosisCvd riskBrains tissue
The invention is based on the surprising discovery that as few as one episode of blast exposure increases the risk of CTE. Blast exposure is associated with chronic traumatic encephalopathy, impaired neuronal function, and persistent cognitive deficits in blast-exposed military veterans and experimental animals. Early diagnosis and assessment of risk permits physicians to prescribe treatment to reduce or slow progression of impairment before the onset of overt symptoms that become apparent decades after an initial insult or trauma to brain tissue. The invention provides methods and compositions for diagnosis and prognosis of individuals at risk of long term complications related to blast injury or concussive injury.
Owner:TRUSTEES OF BOSTON UNIV +1
Detection of Brain Injury
ActiveUS20150344954A1Improve long-term prognosisGood and poor survivalBiocideNervous disorderInjury brainCerebral injury
The present invention provides minimally invasive methods of detecting, diagnosing, and assessing neuronal damage associated with traumatic brain injury (TBI) or chronic traumatic encephalopathy (CTE). Specific species of microRNAs (miRNA), small, noncoding RNA molecules that play gene regulatory functions, are correlated with cellular damage and oxidative stress following TBI or CTE, allowing for rapid, minimally-invasive diagnosis and assessment of brain injury. The early identification and longitudinal assessment of neuronal damage in subjects suffering from or at risk of suffering from a TBI (e.g., football players, boxers, military personnel, fall victims) will improve clinical outcomes by guiding critical medical and behavioral decision making.
Owner:UNIVERSITY OF MONTANA
Protein biomarkers for acute, subacute and chronic traumatic injuries of the central nervous system
Proteins that are differentially expressed or elevated in tissue and biofluids after central nervous system injuries are described. Elevated or reduced levels of the proteins, alone or in various combinations or ratios, can be used to assess severity of central nervous system injury (CNS injury) including traumatic brain injury (TBI), traumatic spinal cord injury (SCI) and chronic traumatic encephalopathy (CTE). Time course measurements post CNS-injury of these proteins can be used to monitor progress or recovery over periods up to several months. Differentiation of acute, subacute and chronic injury can be diagnosed by comparing the protein levels in CNS-injury patients at days 1-3, day 4-10 with levels at day 30-180 in comparison with normal controls.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK +1
Compounds and methods for reducing Tau expression
ActiveUS10407680B2Maintenance and reduction number and volumePreventing or amelioratingOrganic active ingredientsNervous disorderNeuro-degenerative diseaseBiology
Provided are compounds, methods, and pharmaceutical compositions for reducing the amount or activity of Tau mRNA in a cell or animal, and in certain instances reducing the amount of Tau protein in a cell or animal. Such compounds, methods, and pharmaceutical compositions are useful to ameliorate at least one symptom of a neurodegenerative disease. Such symptoms include loss of memory, loss of motor function, and increase in the number and / or volume of neurofibrillary inclusions. Such neurodegenerative diseases include tauopathies, Alzheimer's Disease, Fronto-temporal Dementia (FTD), FTDP-17, Progressive Supranuclear Palsy (PSP), Chronic Traumatic Encephalopathy (CTE), Corticobasal Ganglionic Degeneration (CBD), Epilepsy, and Dravet's Syndrome.
Owner:BIOGEN MA INC
Compounds and methods for reducing tau expression
ActiveUS20180119145A1Easy maintenanceMaintenance and reduction numberOrganic active ingredientsNervous disorderInclusion bodiesS syndrome
Provided are compounds, methods, and pharmaceutical compositions for reducing the amount or activity of Tau mRNA in a cell or animal, and in certain instances reducing the amount of Tau protein in a cell or animal. Such compounds, methods, and pharmaceutical compositions are useful to ameliorate at least one symptom of a neurodegenerative disease. Such symptoms include loss of memory, loss of motor function, and increase in the number and / or volume of neurofibrillary inclusions. Such neurodegenerative diseases include tauopathies, Alzheimer's Disease, Fronto-temporal Dementia (FTD), FTDP-17, Progressive Supranuclear Palsy (PSP), Chronic Traumatic Encephalopathy (CTE), Corticobasal Ganglionic Degeneration (CBD), Epilepsy, and Dravet's Syndrome.
Owner:BIOGEN MA INC
Detection of traumatic brain injury
ActiveUS9605315B2Improve long-term prognosisGood and poor survivalNervous disorderSugar derivativesInjury brainCvd risk
The present invention provides minimally invasive methods of detecting, diagnosing, and assessing neuronal damage associated with traumatic brain injury (TBI) or chronic traumatic encephalopathy (CTE). Specific species of microRNAs (miRNA), small, noncoding RNA molecules that play gene regulatory functions, are correlated with cellular damage and oxidative stress following TBI or CTE, allowing for rapid, minimally-invasive diagnosis and assessment of brain injury. The early identification and longitudinal assessment of neuronal damage in subjects suffering from or at risk of suffering from a TBI (e.g., football players, boxers, military personnel, fall victims) will improve clinical outcomes by guiding critical medical and behavioral decision making.
Owner:UNIVERSITY OF MONTANA
Pyrrolopyrimidine derivatives
InactiveUS20050277773A1Strong inhibitory activityAntibacterial agentsBiocideMedicineNeuro-degenerative disease
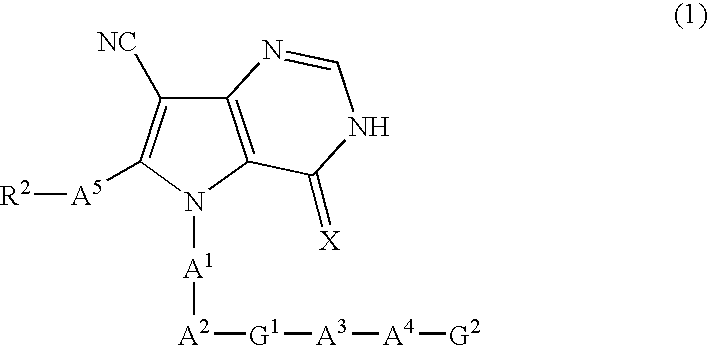
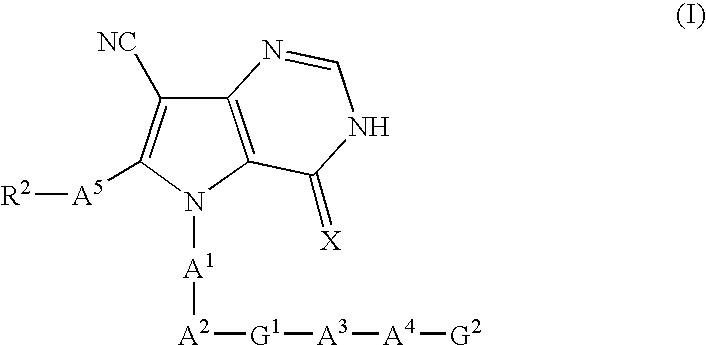
The invention provides pyrrolo[3,2-d]pyrimidine derivatives represented by formula (I), and their medically acceptable salts. The compounds of the invention exhibit GSK-3 inhibiting activity and are therefore expected to be useful as therapeutic or prophylactic agents for conditions in which GSK-3 is implicated, such as diabetes, diabetes complications, Alzheimer's disease, neurodegenerative diseases, manic depression, traumatic encephalopathy, alopecia, inflammatory diseases, cancer and immune deficiency.
Owner:TEIJIN LTD
Compounds and methods for reducing tau expression
InactiveUS20190211332A1Maintaining and improving motor functionReduce maintenanceNervous disorderDNA/RNA fragmentationBiologyS syndrome
Provided are compounds, methods, and pharmaceutical compositions for reducing the amount or activity of Tau mRNA in a cell or animal, and in certain instances reducing the amount of Tau protein in a cell or animal. Such compounds, methods, and pharmaceutical compositions are useful to ameliorate at least one symptom of a neurodegenerative disease. Such symptoms include loss of memory, loss of motor function, and increase in the number and / or volume of neurofibrillary inclusions. Such neurodegenerative diseases include tauopathies, Alzheimer's Disease, Fronto-temporal Dementia (FTD), FTDP-17, Progressive Supranuclear Palsy (PSP), Chronic Traumatic Encephalopathy (CTE), Corticobasal Ganglionic Degeneration (CBD), Epilepsy, and Dravet's Syndrome.
Owner:BIOGEN IDEC MA INC
Micro-rna, autoantibody and protein markers for diagnosis of neuronal injury
ActiveUS20170242041A1Microbiological testing/measurementImmunoglobulins against animals/humansDiseaseProtein markers
Processes and materials are provided for the detection, diagnosis, or determination of the severity of a neurological injury or condition, including traumatic brain injury, multiple-organ injury, stroke, Alzeimer's disease, Parkinson disease and Chronic Traumatic Encephalopathy (CTE). The processes and materials include biomarkers detected or measured in a biological sample such as whole blood, serum, plasma, or CSF. Such biomarkers include Tau and GFAP proteins, their proteolytic breakdown products, brain specific or enriched micro-RNA, and brain specific or enriched protein directed autoantibodies. The processes and materials are operable to detect the presence of absence of acute, subacute or chronic brain injuries and predict outcome for the brain injury.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY +1
Methods and kits for diagnosing, assessing or quantitating drug use, drug abuse and narcosis, internuclear ophthalmoplegia, attention deficit hyperactivity disorder (ADHD), chronic traumatic encephalopathy, schizophrenia spectrum disorders and alcohol consumption
The invention provides methods for diagnosing, assessing or quantitating drug use, drug abuse or narcosis or for differentiating drug use, drug abuse or narcosis from brain injury in a subject by tracking eye movement of at least one eye of the subject, analyzing eye movement of at least one eye of the subject, comparing eye movement of at least one eye of the subject the normal or mean eye movement; and, optionally calculating a standard deviation or p value for eye movement of at least one eye of the subject as compared to the normal or mean eye movement.
Owner:NEW YORK UNIV
Isoquinoline derivatives as perk inhibitors
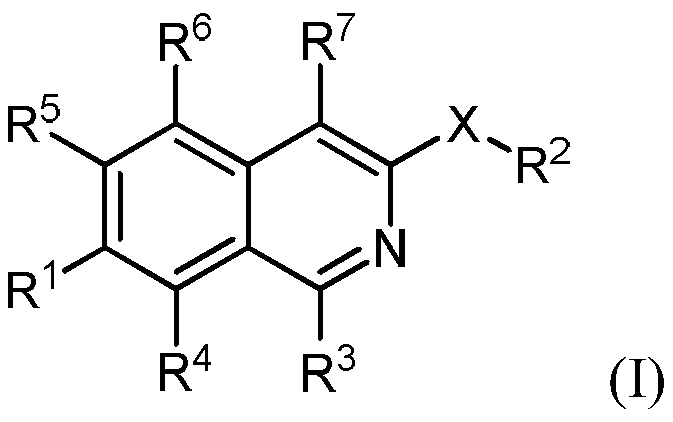
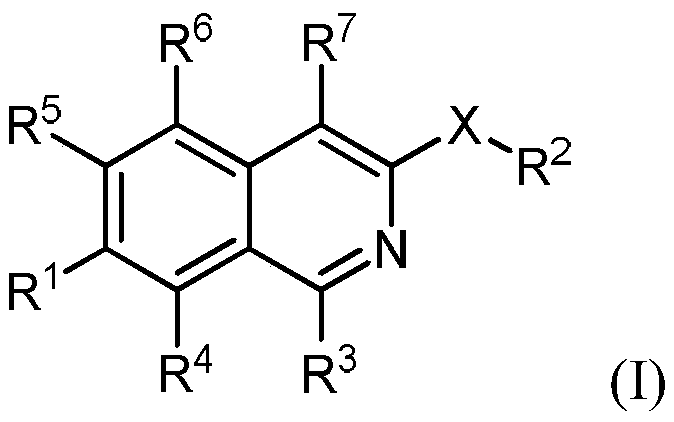
The invention is directed to substituted isoquinoline derivatives and uses thereof. Specifically, the invention is directed to compounds according to Formula I and the use of compounds of Formula (I)in treating disease states: (I) wherein R1, R2, R3, R4, R5, R6, R7 and X are as defined herein. The compounds of the invention are inhibitors of PERK and can be useful in the treatment of cancer, pre-cancerous syndromes and diseases associated with activated unfolded protein response pathways, such as Alzheimer's disease, spinal cord injury, traumatic brain injury, ischemic stroke, stroke, Parkinson disease, diabetes, metabolic syndrome, metabolic disorders, Huntington's disease, Creutzfeldt-Jakob Disease, fatal familial insomnia, Gerstmann-Str ussler-Scheinker syndrome, and related prion diseases, amyotrophic lateral sclerosis, progressive supranuclear palsy, myocardial infarction, cardiovascular disease, inflammation, organ fibrosis, chronic and acute diseases of the liver, fatty liver disease, liver steatosis, liver fibrosis, chronic and acute diseases of the lung, lung fibrosis, chronic and acute diseases of the kidney, kidney fibrosis, chronic traumatic encephalopathy (CTE), neurodegeneration, dementias, frontotemporal dementias, tauopathies, Pick's disease, Neimann-Pick's disease, amyloidosis, cognitive impairment, ather osclerosis, ocular diseases, arrhythmias, in organ transplantation and in the transportation of organs for transplantation. Accordingly, the invention is further directed to pharmaceutical compositions comprising a compound of the invention. The inventionis still further directed to methods of inhibiting PERK activity and treatment of disorders associated therewith using a compound of the invention or a pharmaceutical composition comprising a compound of the invention.
Owner:GLAXOSMITHKLINE INTPROP DEV LTD
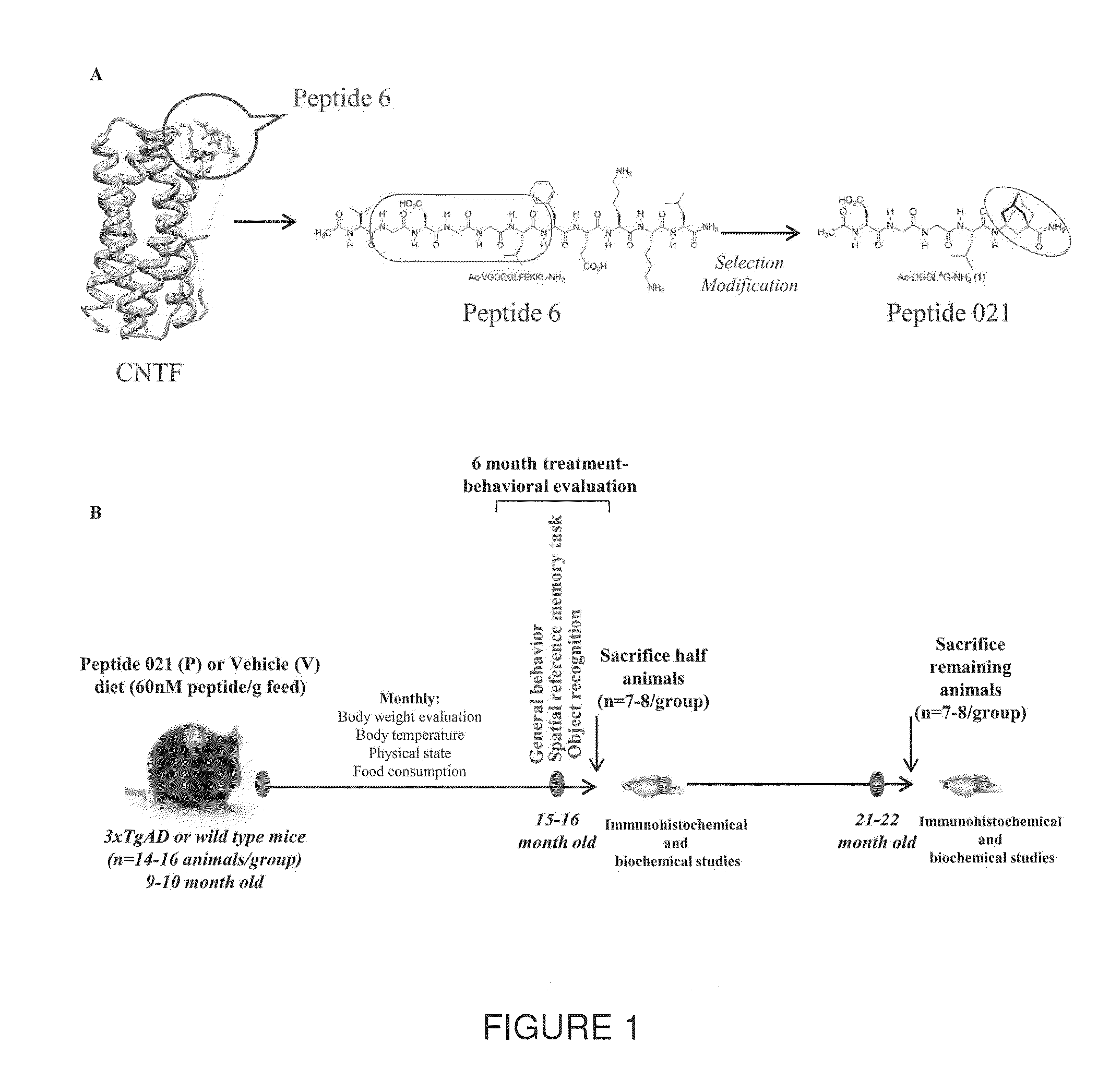
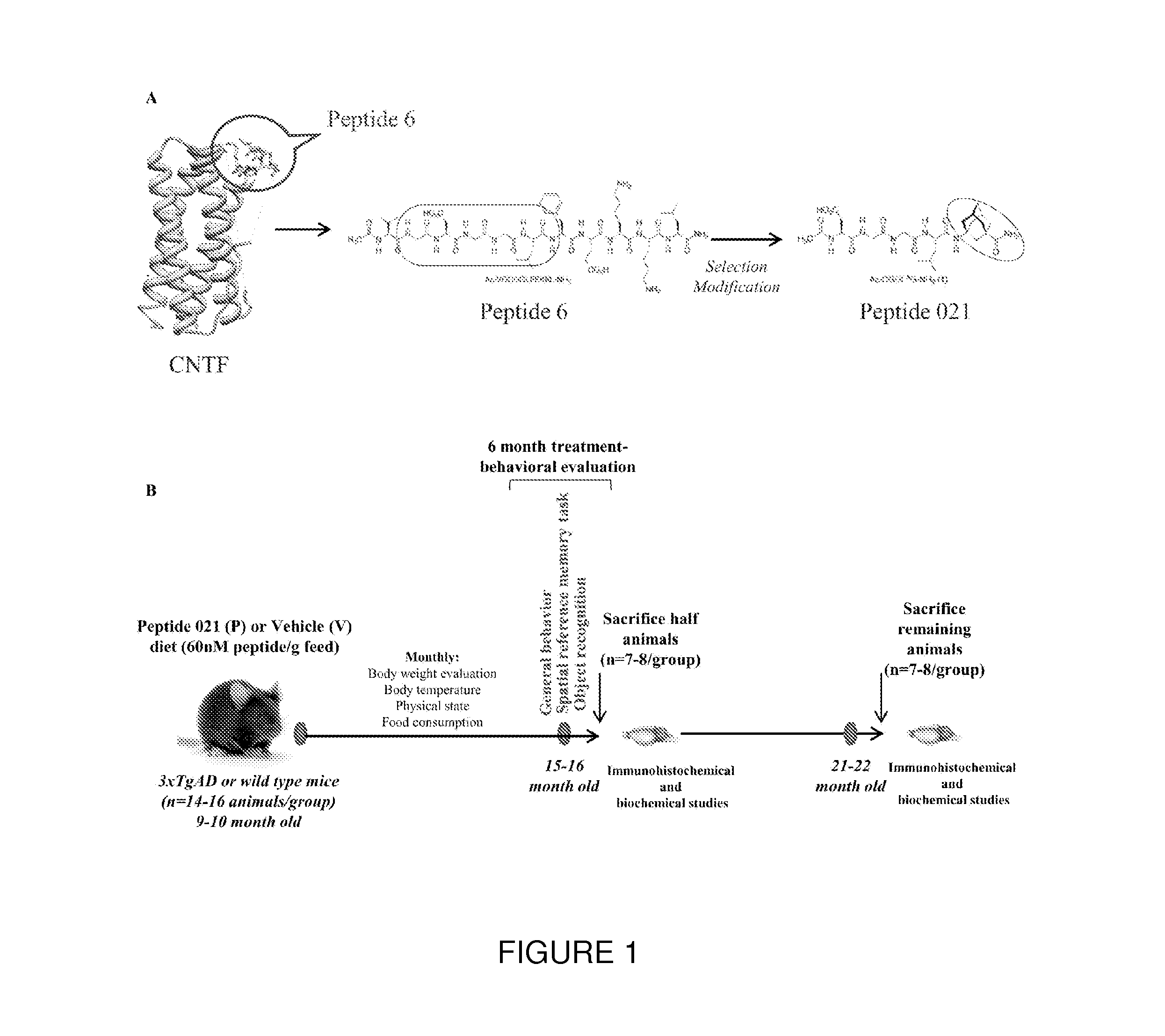
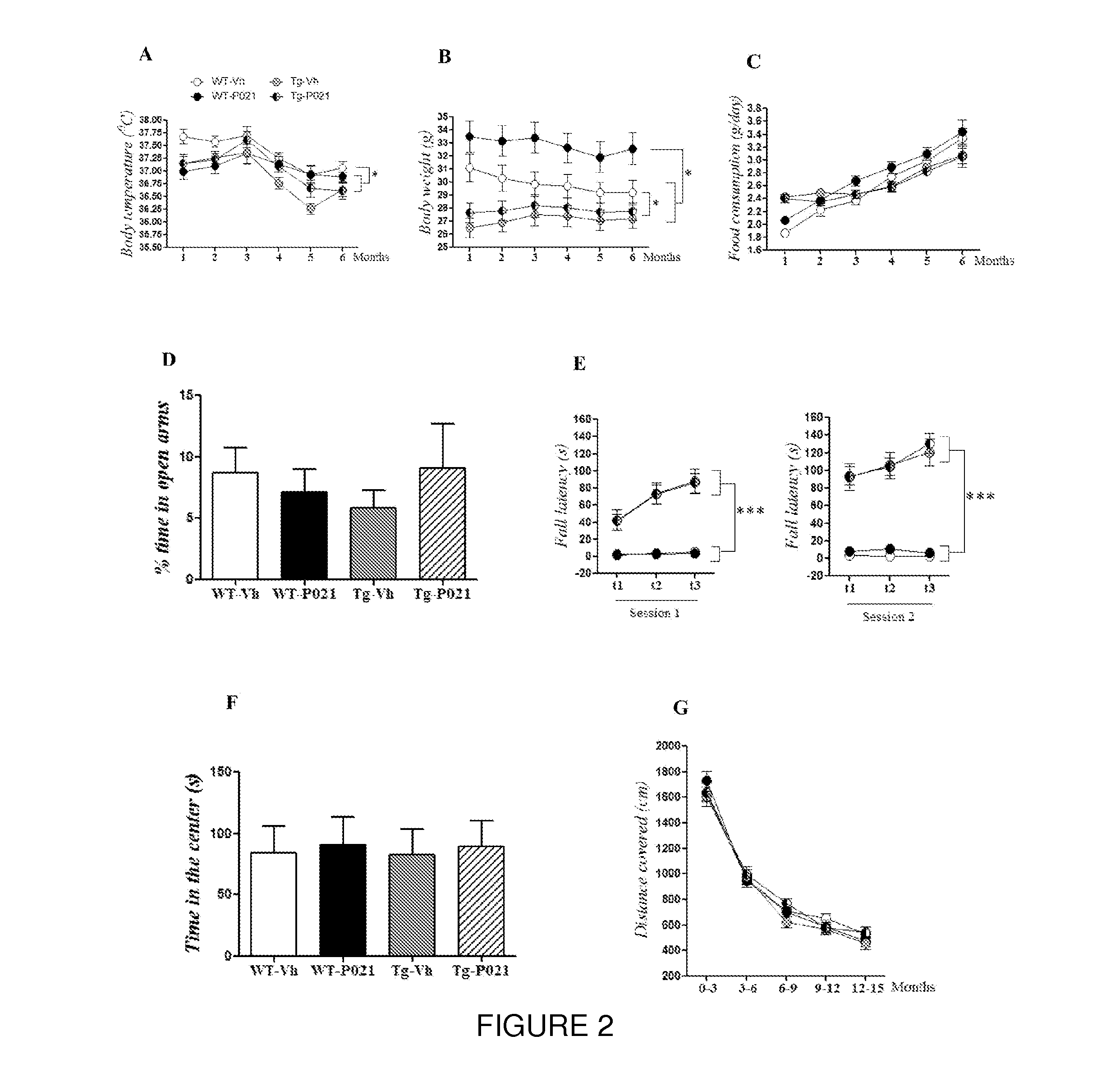
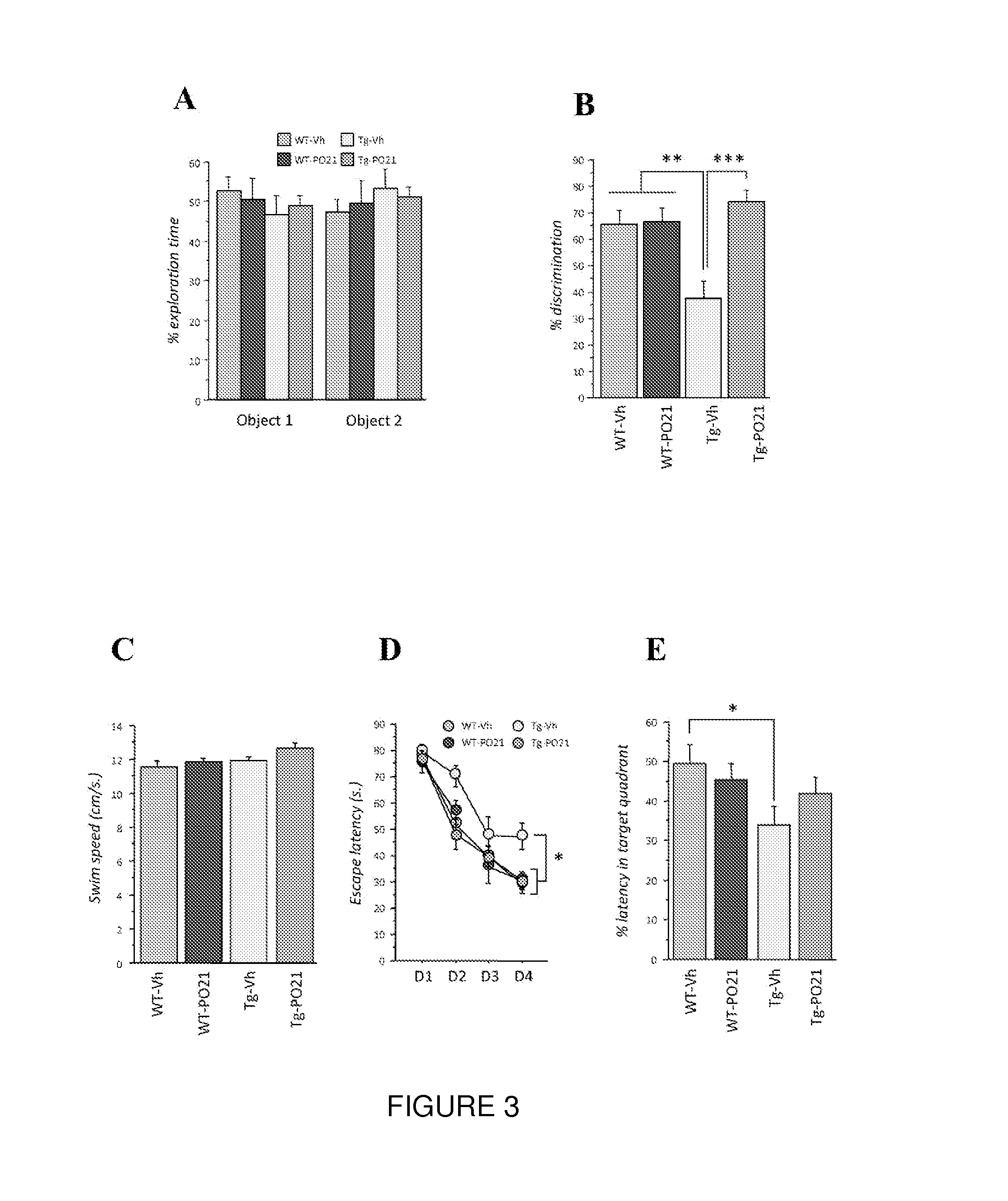
Neurotrophic peptides for the treatment of tauopathies
ActiveUS9327011B2Same orEffective treatmentTetrapeptide ingredientsPharmaceutical non-active ingredientsMedicineParkinsonism dementia
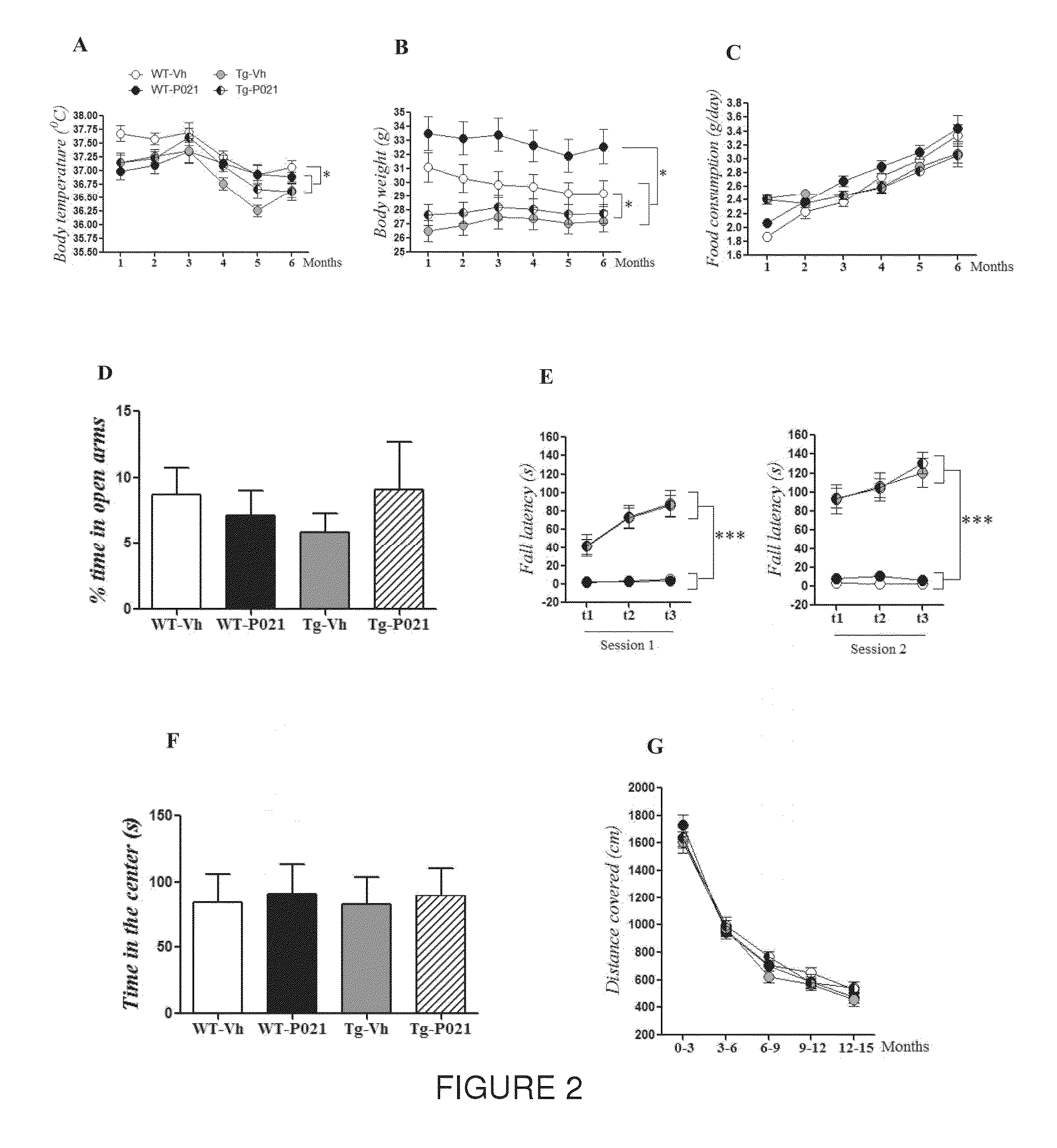
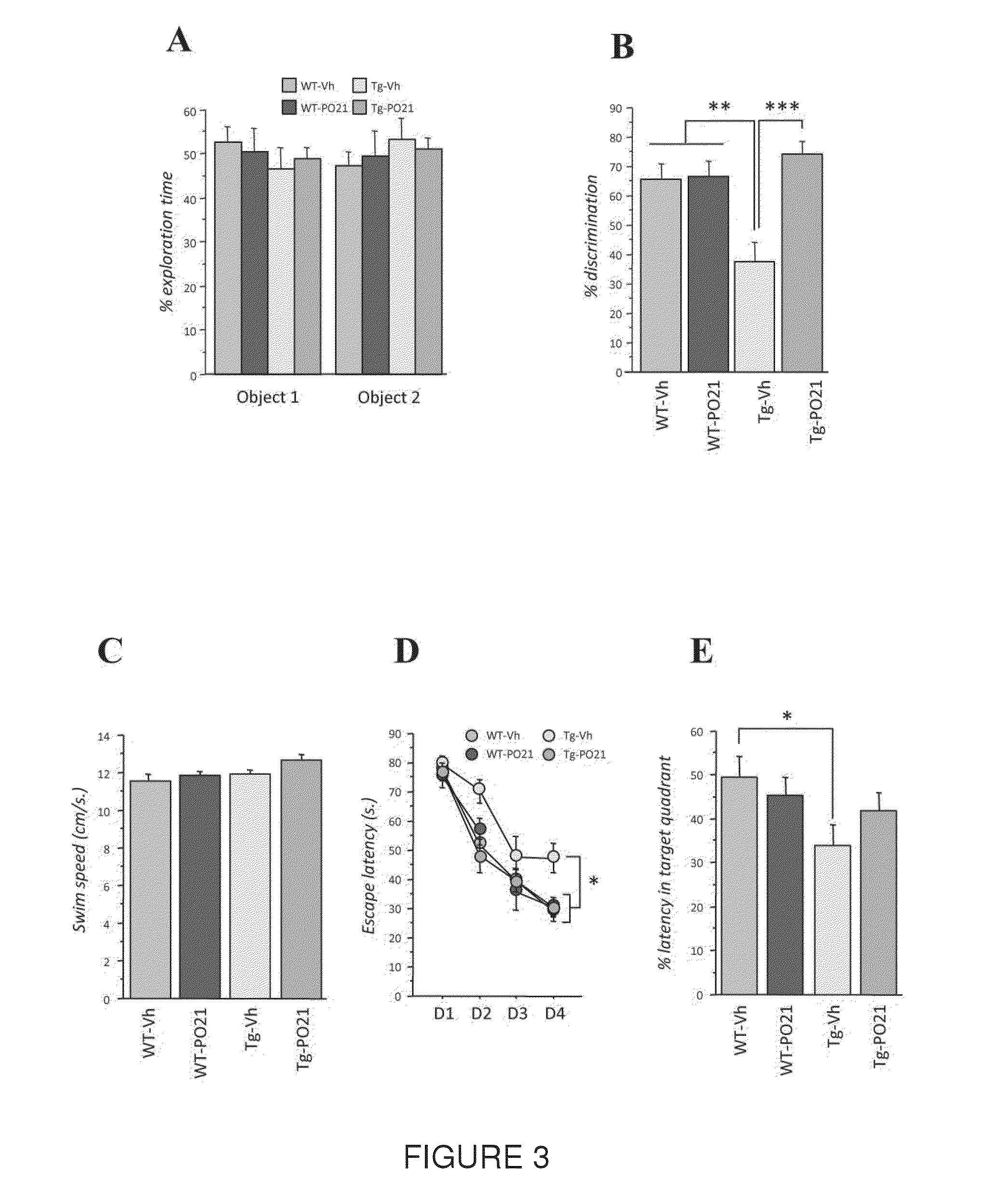
Peptide 6 and in particular, Peptide 021, may be used to treat tauopathies, such as frontotemporal dementia with Parkinsonism linked to chromosome-17 (FTDP-17) tau, corticobasal degeneration, Pick disease, progressive supranuclear palsy, Guam Parkinsonism dementia complex, dementia pugilistica also known as traumatic encephalopathy or traumatic brain injury, ceroid neuronal lipofusinosis, Hallerworden Sptaz disease, Alzheimer's disease, and adults with Down syndrome.
Owner:RES FOUDATION FOR MENTAL HYGIENE INC
Oral formulations and uses thereof
PendingCN111670035AOrganic active ingredientsNervous disorderICP - Intracranial pressurePost-concussion syndrome
Disclosed are therapeutic oral formulations comprising particular substituted pyridine based compounds, their manufacture, and methods and uses of said formulations in treating substance P mediated pathways in the brain such as elevated intracranial pressure or the modification of expression of (hyper)-phosphorylated tau protein (tau) in the brain for indications such as, but not limited to concussion, post-concussive (or post-concussion) syndrome (PCS), chronic traumatic encephalopathy (CTE), traumatic brain injury (TBI) and stroke.
Owner:EUSTRALIS PHARMA LIMITED TRADING AS PRESSURA NEURO
Neurotrophic peptides for the treatment of tauopathies
ActiveUS20140357572A1Same orEffective treatmentNervous disorderMetabolism disorderMedicineCorticobasal degeneration
Peptide 6 and in particular, Peptide 021, may be used to treat tauopathies, such as frontotemporal dementia with Parkinsonism linked to chromosome-17 (FTDP-17) tau, corticobasal degeneration, Pick disease, progressive supranuclear palsy, Guam Parkinsonism dementia complex, dementia pugilistica also known as traumatic encephalopathy or traumatic brain injury, ceroid neuronal lipofusinosis, Hallerworden Sptaz disease, Alzheimer's disease, and adults with Down syndrome.
Owner:RES FOUDATION FOR MENTAL HYGIENE INC
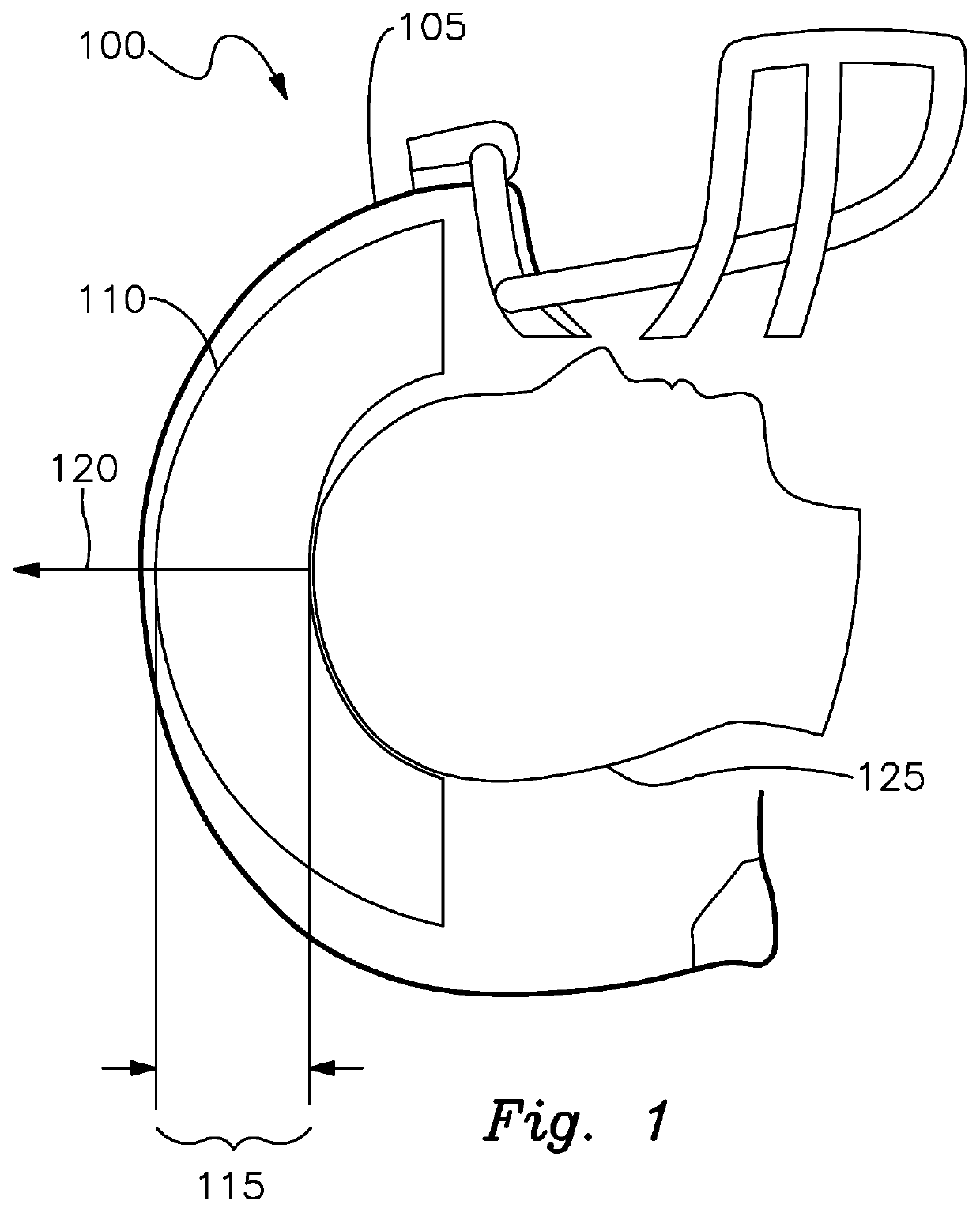
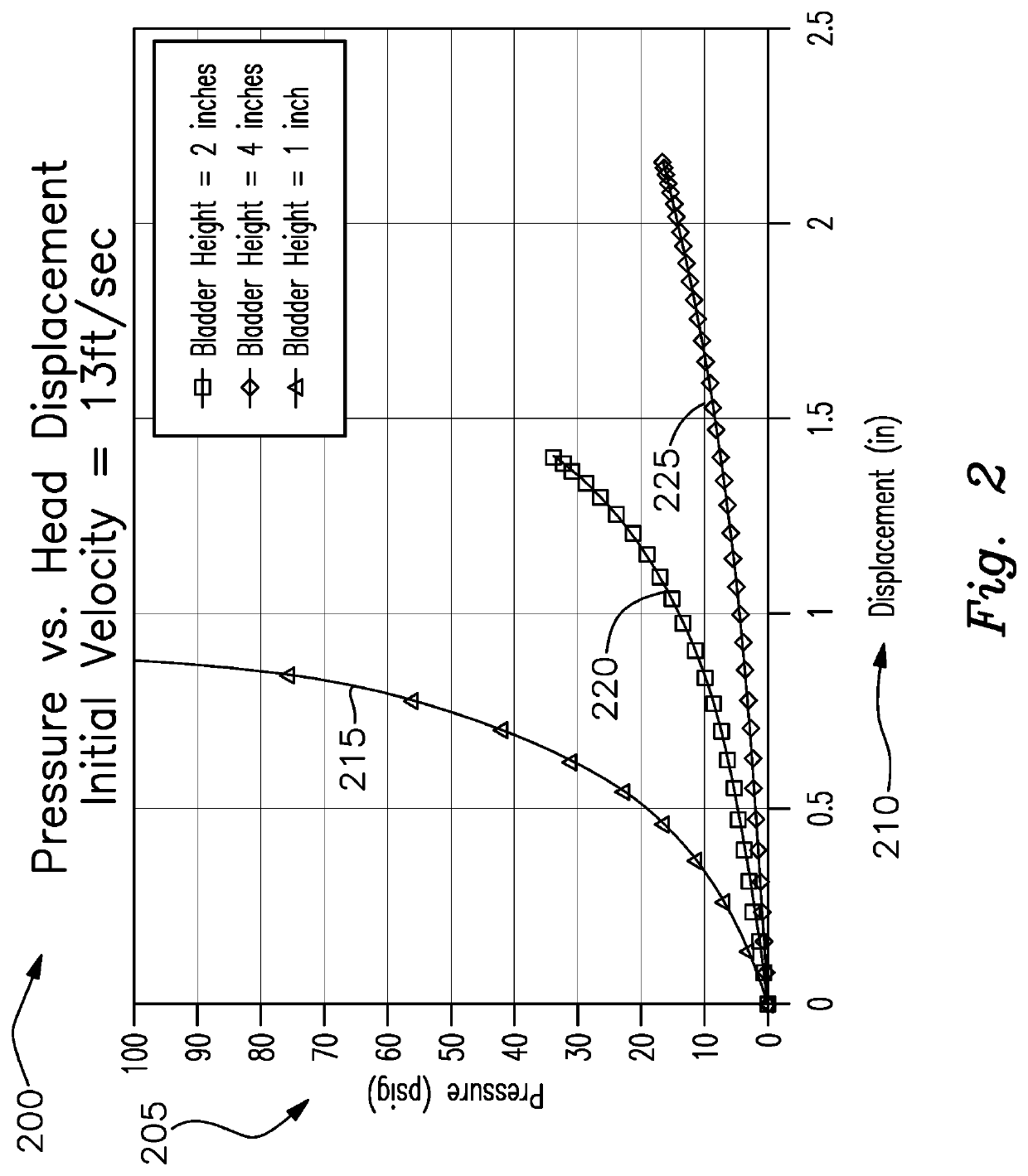
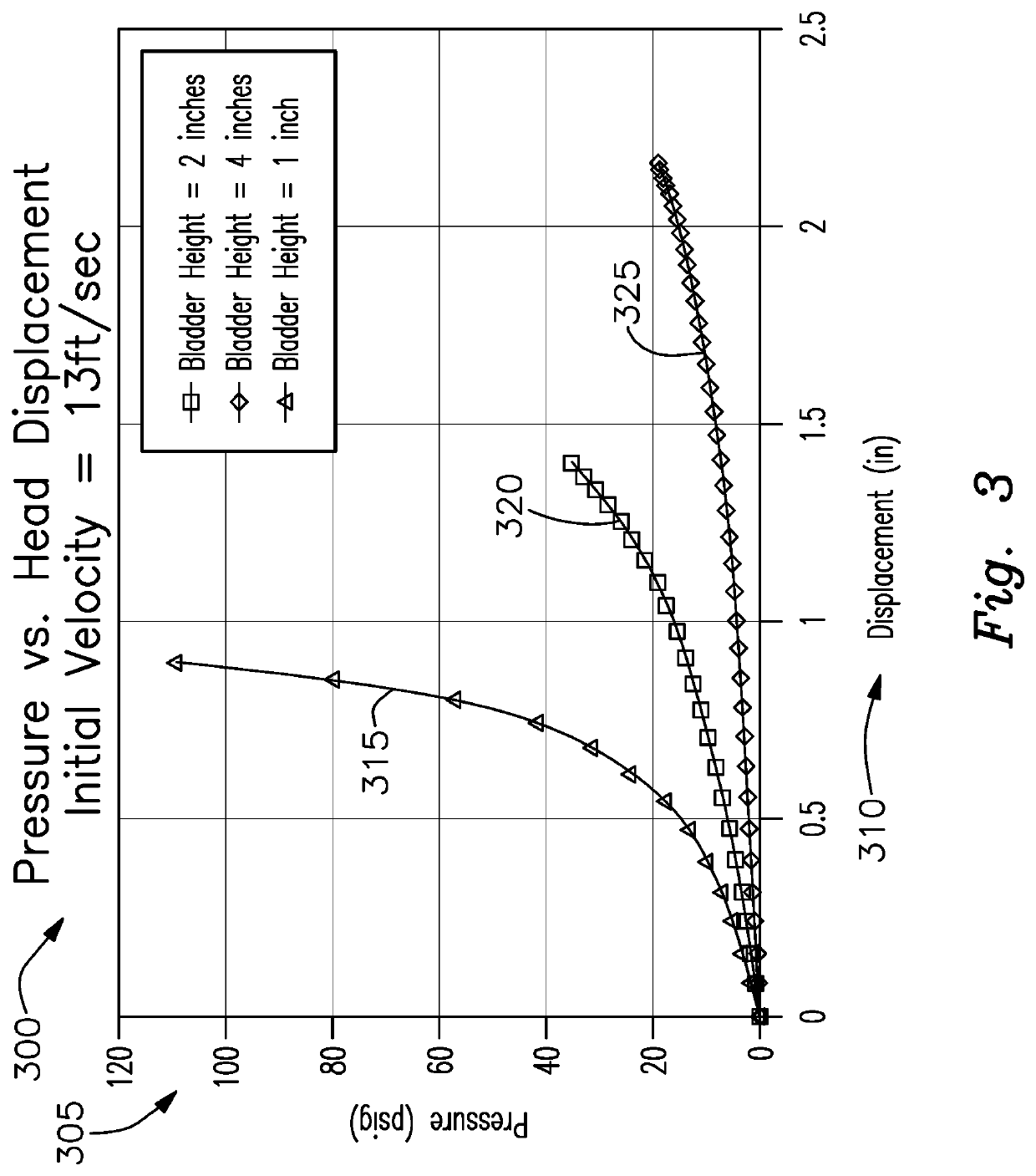
Chronic Traumatic Encephalopathy Limiting Sports Helmet
PendingUS20200281300A1Reduce head injuriesImprove impact performanceSport apparatusHelmetsAccelerometerAtmospheric air
A smart hard shell helmet for protection of head injury having a plurality of air filled inflatable bladders inside the shell, a valve on at least one bladder that responds to a controller, at least one flexible extendible strap extended from the bladder to a one point on the inside of the helmet, accelerometers operably connected to the helmet and wearer's head for sensing g force changes, pressure sensors in the bladders, an electrically actuated valve responsive to pressure and force measurement determined by the controller thresholds being met, and the valve exhausts a portion of the air in the bladder to the atmosphere extending the strap to the second state followed by the strap returning to the first state by drawing atmospheric air through the valve and re-inflating the bladder.
Owner:SHAKESPEARE W JEFFREY
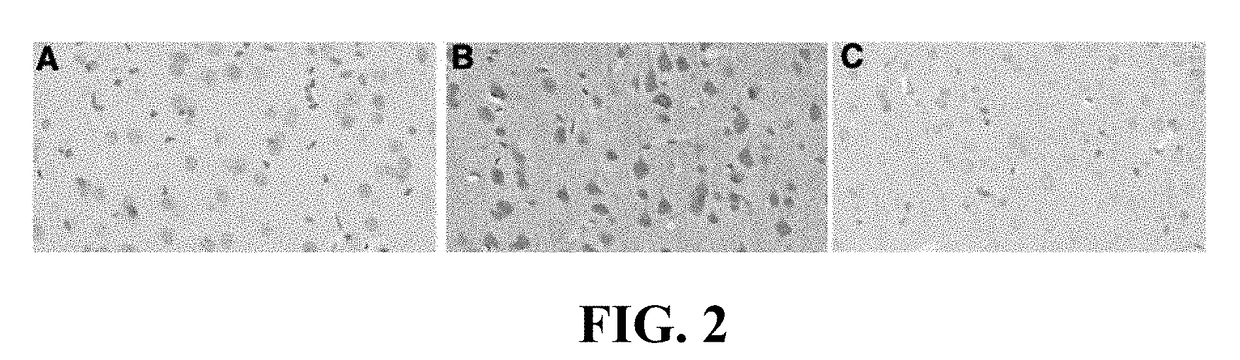
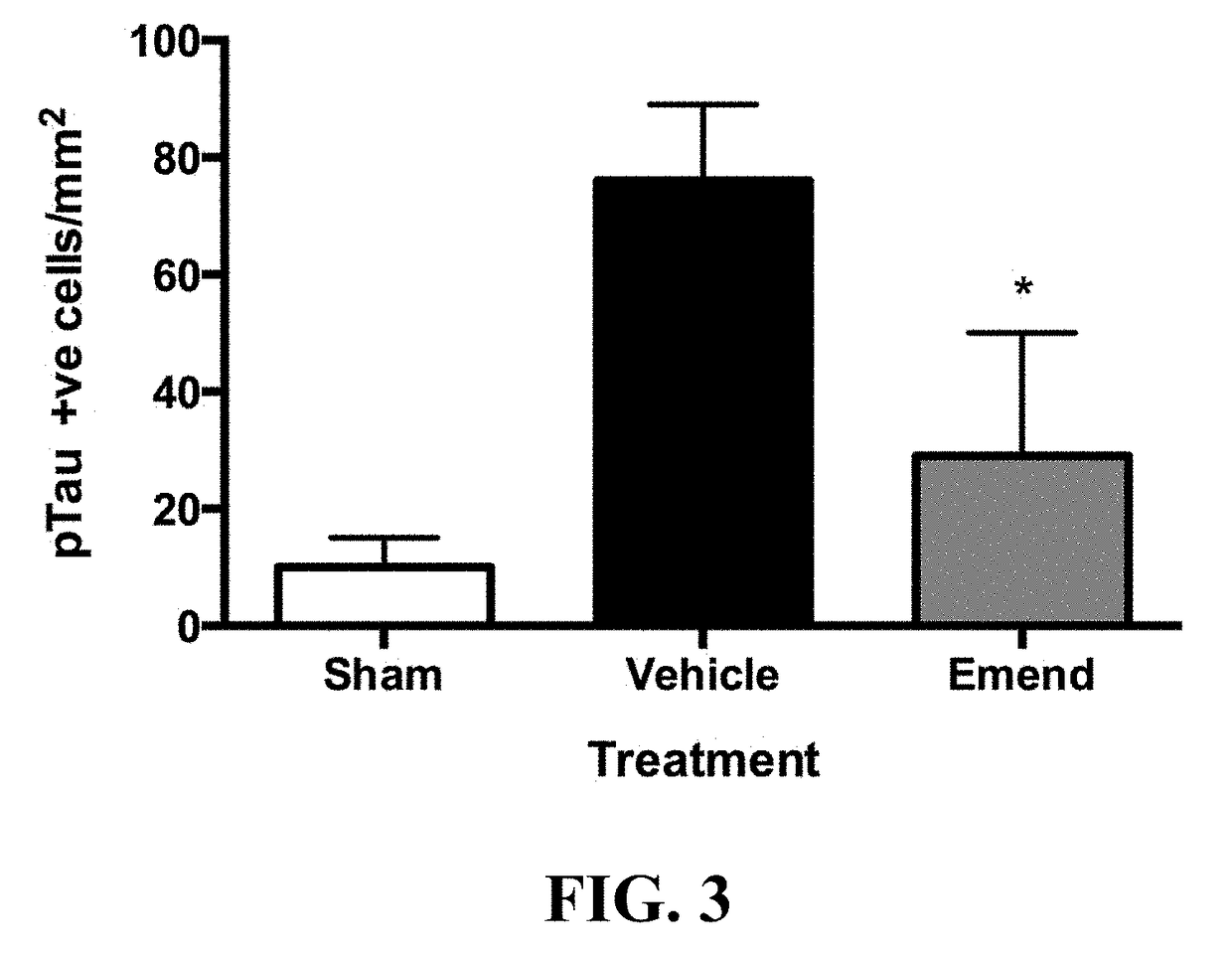
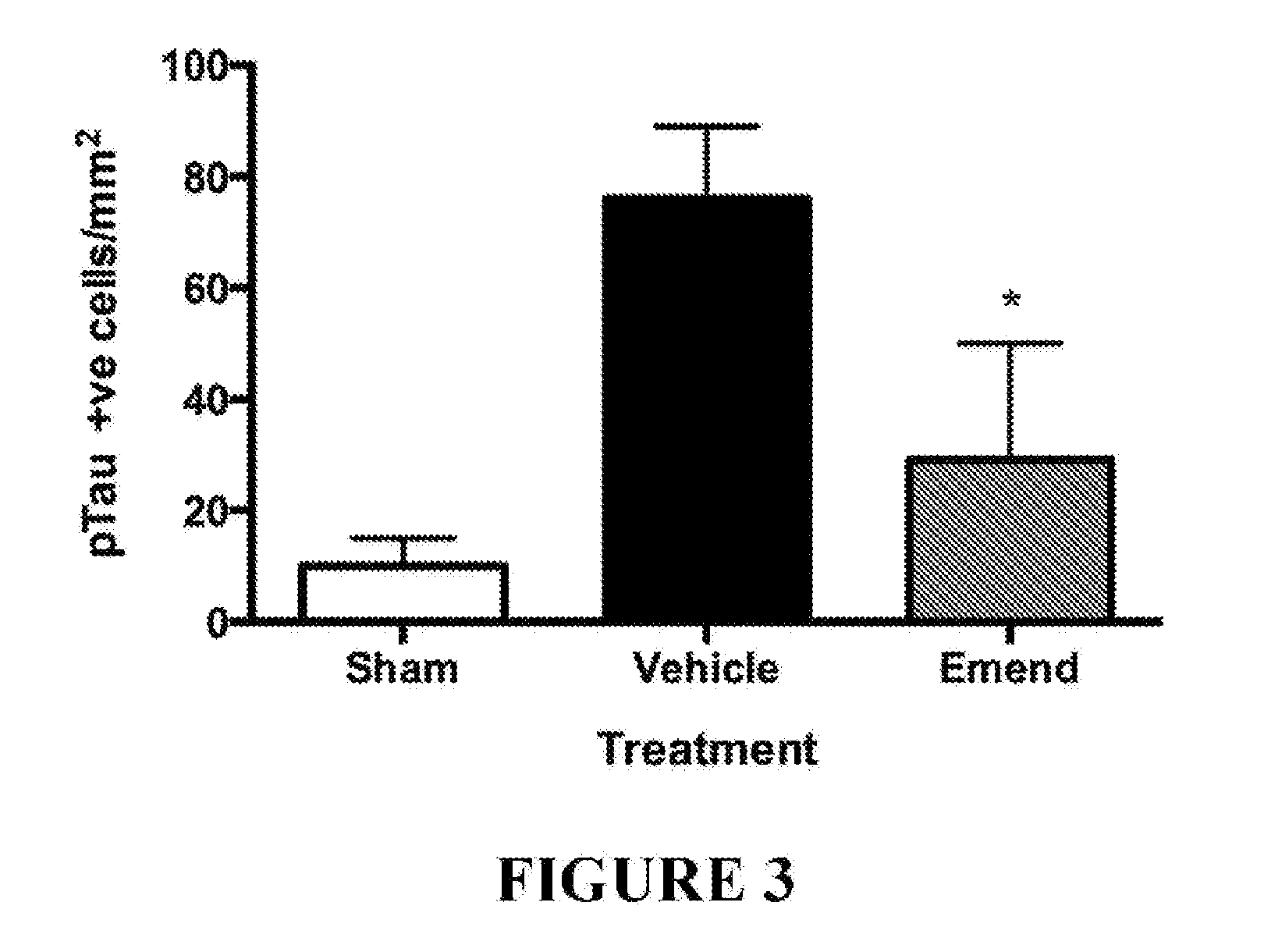
Method for preventing and/or treating chronic traumatic encephalopathy - iii
The present invention relates to a method for the prevention and / or treatment of chronic traumatic encephalopathy comprising administration of an effective amount of aprepitant (otherwise known as emend, fosaprepitant, ivemend, L754030, and ONO-7436). The invention further relates to the inhibition of progression of a disease, condition or state associated with tau hyperphosphorylation, and the treatment of a subject with a concussive injury.
Owner:EUSTRALIS PHARMA LIMITED TRADING AS PRESSURA NEURO
Novel methods of treating a neurodegenerative disease in a mammal in need thereof
ActiveUS20160303107A1High spinal cord concentrationImprove survivalNervous disorderHeterocyclic compound active ingredientsHuntingtons choreaAmyotrophic lateral sclerosis
The present invention provides a method of treating or ameliorating a neurodegenerative disease in a mammal, the method comprising administering to the mammal a therapeutically effective amount of a neurodegenerative disease drug, wherein the drug is a substrate of an ABC transporter inhibitor, wherein the mammal is further administered a therapeutically effective amount of an ABC transporter inhibitor, whereby the neurodegenerative disease is treated in the mammal. In certain embodiments, the neurodegenerative disease comprises at least one selected from the group consisting of spinal cord injury, Alzheimer's disease, Parkinson's disease, Huntington's disease, prion disease, amyotrophic lateral sclerosis, a tauopathy, and chronic traumatic encephalopathy.
Owner:THOMAS JEFFERSON UNIV
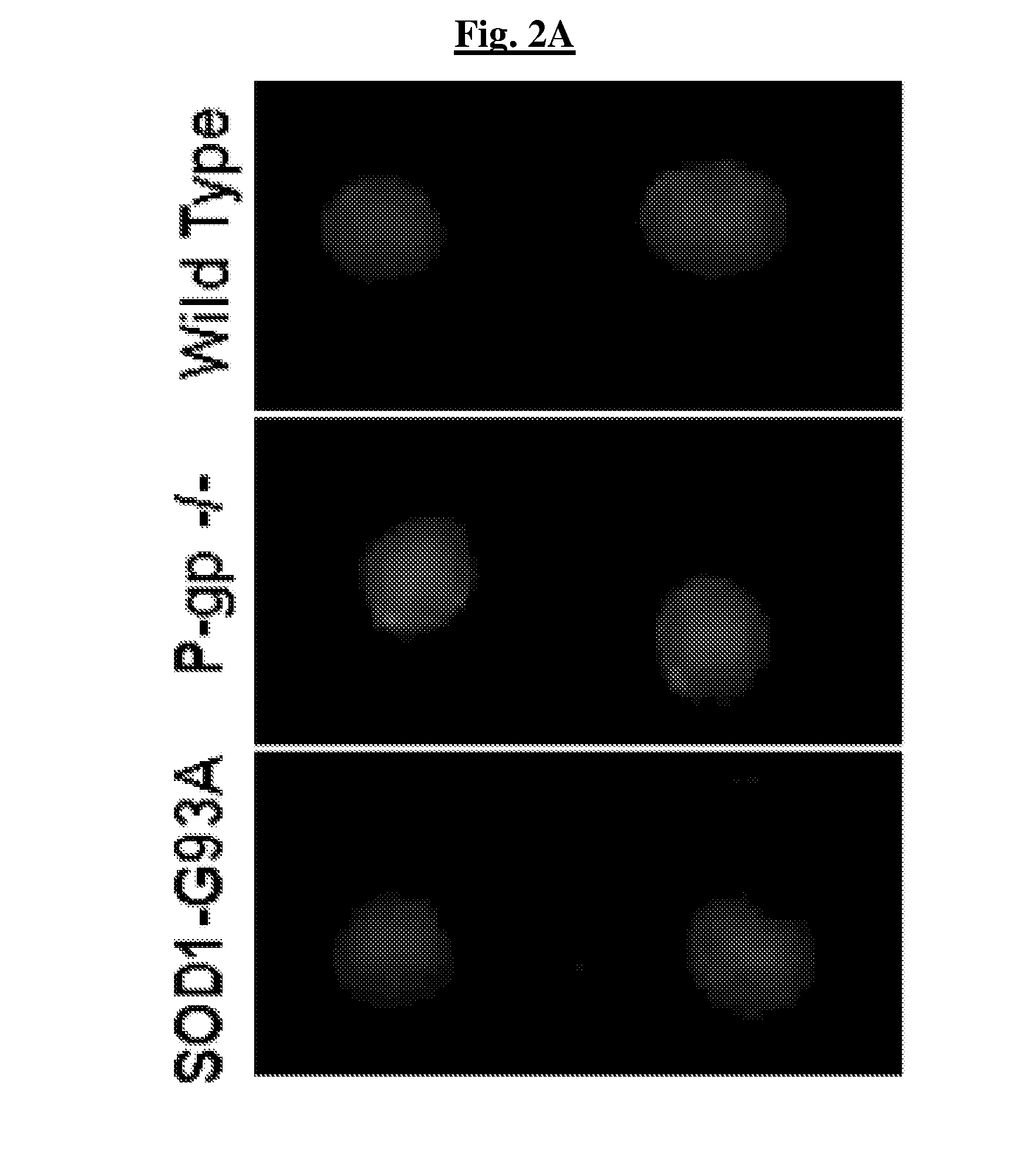
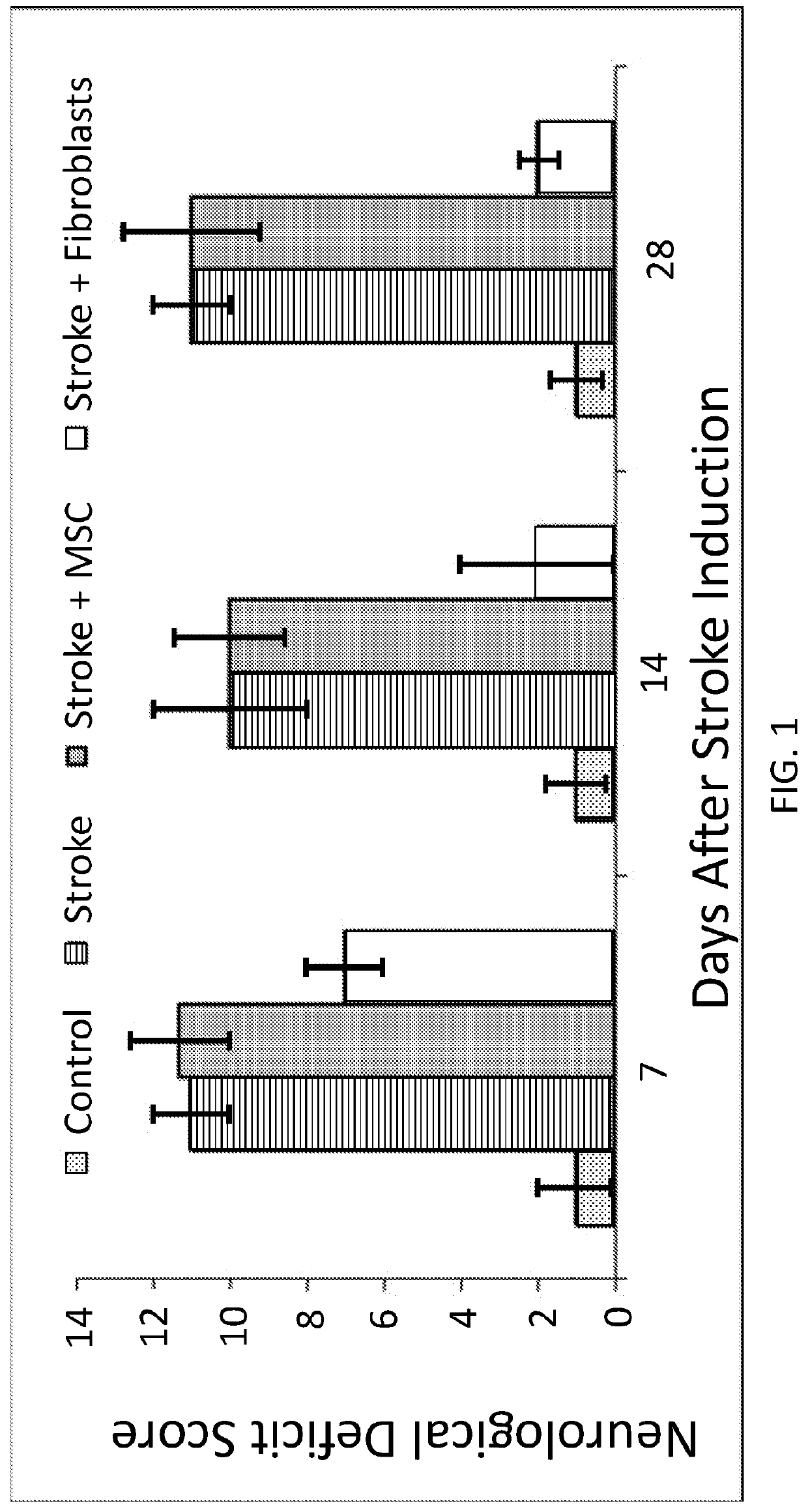
Treatment of cerebral hypoxia including stroke, chronic traumatic encephalopathy, and traumatic brain injury
PendingUS20220023350A1Enhance regenerative activityNervous disorderAntibody ingredientsBrain HypoxiaInjury brain
Disclosed are methods, compositions of matter, and means of treatment or prophylaxis using fibroblasts possessing regenerative properties for the treatment of brain injuries including stroke, transient ischemic injuries, chronic traumatic encephalopathy, traumatic brain injury, and tauopathies. Embodiments of the disclosure administer fibroblasts with regenerative properties either systemically, locally, or a combination of the two prior to, concurrent with, or subsequent to a brain injury. In some embodiments of the disclosure fibroblasts or products thereof, are administered intranasally, intrathecally, and / or intravenously.
Owner:FIGENE
Method for preventing and/or treating chronic traumatic encephalopathy-I
ActiveUS10729677B2Good effectImprove the level ofOrganic active ingredientsNervous disorderPharmacologyChronic traumatic encephalopathy
The present invention relates to a method of preventing and / or treating chronic traumatic encephalopathy.
Owner:EUSTRALIS PHARMA LIMITED TRADING AS PRESSURA NEURO
Method for Preventing and/or Treating Chronic Traumatic Encephalopathy - III
InactiveUS20160136173A1Improved prognosisImprove stateOrganic active ingredientsNervous disorderTau hyperphosphorylationAprepitant
The present invention relates to a method for the prevention and / or treatment of chronic traumatic encephalopathy comprising administration of an effective amount of aprepitant (otherwise known as emend, fosaprepitant, ivemend, L754030, and ONO-7436). The invention further relates to the inhibition of progression of a disease, condition or state associated with tau hyperphosphorylation, and the treatment of a subject with a concussive injury.
Owner:EUSTRALIS PHARMA LIMITED TRADING AS PRESSURA NEURO
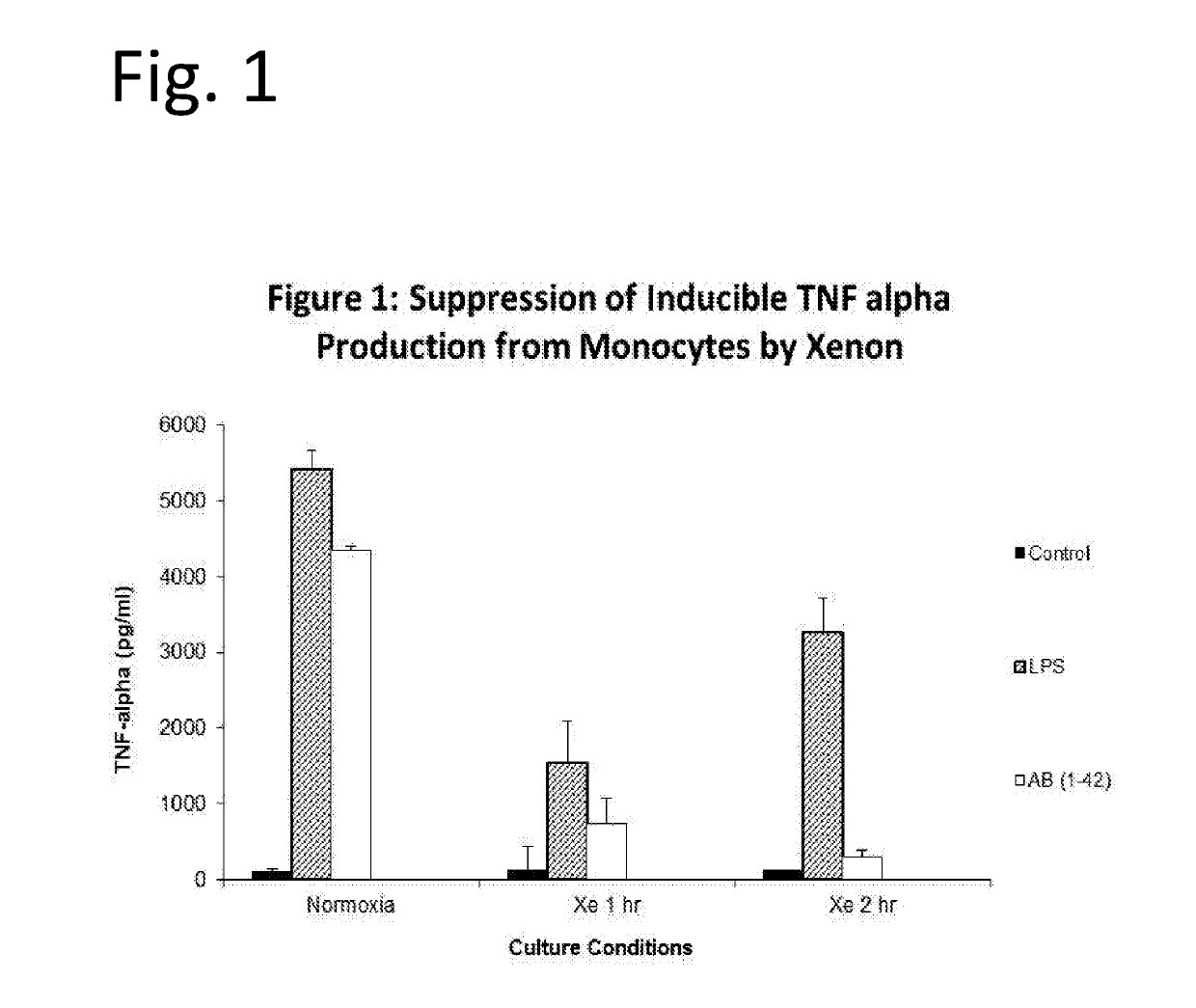
Noble gas treatment of alzheimer's disease and taupathies
InactiveUS20170196906A1Inorganic active ingredientsPharmaceutical delivery mechanismNoble gasBiology
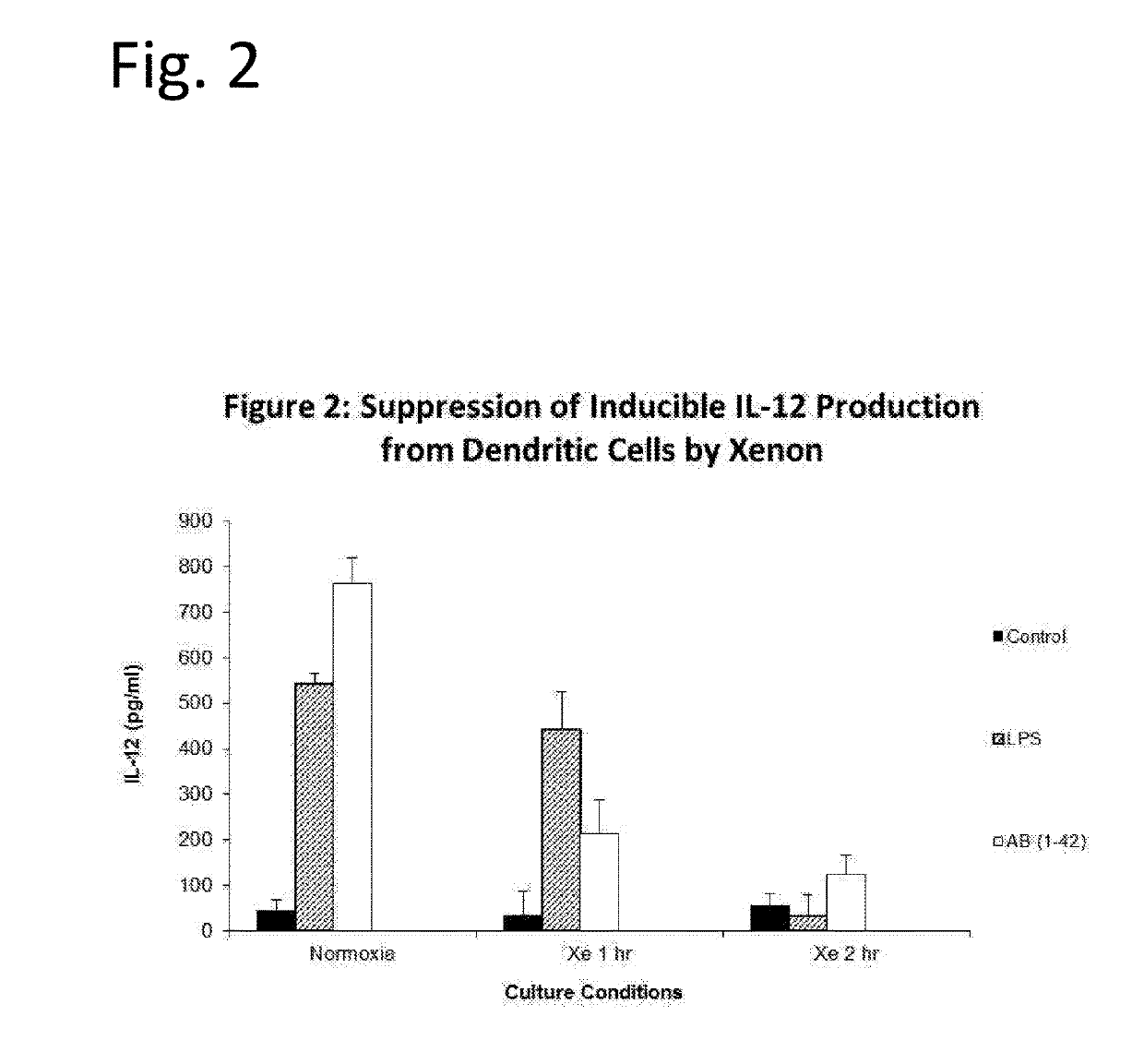
The invention discloses means of utilizing Noble Gases for inhibition of pathogenic processes associated with Alzheimer's Disease progression, as well as reversal of Alzheimer's Disease. In one embodiment compositions containing xenon gas are administered alone or together with therapeutic interventions for treatment of Alzheimer's Disease. In another embodiment, taupathies such as chronic traumatic encephalopathy are treated by administration of xenon or Noble Gas containing mixtures.
Owner:NOBILIS THERAPEUTICS INC
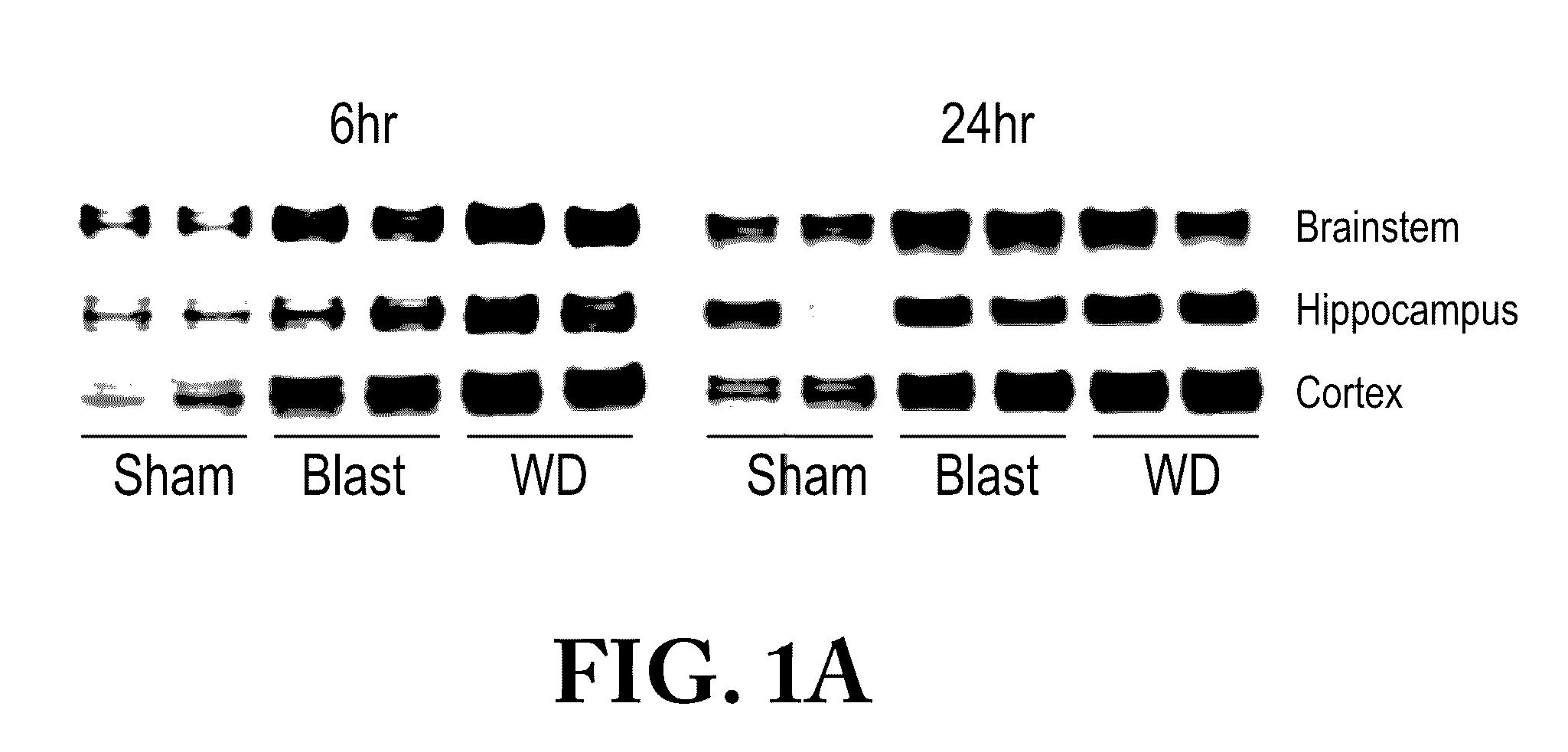
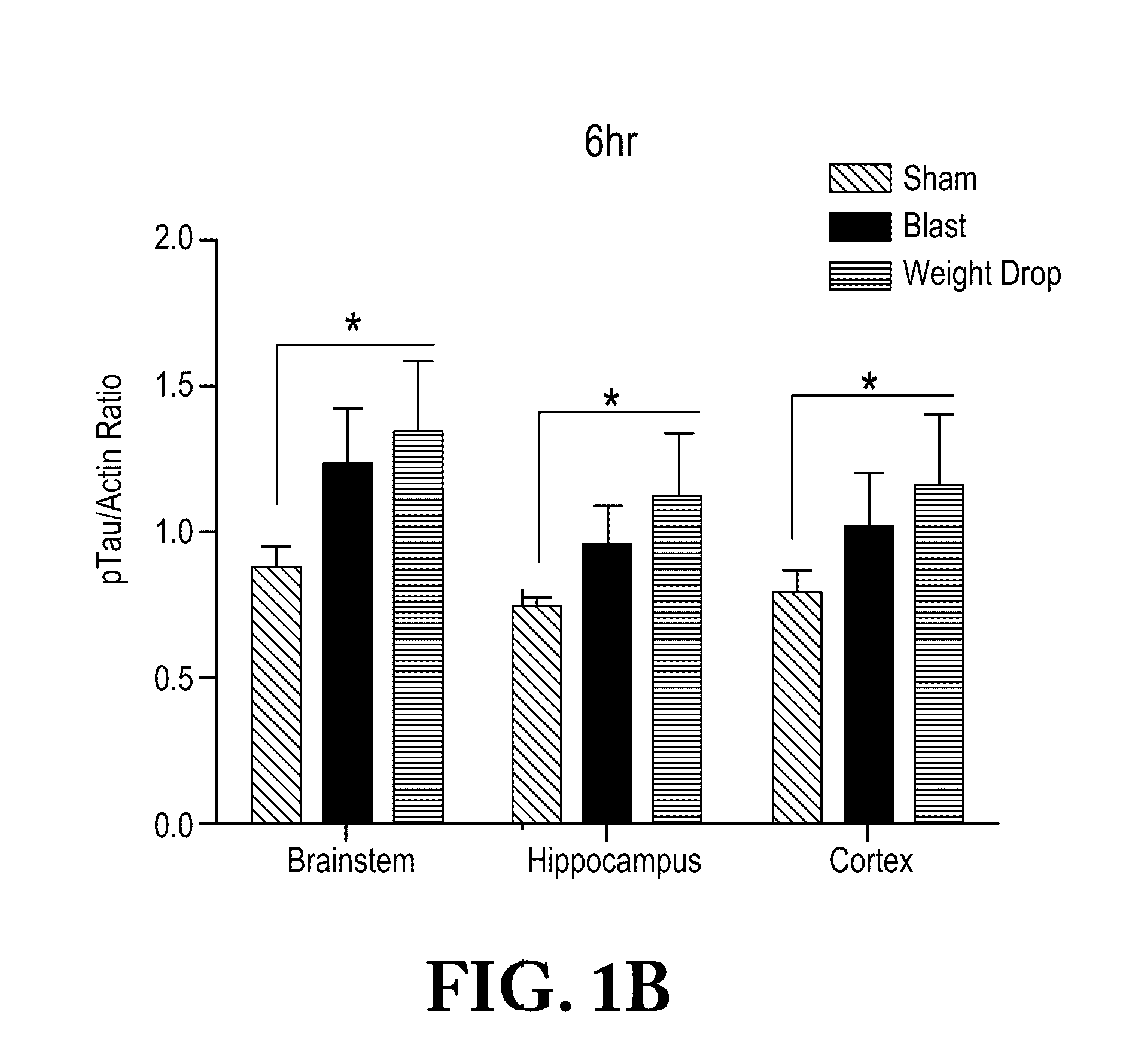
Diagnosis and treatment of tauopathy and chronic traumatic encephalopathy
InactiveUS20170030930A1Reduce expressionReduced activityHydrolasesPeptide/protein ingredientsBiomarker (petroleum)Normal range
A method of diagnosing TBI-induced tauopathy / chronic traumatic encephalopathy (CTE) by obtaining control samples from a control patients that have not been exposed to TBI and recording a normal range of tissue non-specific alkaline phosphatase (TNAP) or total alkaline phosphatase (AP) activity. Then obtaining samples from object patients that have been exposed to TBI. Comparing the biomarker, TNAP / AP, levels of said object patients to the controls. Then determining if the object patient has been exposed to TBI if the TNAP / AP levels are decreased below the normal range. Treating the patient by increasing the level of TNAP enzyme in the brain to within a normal range or modifying the TNAP enzyme activity so that it regains normal activity.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
Mobile phone for treating a patient with dementia
ActiveUS10293160B2Improve the level ofSlowing and arresting progressionUltrasonic/sonic/infrasonic diagnosticsInertial sensorsRechargeable cellPhysical therapy
Devices, systems and methods are disclosed that allow a patient to self-treat neurodegenerative diseases, such as dementia, Alzheimer's disease, ischemic stroke, post-concussion syndrome, chronic traumatic encephalopathy and the like by electrical noninvasive stimulation of a vagus nerve. The system comprises a handheld stimulator that is applied to the surface of the patient's neck, wherein the stimulator comprises or is joined to a smartphone. A camera of the smartphone may be used to position and reposition the stimulator to a particular location on the patient's neck. The system may also comprise a base station that is used to meter the charging of a rechargeable battery within the stimulator. The base station and stimulator transmit data to one another regarding the status of a stimulation session.
Owner:ELECTROCORE
Treatment of chronic traumatic encephalopathy
The invention relates to compounds, compositions and methods to effectively treat traumatic brain injury (TBI) and chronic traumatic encephalopathy (CTE). As a result of administering a therapeutically effective amount of 3-phenyl-N-[2,2,2-trichloro-1-[[(8-quinolinylamino) thioxomethyl] amino] ethyl]-2-propenamide and / or guanabenz, the effects of traumatic brain injury are mitigated and / or the development of chronic traumatic encephalopathy is reduced or precluded.
Owner:CTEC LLC
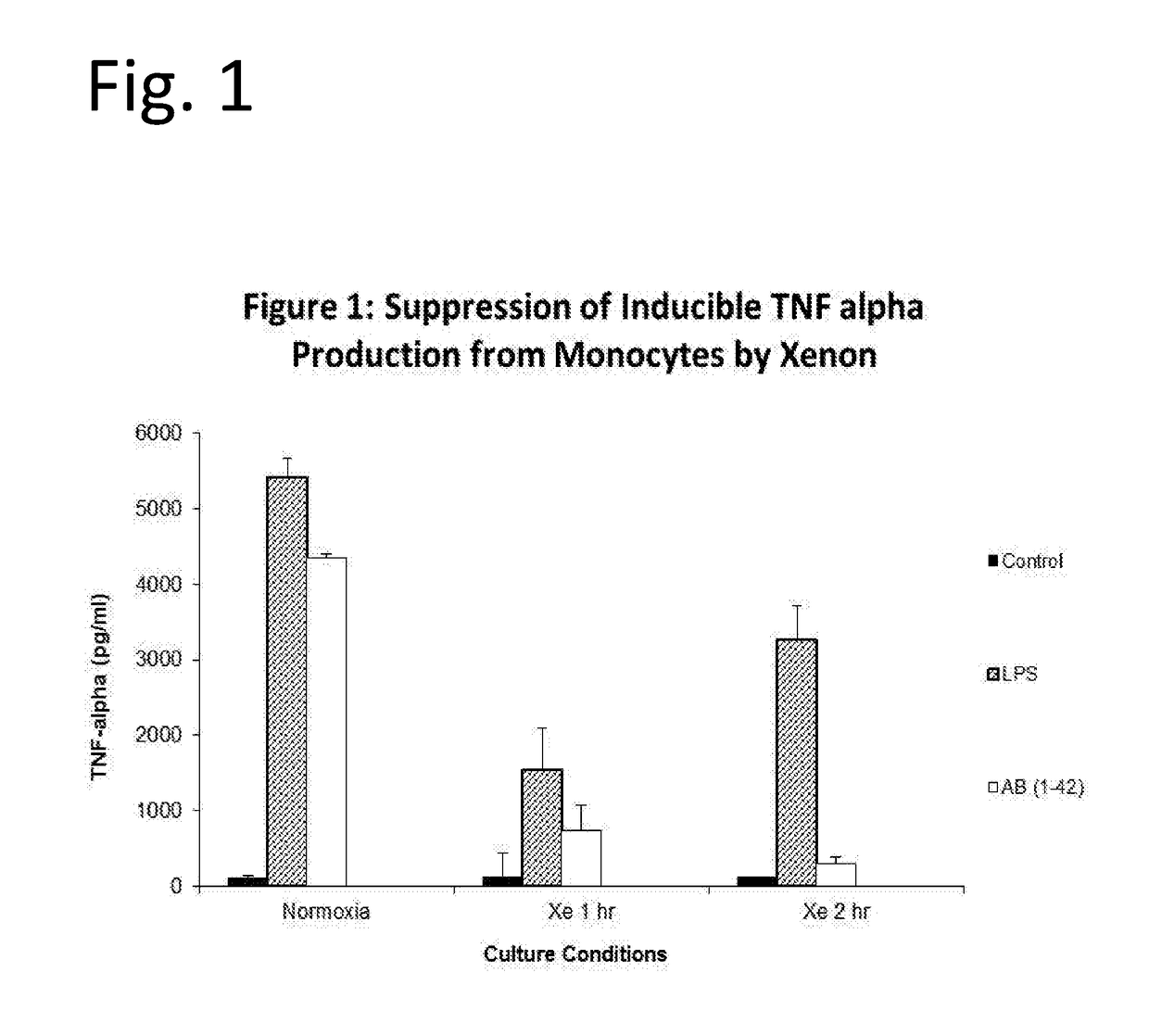
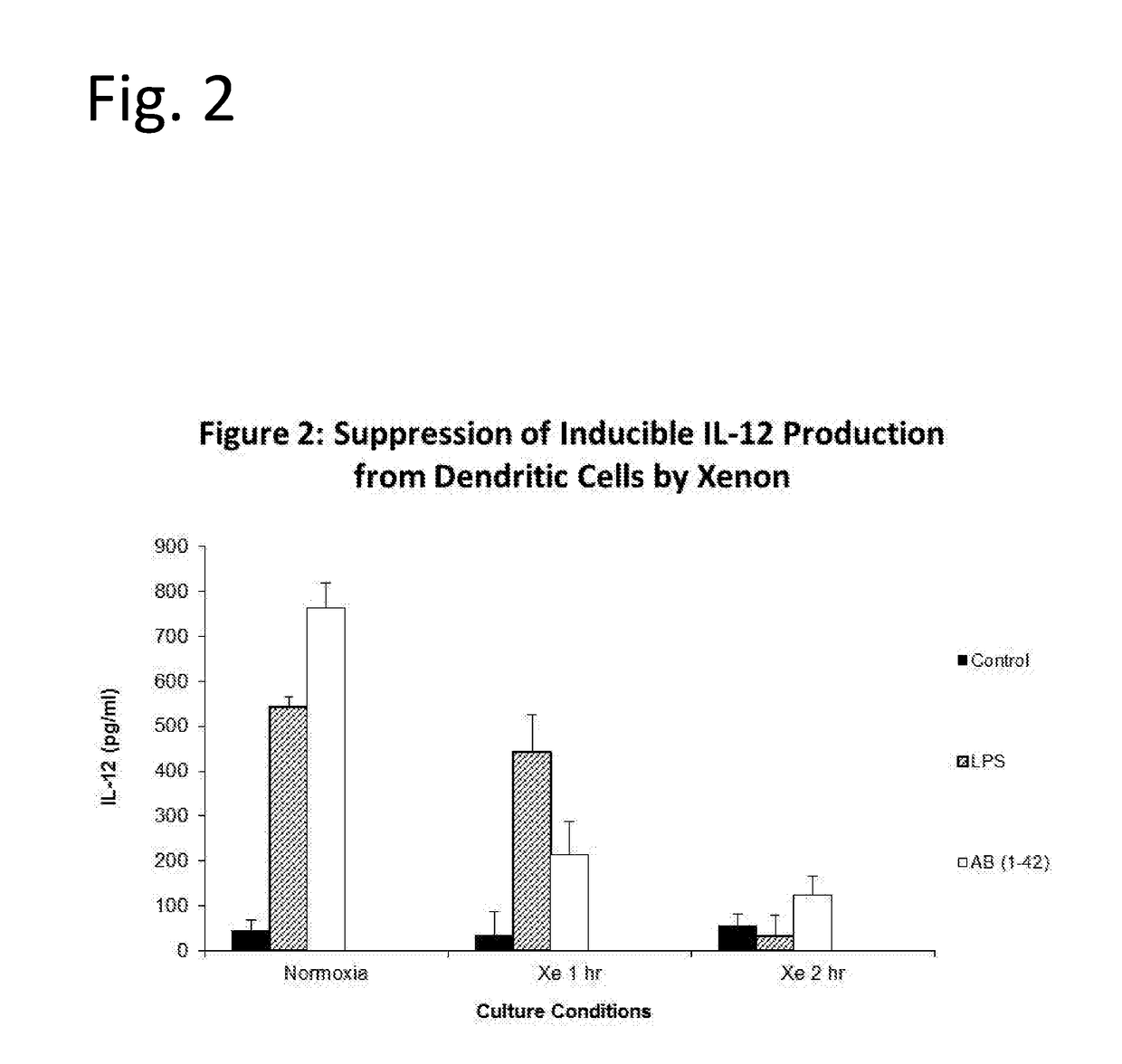
Noble gas modulation of il-12
InactiveUS20190125786A1Inorganic active ingredientsPharmaceutical delivery mechanismNoble gasMedicine
The invention discloses means of utilizing Noble Gases for inhibition of pathogenic processes associated with Alzheimer's Disease progression, as well as reversal of Alzheimer's Disease. In one embodiment compositions containing xenon gas are administered alone or together with therapeutic interventions for treatment of Alzheimer's Disease. In another embodiment, taupathies such as chronic traumatic encephalopathy are treated by administration of xenon or Noble Gas containing mixtures.
Owner:NOBILIS THERAPEUTICS INC
Chemical Compounds
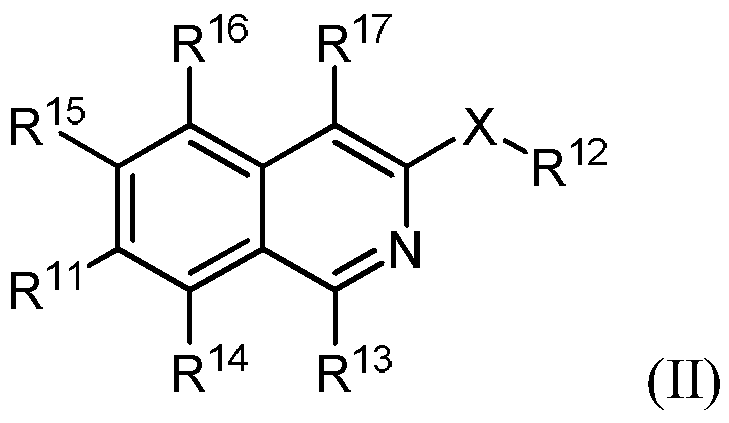
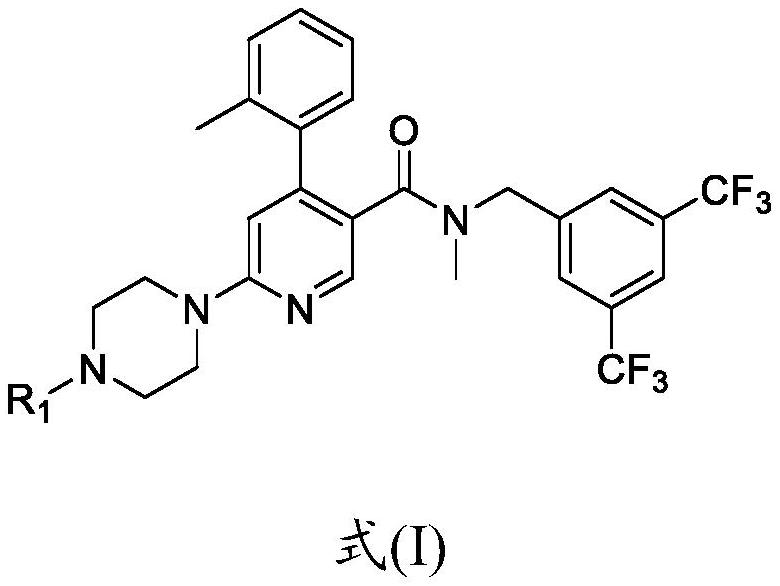
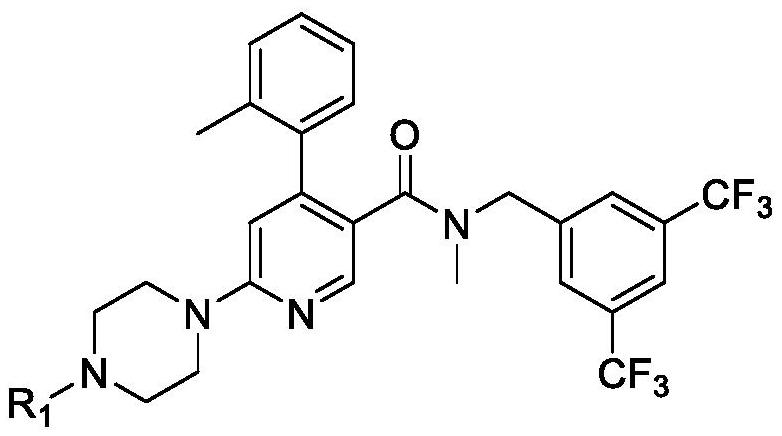
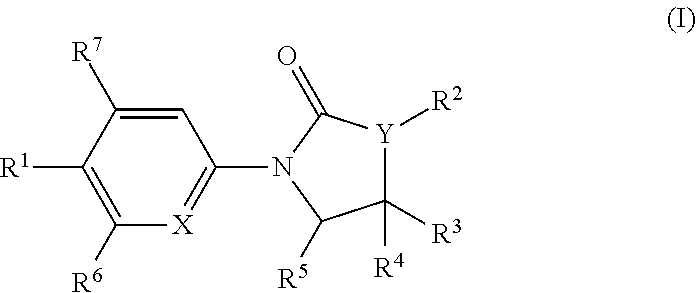
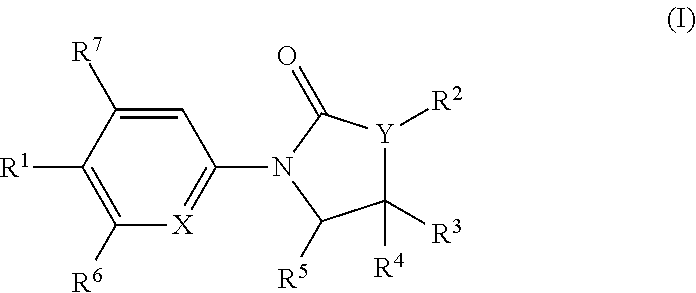
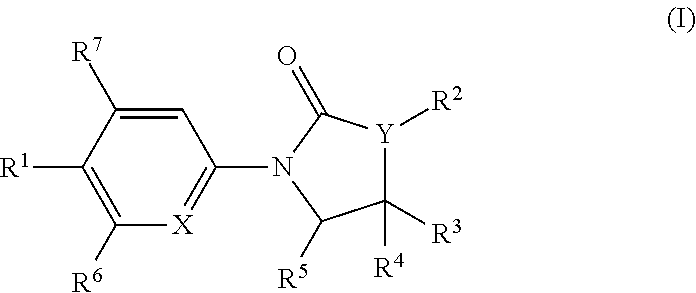
The invention is directed to substituted pyrrolidinone and imidazolidinone derivatives. Specifically, the invention is directed to compounds according to Formula I:wherein R1, R2, R3, R4, R5, R6, R7, X and Y are as defined herein.The compounds of the invention are inhibitors of PERK and can be useful in the treatment of cancer, pre-cancerous syndromes and diseases / injuries associated with activated unfolded protein response pathways, such as Alzheimer's disease, spinal cord injury, traumatic brain injury, ischemic stroke, stroke, Parkinson's disease, diabetes, metabolic syndrome, metabolic disorders, Huntington's disease, Creutzfeldt-Jakob Disease, fatal familial insomnia, Gerstmann-Sträussler-Scheinker syndrome, and related prion diseases, amyotrophic lateral sclerosis, progressive supranuclear palsy, myocardial infarction, cardiovascular disease, inflammation, organ fibrosis, chronic and acute diseases of the liver, fatty liver disease, liver steatosis, liver fibrosis, chronic and acute diseases of the lung, lung fibrosis, chronic and acute diseases of the kidney, kidney fibrosis, chronic traumatic encephalopathy (CTE), neurodegeneration, dementias, frontotemporal dementias, tauopathies, Pick's disease, Neimann-Pick's disease, amyloidosis, cognitive impairment, atherosclerosis, ocular diseases, arrhythmias, in organ transplantation and in the transportation of organs for transplantation. Accordingly, the invention is further directed to pharmaceutical compositions comprising a compound of the invention. The invention is still further directed to methods of inhibiting PERK activity and treatment of disorders associated therewith using a compound of the invention or a pharmaceutical composition comprising a compound of the invention.
Owner:GLAXOSMITHKLINE INTELLECTUAL PROPERTY (NO 2) LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com