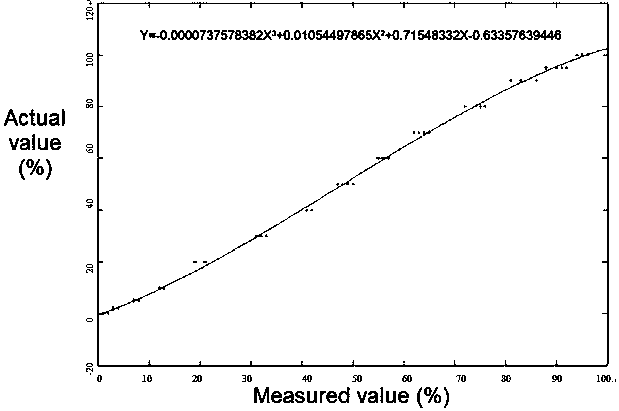
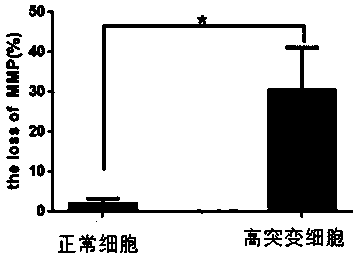
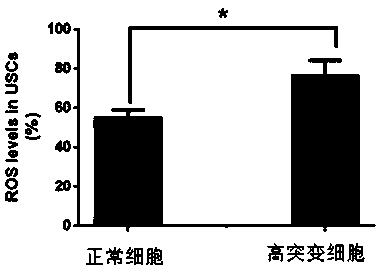
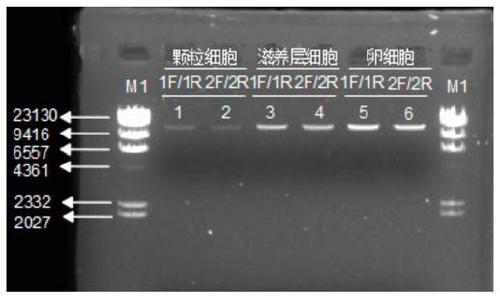
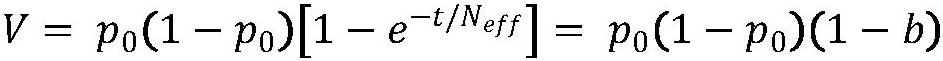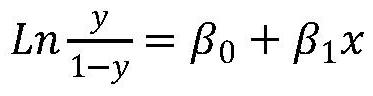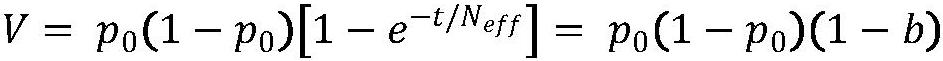Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
38 results about "Mitochondrial mutation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Mitochondrial diseases are sometimes (about 15% of the time) caused by mutations in the mitochondrial DNA that affect mitochondrial function. Other mitochondrial diseases are caused by mutations in genes of the nuclear DNA, whose gene products are imported into the mitochondria (mitochondrial proteins) as well as acquired mitochondrial conditions.
Implementation of a mitochondrial mutator
InactiveUS20060248613A1Sugar derivativesOther foreign material introduction processesPolynucleotideMitochondrial mutation
Plant MSH1 polynucleotides and polypeptides are described. Also described are methods for the use and modulation of such MSH1 polynucleotides and polypeptides.
Owner:BOARD OF RGT UNIV OF NEBRASKA
Kit for detecting regulon of Leber disease and use thereof
InactiveCN102747152ARelieve painSolve the problem of extracting DNAMicrobiological testing/measurementPositive controlMitophagy
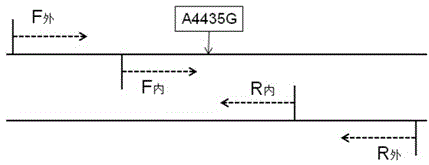
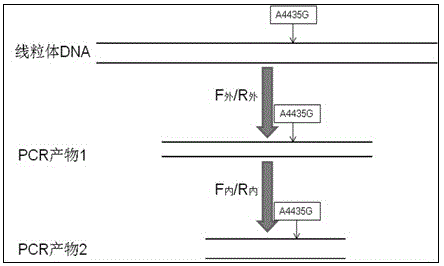
The invention provides a kit for detecting mitochondrial DNA mutation related to a regulon of a Leber disease, wherein the kit consists of primer sequences, reagents for extracting genome DNA of samples, reagents for PCR amplification reaction, a positive control sample, a negative control sample and an instruction, restrictive endonucleases comprise a restrictive endonuclease Nla III, and the positive control sample is a restriction enzyme digested sample in which 4435th site of a mitochondrial sequence occur A to G mutation. The kit provided by the invention can be applied in the detection of mitochondrial DNA mt DNAA4435G mutation related to the regulon of the Leber disease. According to the kit, specificity and stability of detection results can be ensured, cost is lower than that of other detection methods, and the detection process is simple, quick, accurate and economic, and thus is suitable for detecting LHON-related gene mutation.
Owner:ZHEJIANG UNIV
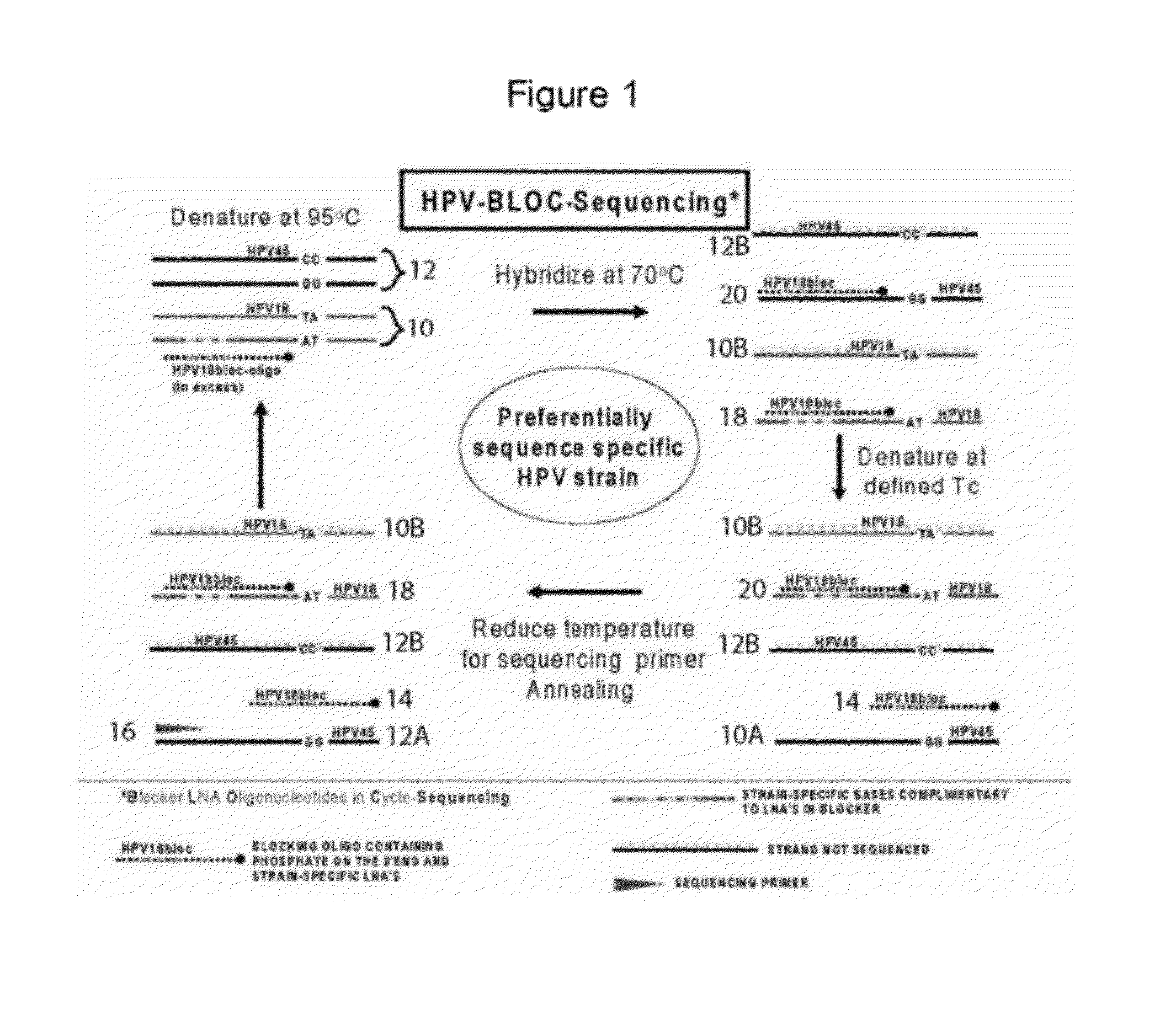
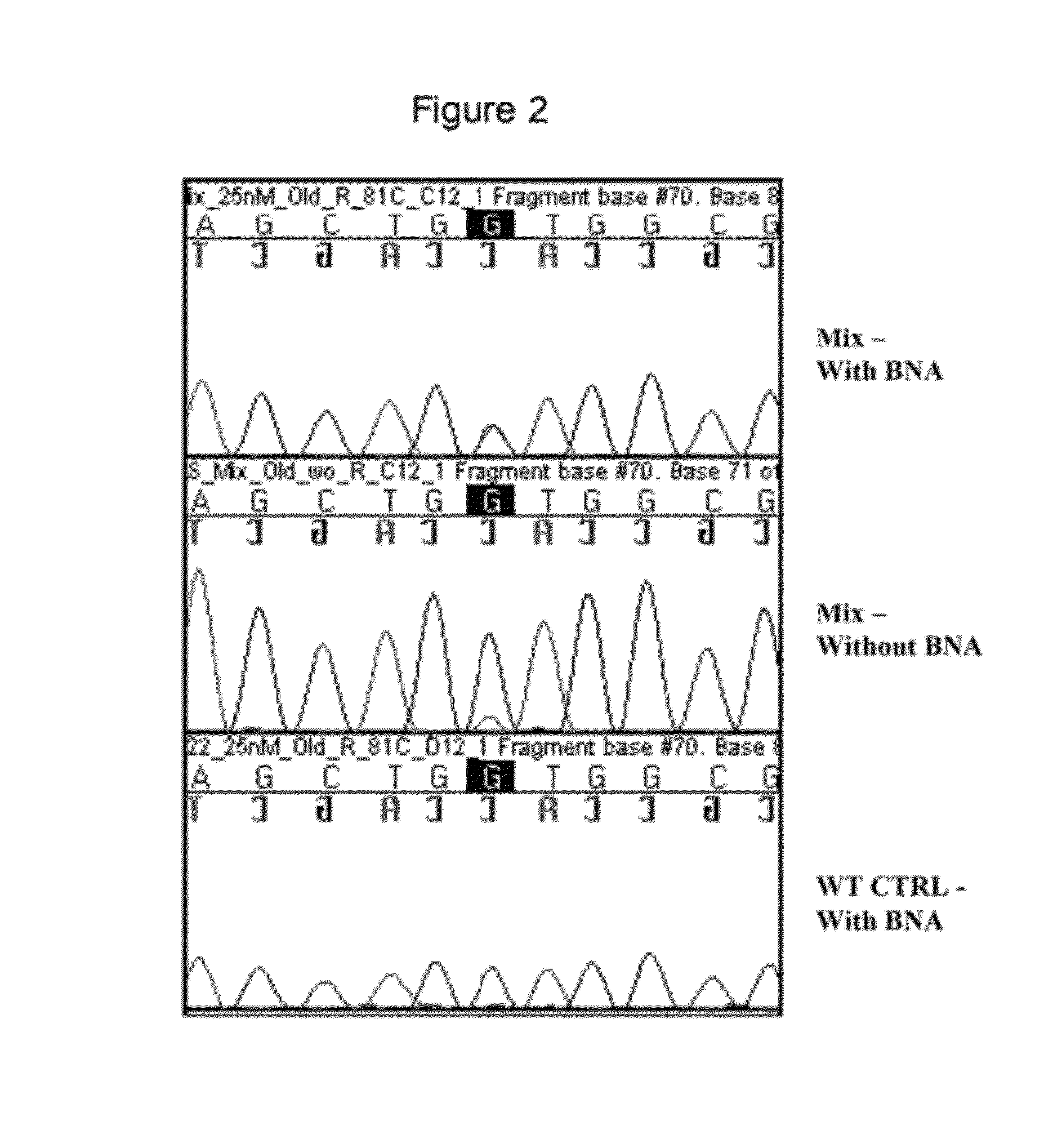
Kit and method for sequencing a target DNA in a mixed population
InactiveUS20120225421A1Reduce the temperatureMicrobiological testing/measurementMaterial analysis by optical meansCancer therapyDNA
Methods and kits for sequencing a target DNA sequence in a sample having a related reference sequence are provided herein. In particular, kits and methods for sequencing cancer and cancer therapy associated mutations are described. Also provided are kits and methods for detecting mitochondrial mutations and for differentiating between closely related viral strains.
Owner:TRANSGENOMIC
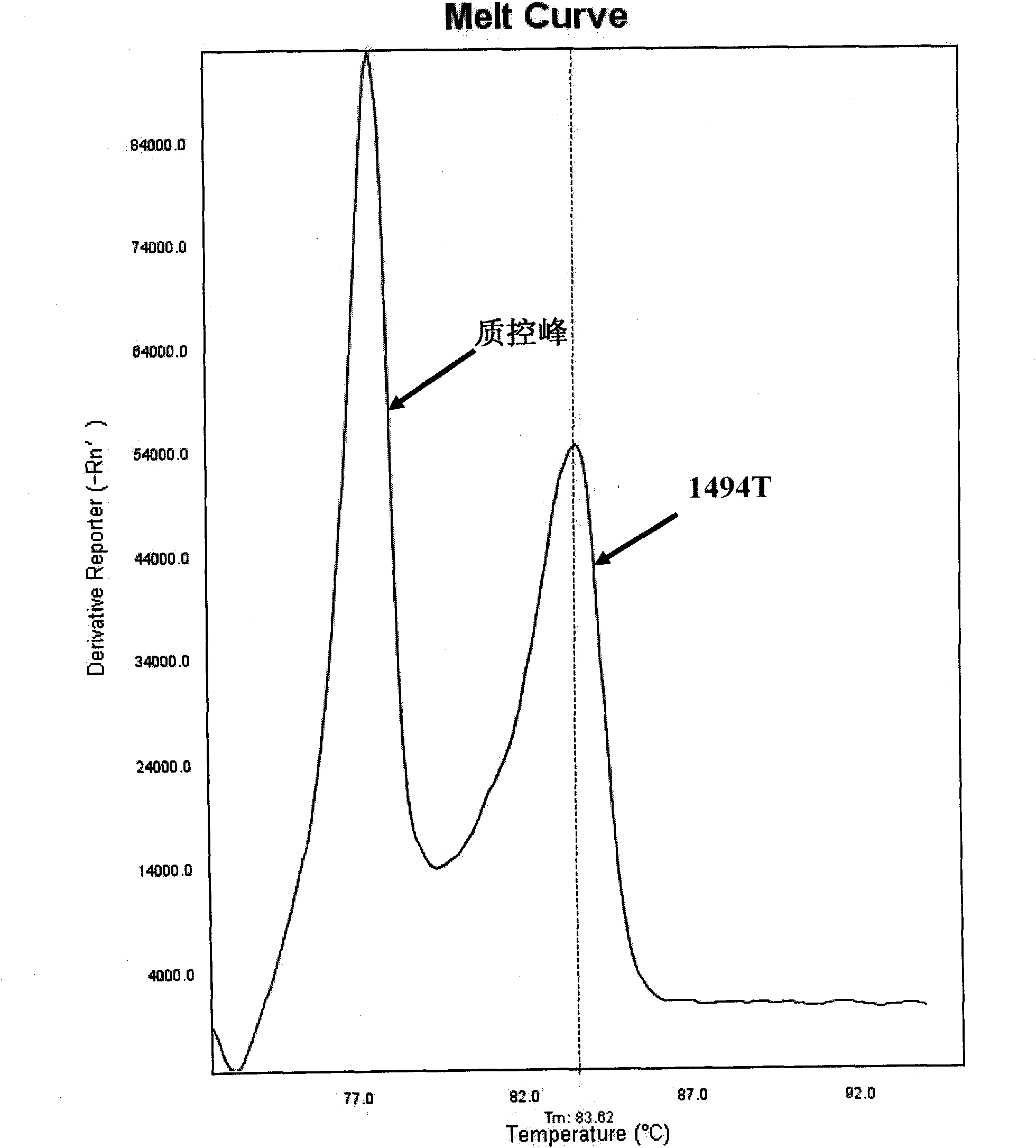
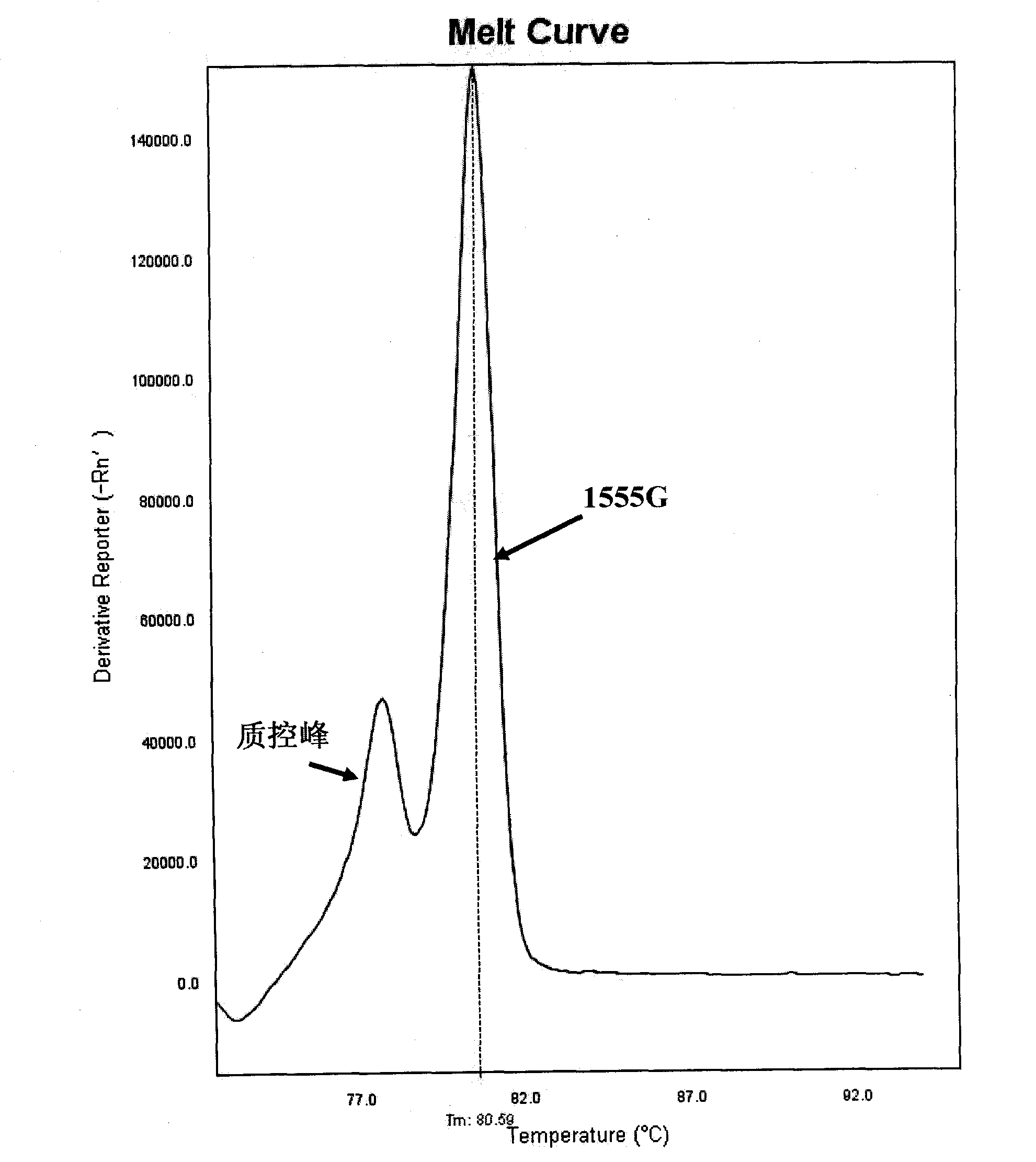
Method for detecting mitochondrial mutations and kit thereof
ActiveCN102154481AEasy to operateShorten the timeMicrobiological testing/measurementMultiplex pcrsMitochondrial mutation
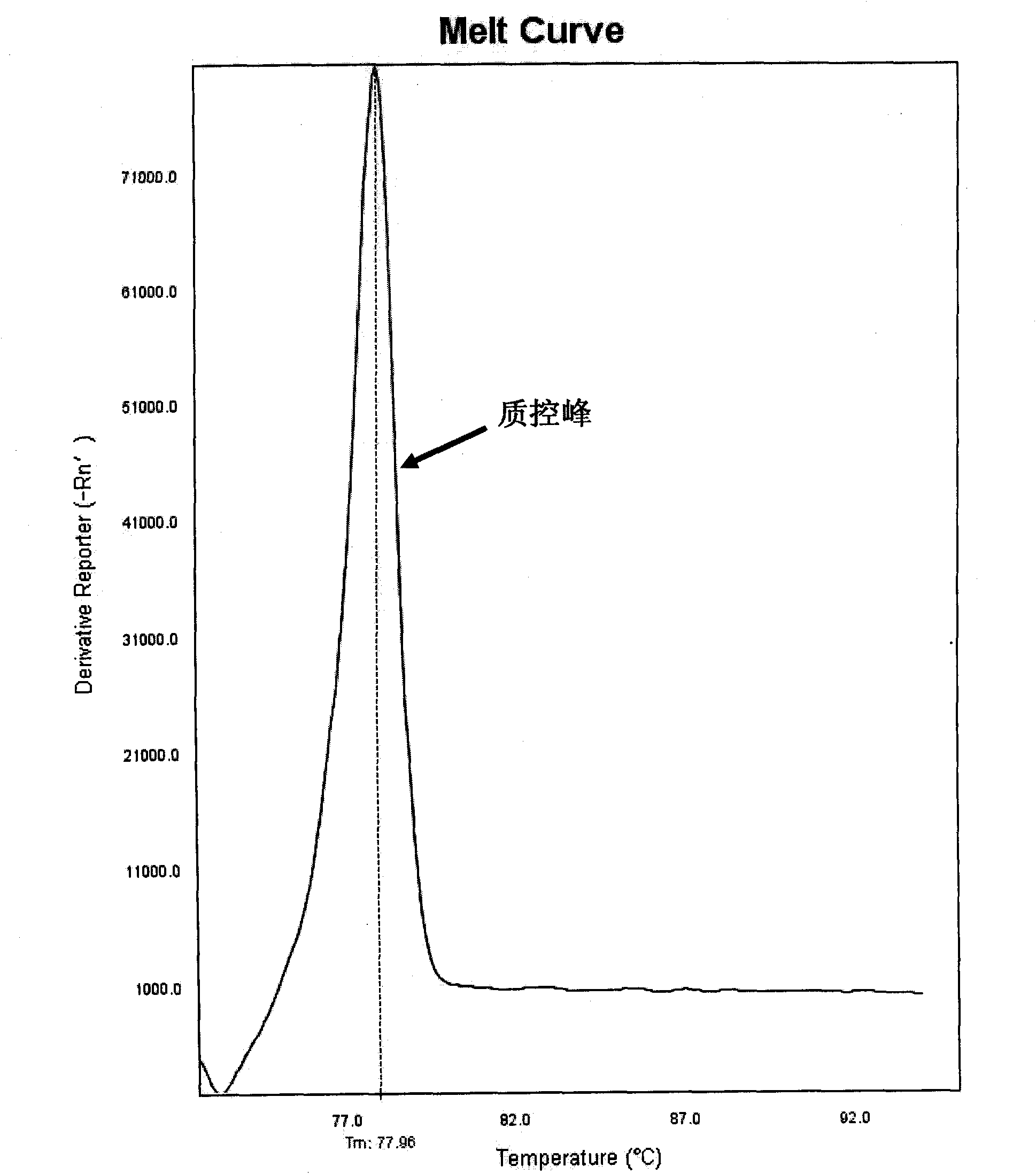
The invention discloses a method for detecting mitochondrial mutations and a kit thereof. The method is used for detecting 1494T and 1555G mutations, five primers are shown as a sequence one, a sequence two, a sequence three, a sequence four and a sequence five in a primer sequence table, the primers are used for multiple polymerase chain reaction (PCR) amplification and melting curve analysis, and whether corresponding mutations happen is judged according to a melting peak at the specific temperature. Meanwhile, the invention also provides a kit special for the method.
Owner:智海生物工程(北京)股份有限公司
Implementation of a mitochondrial mutator
InactiveUS20060248614A1Suppressing ectopic recombinationInhibitory activitySugar derivativesOther foreign material introduction processesMitochondrionPolynucleotide
Plant MSH1 polynucleotides and polypeptides are described. Also described are methods for the use and modulation of such MSH1 polynucleotides and polypeptides.
Owner:MACKENZIE SALLY +1
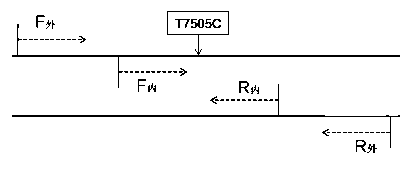
Kit for detecting deaf related mitochondrial T7505C mutation, and application thereof
ActiveCN103266169ARelieve painSolve the problem of extracting DNAMicrobiological testing/measurementA-DNADNA extraction
The invention provides a kit for detecting deaf related mitochondrial DNA T7505C mutation. The kit is composed of a DNA extraction mixed liquid, a PCR mixed liquid for T7505C fragment amplification, a pair of outer primers designed against T7505C, a pair of inner primers designed against the T7505C, a restrictive endonuclease, a positive contrast, a negative contrast and a kit body. A protease K digestion cracking method is utilized in the invention to rapidly extract genome DNA from a small amount of specimens, so a problem of the traditional extraction of DNA from the blood of a deaf patient is solved, the pains of a detected person is mitigated, and a trans-regional sample transmission problem is also solved. The kit has the advantages of low application cost, simple and rapid detection flow, visual result interpretation, ensuring of the specificity and the stability of the detection result, and realization of the application in the detection of the deaf related mitochondrial tRNAGlnT7505C mutation.
Owner:ZHEJIANG UNIV
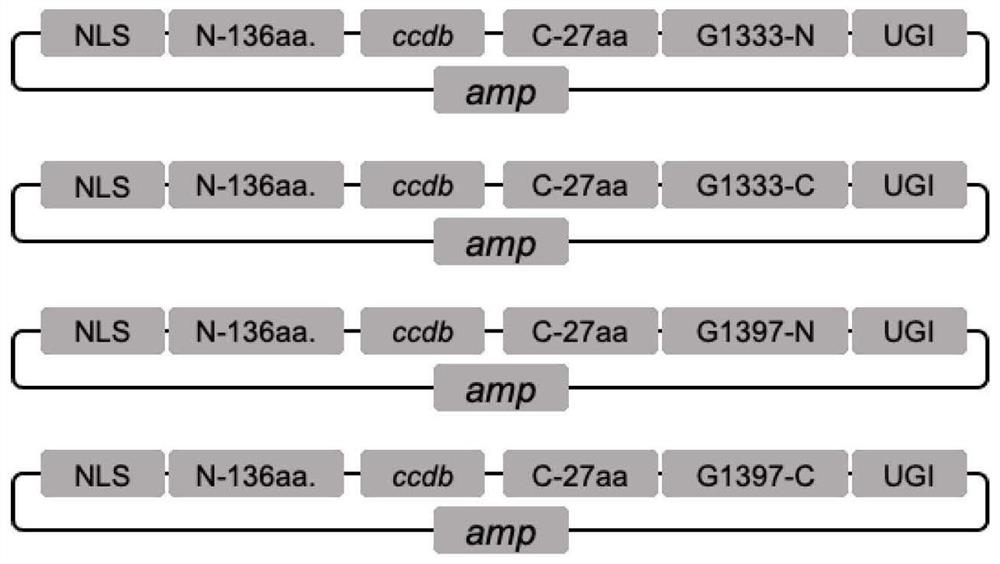
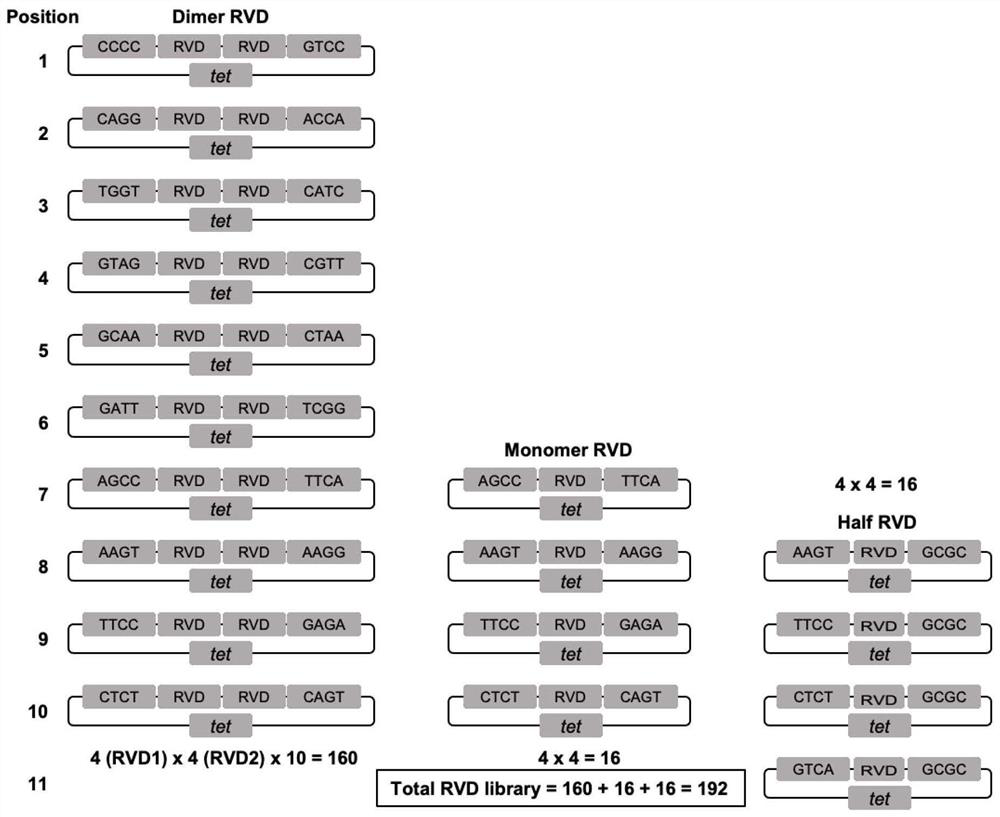
Mitochondrial DNA editing system based on TALE assembly
ActiveCN113403341AImprove assembly efficiencySimple processPeptide librariesNucleic acid vectorGenome editingMitochondrial localization
The invention belongs to the technical field of gene editing, and discloses a mitochondrial DNA editing system based on TALE assembly. The editing system comprises an RVD library, a mitochondrial localization skeleton carrier and a cell nucleus localization skeleton carrier, and the RVD library comprises at least 192 RVD modules including 160 double RVD modules, 16 single RVD modules and 16 half RVD modules. According to the editing system, the assembly efficiency of a TALE module can be remarkably improved, and the assembly cost and time of the TALE module can be remarkably reduced. According to the mitochondrial DNA editing system based on the TALE assembly, the assembly method is easy to operate and suitable for application and popularization. By means of the method, mitochondrial DNA editing can be rapidly and efficiently achieved, and the method is used for manufacturing various animal models so as to simulate diseases caused by human mitochondrial DNA mutation; the method can be potentially applied to gene therapy of mitochondrial DNA mutation diseases.
Owner:NANJING MEDICAL UNIV
Mitochondrial complex I mutations in parkinson's disease and aging
ActiveUS20060194239A1High frequency of mutationHigh mutational burdenSugar derivativesMicrobiological testing/measurementMitochondrial Complex IParkinson's disease
The present invention provides methods for diagnosing and treating Parkinson's disease based on mitochondrial mutations. The present invention also provides methods for diagnosing and treating other diseases and disorders based on mitochondrial mutations.
Owner:GENE SOLUTIONS
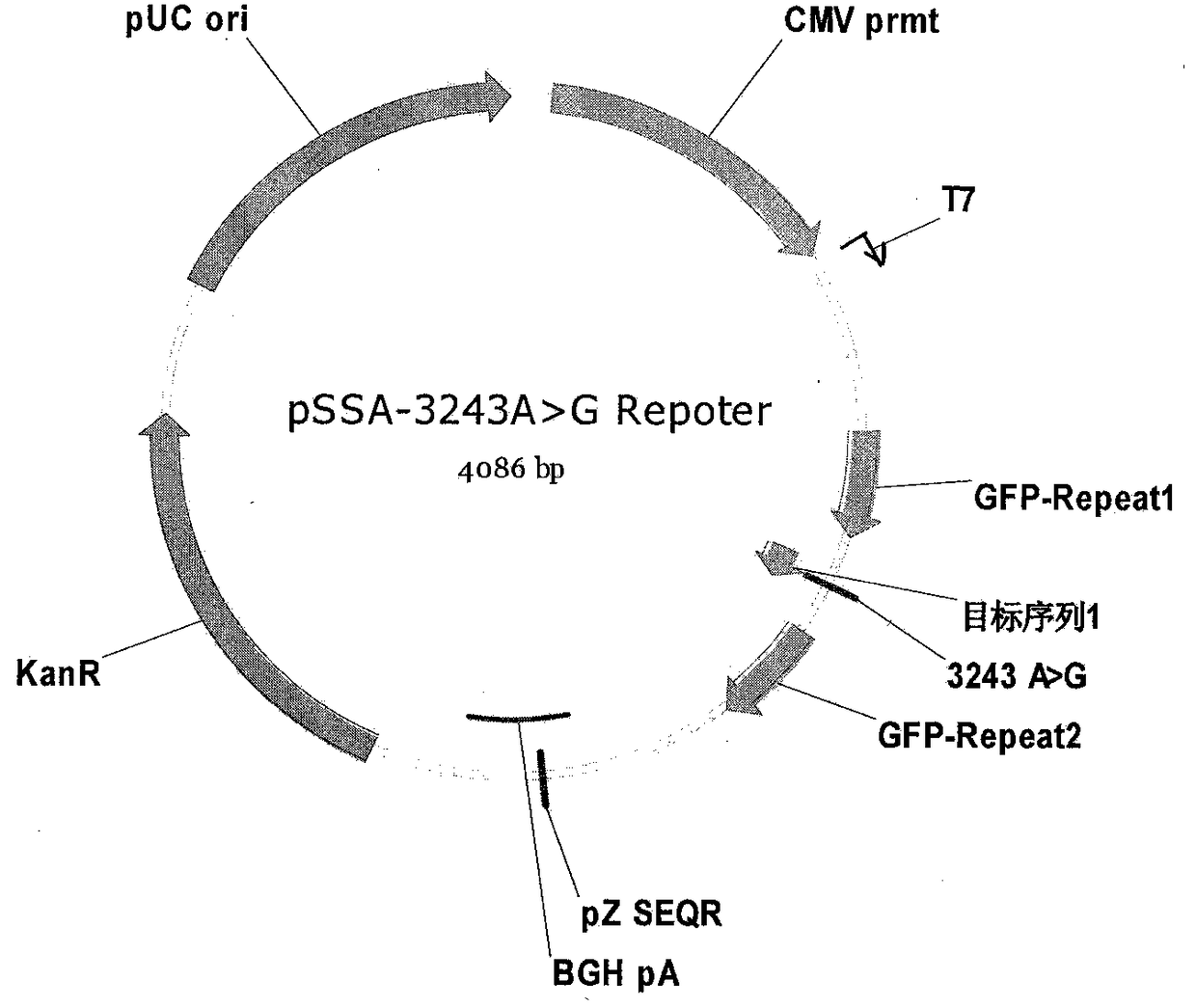
Preparation method of high-mutation urine stem cells in mitochondria mt.3243A > G mutation crowds
PendingCN111269883ASignificant technological progressCompound screeningCell dissociation methodsNew medicationsPrimary cell
The invention provides a preparation method of high-mutation urine stem cells in mitochondria mt.3243A > G mutation crowds. The method comprises the following steps: collecting urine cells of mitochondria mt.3243A > G mutant population; obtaining a sediment red tube by adopting a mode of centrifuging and adding a PBS to blow, beat and mix uniformly, obtaining P1-generation high-mutation urine stemcells are obtained, and obtaining the mitochondria mt.3243A > G mutation urine stem cells with the high mutation rate of 95% or above through digestion passage and mutation rate detection. The methodhas the advantages that the method can be obtained in a non-invasive mode, and the method can be obtained regardless of the gender, age and health condition of the patient. As a primary cell, the cell line maintains the basic properties of the original cell. The invention provides a cell application platform for researching the pathogenesis of mt.3243A > G mutant cells in the future and screeningnew drugs aiming at mt.3243A > G.
Owner:SHANGHAI EAST HOSPITAL EAST HOSPITAL TONGJI UNIV SCHOOL OF MEDICINE
Construction method for human single cell mitochondrial high-throughput sequencing library and kit for library construction
ActiveCN111172157AEnabling ring-wide point mutationsEliminate distractionsMicrobiological testing/measurementLibrary creationPhosphorylationCell layer
The invention provides a construction method for a human single cell mitochondrial high-throughput sequencing library and a kit for library construction. The kit includes mitochondrial genome full-loop amplification primers, single cell lysate, terminal repaired, phosphorylated and 3' added adenylate components of high-throughput library construction, adaptors of high-throughput library construction and forward and reverse library amplification primers. The method realizes the high-throughput sequencing library construction of single or several cell mitochondrial genomes DNA in a mitochondrialgenome complete full-loop amplification based system, and high miss rates and high-proportion false positive site detection rates brought by conventional single cell amplification systems can be evaded, so that massive disadvantages in mitochondrial mutation detection can be got rid of at the single cell level, high sensitivity and accuracy detection can be realized; and as the method adopts themode of full-loop and full-length mitochondrial amplification, the large fragment duplication and deletion of mitochondrial DNA can be discovered while mutation sites are detected.
Owner:福州福瑞医学检验实验室有限公司
Mitochondrial genome editing
InactiveUS20160304854A1Avoid spreadingReduce in oocyteGenetically modified cellsArtificial cell constructsGenome editingGenetics
Methods of preventing the transmission of a mitochondrial disease, disorder, or condition using mitochondria-targeted enzymes or mRNA encoding mitochondria-targeted enzymes. The methods as described herein can specifically eliminate mitochondrial DNA (mtDNA) mutations in the germline.
Owner:GENOVA LAB LLC
Method and kit for detection of mutations in mitochondrial dna
InactiveUS20060078881A1Flexibility in primer designMicrobiological testing/measurementMutation frequencyMitochondrion
The present invention is within the medical field. More precisely, the invention relates to a method and kit for defection of mutations / polymorphisms in human mitochondrial DNA sequences and specifically to the use of mitochondrial DNA variants (polymorphisms) with high mutation frequency to be employed in the comparison of biological samples with samples of known origin in the purpose of, for example, human identification or forensic genetics.
Owner:ALLEN MARIE +2
Inherited Mitochondrial Dna Mutations in Cancer
InactiveUS20080280294A1Increase chanceSugar derivativesMicrobiological testing/measurementProstate cancerFhit gene
A method is provided for identifying a subject likely to have, or at risk of developing a disease condition correlated with increased reactive oxygen species (ROS), including cancer, by identifying in the subject a missense mutation in a nucleic acid of Complex III, IV and / or V of the OXPHOS system. This invention also provides a method of identifying a likelihood of having a heritable predisposition to cancer by detecting a homoplasmic missense mutation in non-tumor tissue of an OXPHOS system gene. This invention also provides a method for detecting likelihood of having cancer, predisposition to cancer, and likelihood of passing a predisposition to cancer to progeny involving identifying in non-tumor tissue of the subject a missense mutation in a complex III, IV and / or V gene of the mitochondrial OXPHOS system. The mutation may be a nuclear or mitochondrial mutation. The invention has been exemplified with respect to prostate cancer. When the mutation is homoplasmic in non-tumor tissue this is an indication it is an inherited and inheritable trait, and that the subject is likely to pass on the mutation to her progeny in the case of mutations in mitochondrial DNA or his or her progeny in the case of mutations in nuclear DNA. Both homoplasmic and heteroplasmic mutations in non-tumor tissue can indicate the presence of cancer.
Owner:EMORY UNIVERSITY
Method of detecting or quantitatively determining mitochondrial dna 3243 variation, and kit therefor
ActiveUS20060188886A1Efficient detectionMicrobiological testing/measurementFermentationFluorescenceQuantitative determination
A method for detecting a DNA having the mitochondrial DNA 3243 mutation, using quantitative PCR using a primer having a nucleotide sequence complementary to a nucleotide sequence starting from the nucleotide number 243 in the nucleotide sequence of SEQ ID NO: 2 and having a length of 12 to 30 nucleotides, as well as, a method for detecting a DNA having the mitochondrial DNA 3243 mutation, using a nucleic acid probe of which end is labeled with a fluorescent dye, and in which fluorescence of the fluorescent dye decreases upon hybridization, wherein the nucleic acid probe has a nucleotide sequence complementary to a nucleotide sequence starting from the nucleotide number 230 in the nucleotide sequence of SEQ ID NO: 2 and having a length of 14 to 40 nucleotides, and the 3′ end of the probe is labeled with the fluorescent dye.
Owner:ARKRAY INC
Primer pair, probe set and kit for detecting mitochondrial obesity gene mutation
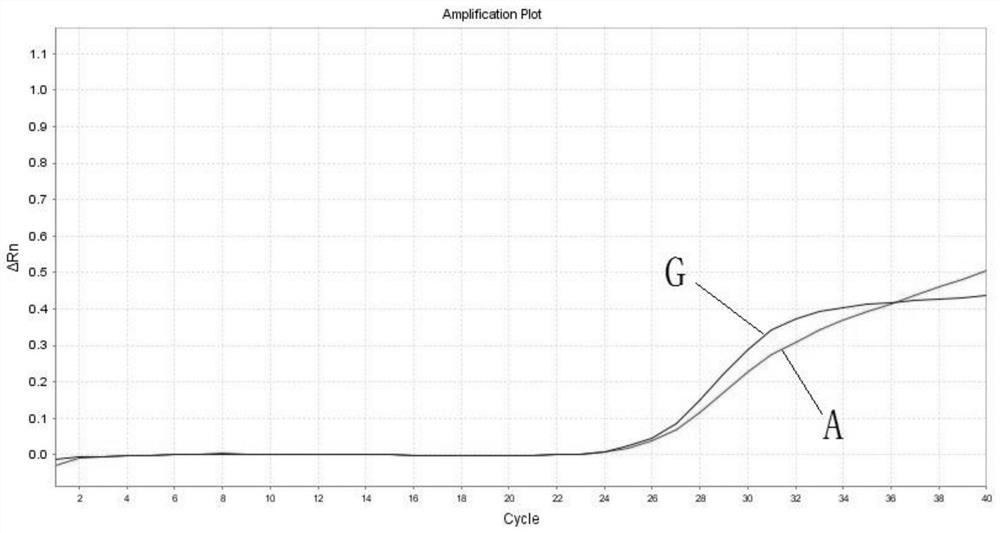
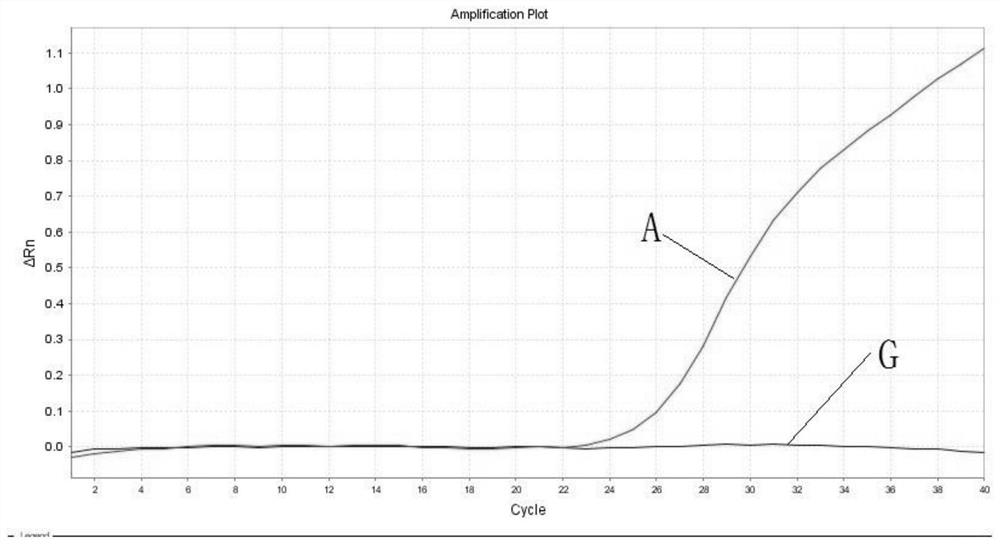
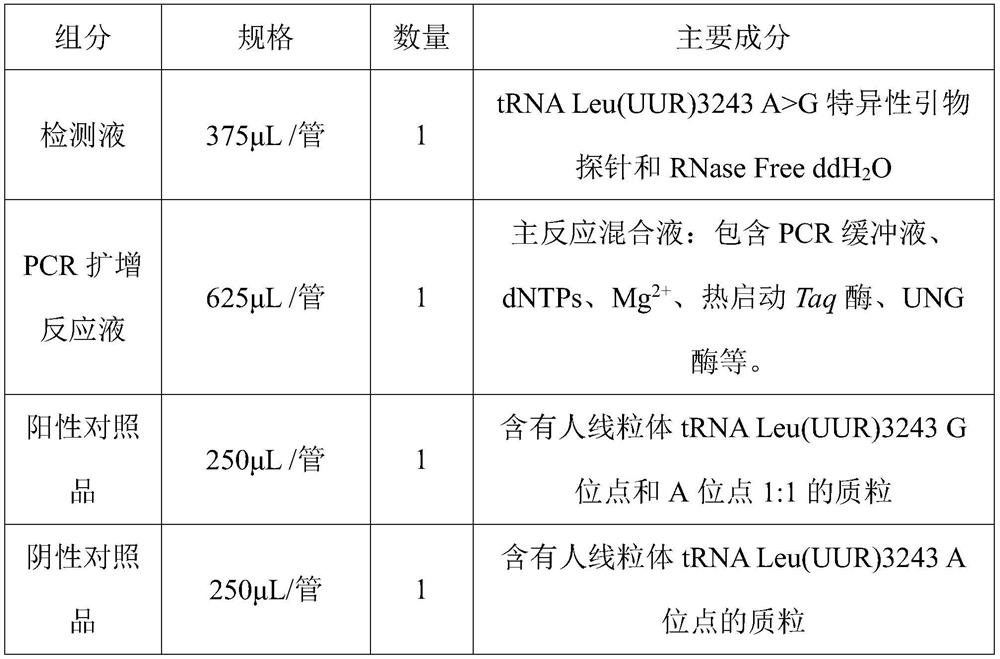
PendingCN113322317AQuick checkAccurate detectionMicrobiological testing/measurementDNA/RNA fragmentationGenes mutationDisease
The invention belongs to the technical field of biological detection, and discloses a primer pair, a probe set and a kit for detecting mitochondrial obesity gene mutation. The kit takes human DNA as a formwork, fluorescence quantitative PCR amplification is carried out to obtain an amplification curve, and a mitochondrial tRNA Leu (UUR) 3243A more than G mutation site can be quickly, accurately and sensitively detected. The kit is further provided with a wild type site probe, and the relative ratio of a mutant type to a wild type is obtained through Ct values of the mutant type and the wild type probe. The blank of clinically detecting the tRNA Leu (UUR) 3243A more than G mutation site is filled, and timely auxiliary data is provided for subsequent treatment of related diseases. The kit is good in repeatability and high in precision. The kit is short in detection period, detection can be completed within 45 minutes at the soonest, the detection time is saved, the efficiency is improved, and the kit is an efficient auxiliary detection means. In the application of non-diagnosis purposes, detection can be completed only by mixing the kit and a collected sample through a fluorescence quantitative PCR instrument, the operation requirement is simple, and the clinical popularization performance is high.
Owner:北京华诺奥美基因生物科技有限公司
Mitochondrial ND5 gene mutations in Parkinson's disease
The present invention provides methods for diagnosing and treating Parkinson's disease based on mitochondrial mutations. The present invention also provides methods for diagnosing and treating other diseases and disorders based on mitochondrial mutations.
Owner:GENE SOLUTIONS
Mitochondrial mutations and rearrangements as a diagnostic tool for the detection of sun exposure, prostate cancer and other cancers
Mitochondrial DNA deletions useful for the detection of cancers and sun exposure are provided. In particular, methods and kits for detecting mitochondrial DNA deletions for the early detection, diagnosis and progression of prostate cancer, sun exposure and non-melonoma skin cancer are provided.
Owner:MITOMICS
Primers, method and kit for whole genome detection of human mitochondria
PendingCN110885880AImprove uniformityStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationRare mutationsMitochondrial mutation
The invention discloses primers, method and kit for whole genome detection of human mitochondria. The primers comprise forward and reverse primers for amplifying 37 gene coding regions of a mitochondrial whole genome (16569bp), and the base sequences of the forward and reverse primers are shown in the formulas of SEQ ID NO: 3-222. A detection method based on the NGS technology comprises the following steps: combining specific primers and a target template DNA sequence, amplifying a target region of a to-be-detected sample by using universal primers, purifying a library by using magnetic beads,performing high-throughput sequencing on the obtained library, and analyzing mutation of the mitochondrial gene. According to the primers, the method and the kit disclosed by the invention, the raremutation of mitochondrial DNA can be accurately detected, a high cost performance, least manual operation and ultrahigh sensitivity are realized, guidance for drug selection of familial mitochondrialpatients and genetic susceptibility is provided, mitochondrial diseases caused by mitochondrial DNA mutation assists diagnosis and the onset risk is effectively reduced.
Owner:杭州联川基因诊断技术有限公司
Method for transforming exogenous mitochondrion into mammal cells
ActiveCN104630149ANo source limitationsHigh purityGenetically modified cellsArtificial cell constructsMitochondrial mutationEndocytosis
Disclosed are synthetic mitochondria obtained by introducing exogenous DNA into mitochondria or mitochondrial shells. Cells containing exogenous mitochondria are then obtained by introducing the synthetic mitochondria into mammalian cells via endocytosis, thereby allowing the exogenous mitochondria to perform effectively within the cells. After being introduced, synthetic mitochondrial DNA genes can be expressed stably, and passaged effectively. The method for introducing exogenous mitochondria into cells can serve as a new mitochondrial molecular cloning method, performing gene knockout, gene knock-in, gene rearrangement etc. within mitochondria, thereby enabling any molecular cloning of mammalian mitochondrial DNA to be engineered, which has significant implications for the treatment of diseases caused by mitochondrial DNA mutations.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
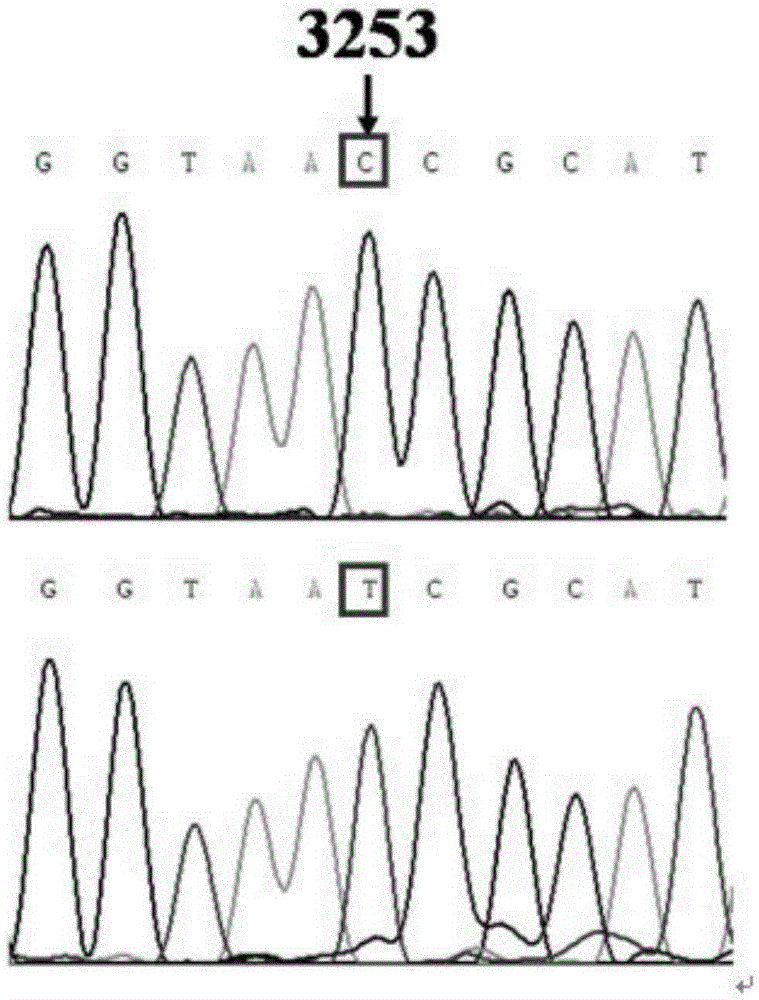
Method and kit for mutation detection of mitochondrion tRNA<Leu(UUR)>3253T>C
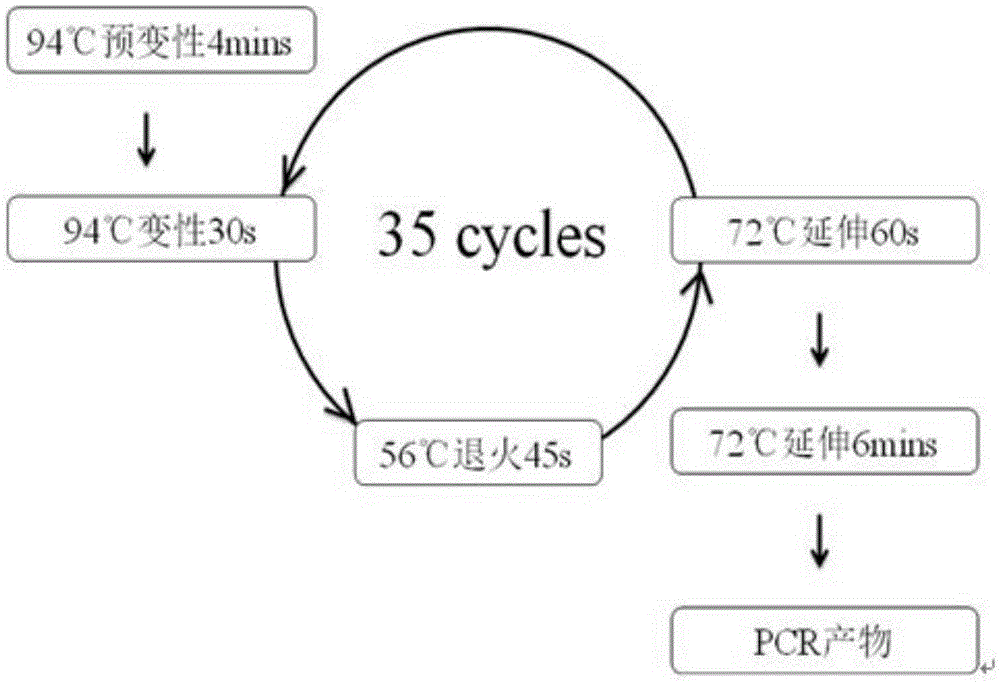
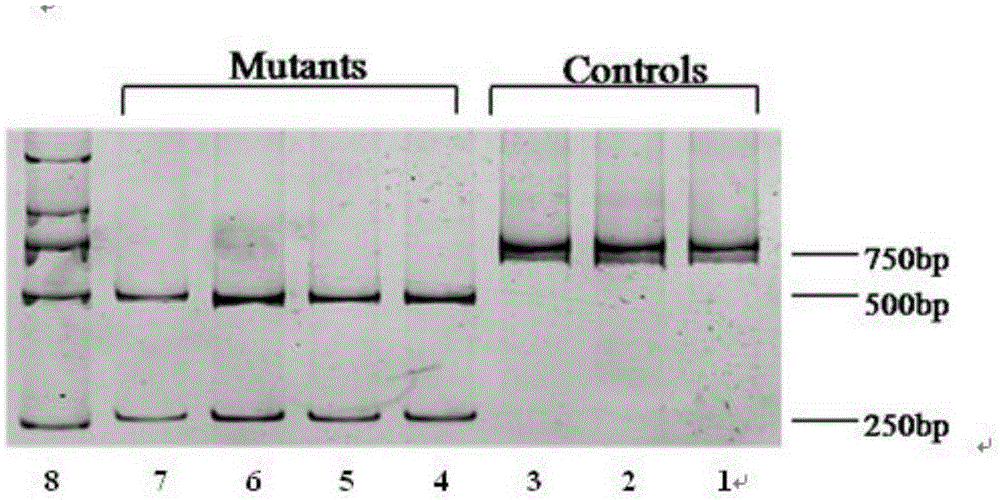
ActiveCN105274242AClear analysis of test resultsIntuitive analysis of test resultsMicrobiological testing/measurementEnzymeMutation detection
The invention discloses a method and a kit for mutation detection of a mitochondrion tRNA<Leu(UUR)>3253T>C. The method includes DNA (deoxyribonucleic acid) extraction, specific primer design, specific band PCR (polymerase chain reaction) amplification, specific restriction enzyme BstEII digestion, agarose gel electrophoresis and the like. Results can be obtained within a few hours, and one enzyme is only capable of identifying one specific deoxyribonucleotide sequence, so that accuracy of the results is realized. The invention further provides a kit for mutation detection of the mitochondrion tRNA<Leu(UUR)>3253T>C related to primary hypertension. The method and the kit for mutation detection of the mitochondrion tRNA<Leu(UUR)>3253T>C have the advantages of low requirements on instruments for detection, low cost of required reagents, simplicity and easiness in operation, stability of the method, high specificity and the like.
Owner:WENZHOU MEDICAL UNIV
Fluorescent quantitative PCR (Polymerase Chain Reaction) detection kit for mutation A1555G of mitochondrial deafness and application of fluorescent quantitative PCR detection kit
InactiveCN107287303AAccurate diagnosisRapid diagnosisMicrobiological testing/measurementFluoProbesNucleotide
The invention provides a fluorescent quantitative PCR (Polymerase Chain Reaction) detection kit for common mutation A1555G of mitochondrial deafness. The kit comprises quantitative PCR reaction solutions, a first positive control, a second positive control, a negative control, a description and a box body, wherein the quantitative PCR reaction solutions contain a PCR buffer solution, MgCl2, dNTPs, heat-resistant DNA polymerase, an upstream amplification primer, a downstream amplification primer, a first fluorescent probe and a second fluorescent probe. According to the kit provided by the invention, two MGB probes are designed for A>G mono-base mutation of a 1555th locus of mtDNA, and whether the locus is subjected to A>G mutation or not is determined through detecting 1555th nucleotide of a mitochondrion correlated to deafness by fluorescent quantitative PCR, so that the kit has the advantages of simplicity, convenience, rapidness, accuracy and the like, can be applied to the clinical diagnosis and control of deafness related to mitochondrial mutation A1555G and can also be used for providing a foundation for preventing and treating the mitochondrial deafness induced by the mutation A1555G.
Owner:ZHEJIANG UNIV
Fluorescent quantitative PCR detection kit for mitochondrial deafness C1494T mutation and application thereof
The invention provides a fluorescent quantitative PCR detection kit for mitochondrial deafness C1494T common mutation C1494T. The fluorescent quantitative PCR detection kit comprises a quantitative PCR reaction liquid, a first positive reference substance, a second positive reference substance, a negative reference substance, a direction and a case body, wherein the quantitative PCR reaction liquid contains a PCR buffer solution, MgCl2, dNTPs, heat-proof DNA polymerase, an upstream amplimer, a downstream amplimer, a first fluorescent probe and a second fluorescent probe. Two MGB probes are designed with respective to C>T single base mutation of the 1494 site of mtDNA, the deafness-related mitochondrial 1555 site nucleotide is detected through fluorescent quantitation PCR, and whether the site is subjected to C>T mutation or not is determined. The fluorescent quantitative PCR detection kit has the advantages of being simple, convenient, quick and accurate, can be applied to clinical diagnosis and treatment of mitochondrial C1494T mutation-related deafness and provides references for prevention and treatment of mitochondrial deafness caused by C1494T mutation.
Owner:ZHEJIANG UNIV
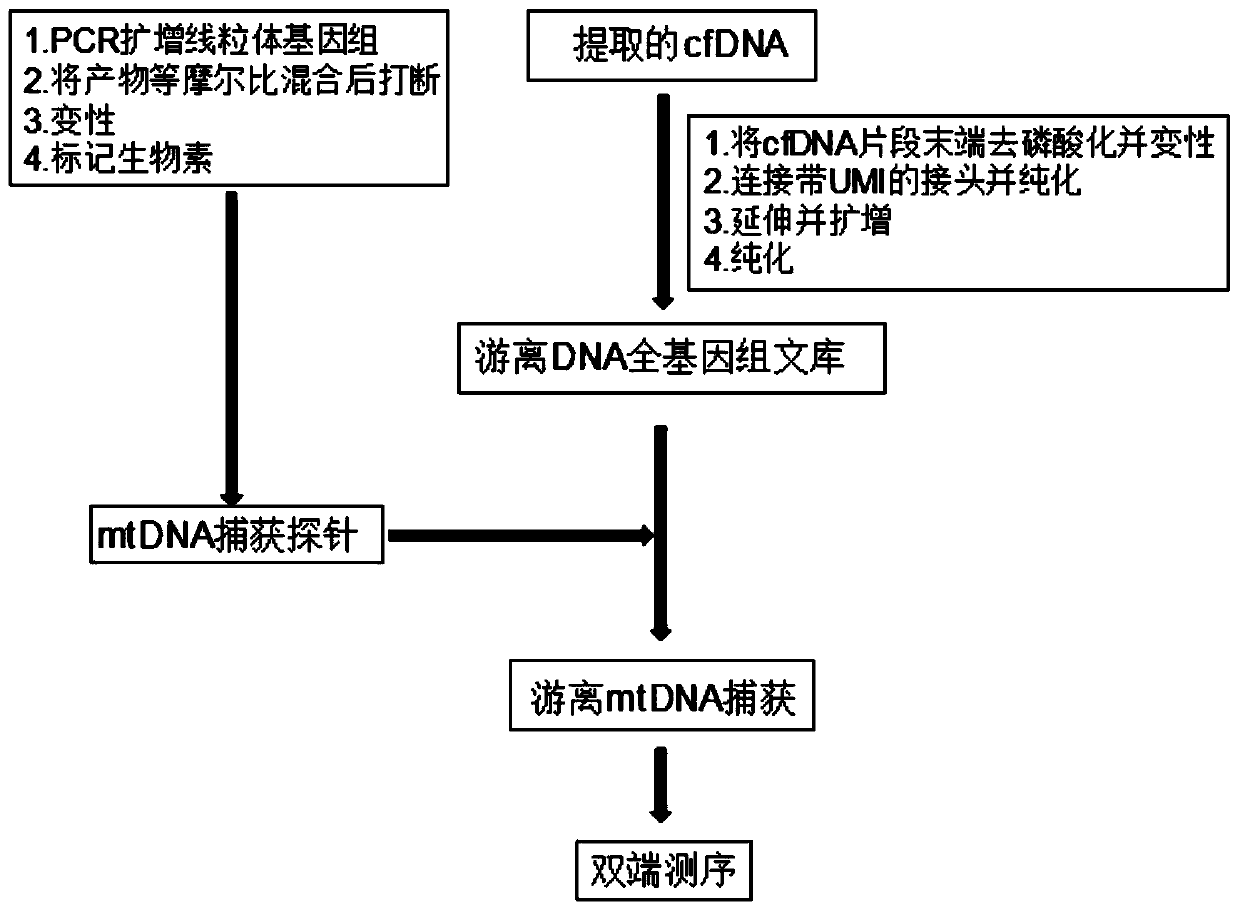
Free mitochondrial DNA mutation detection technique based on bibasic sequencing technique
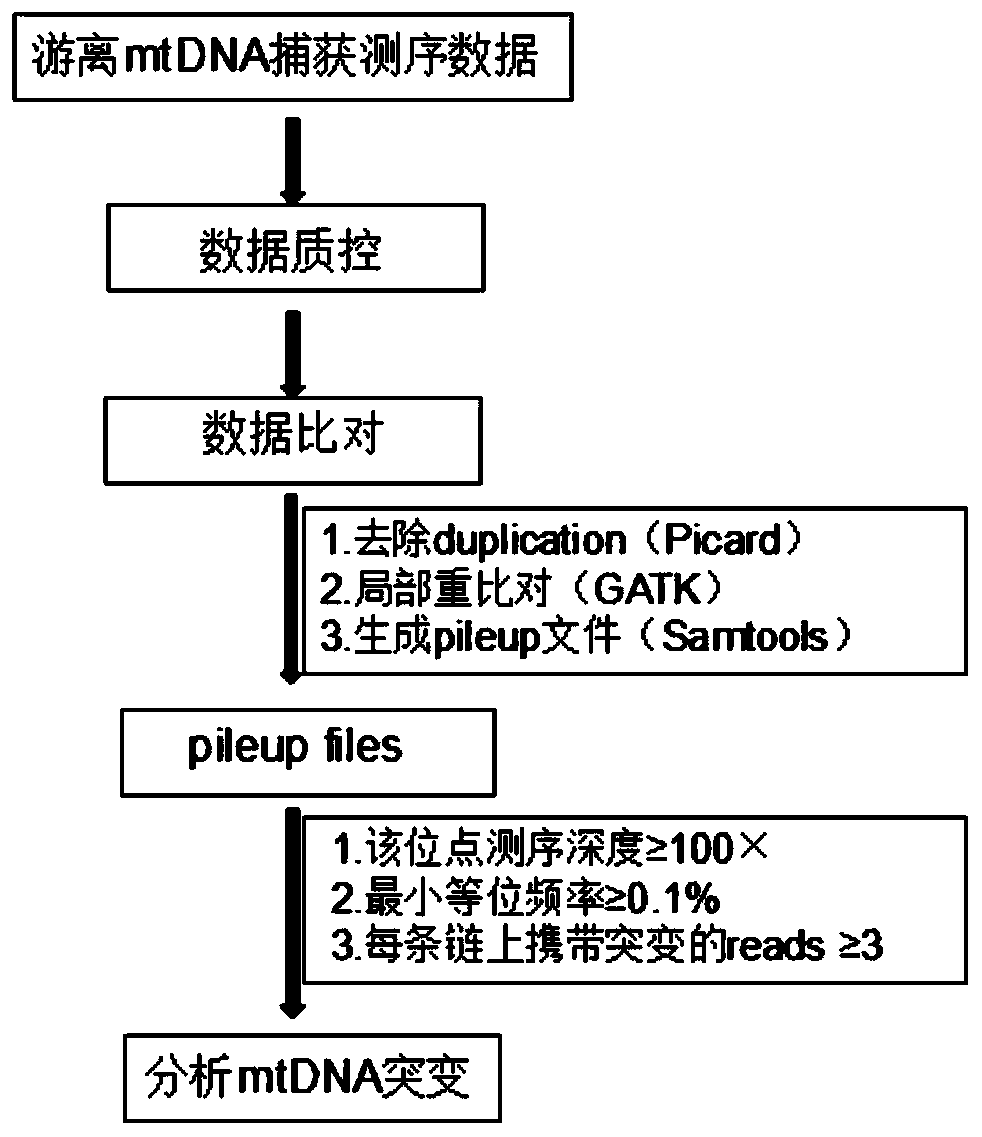
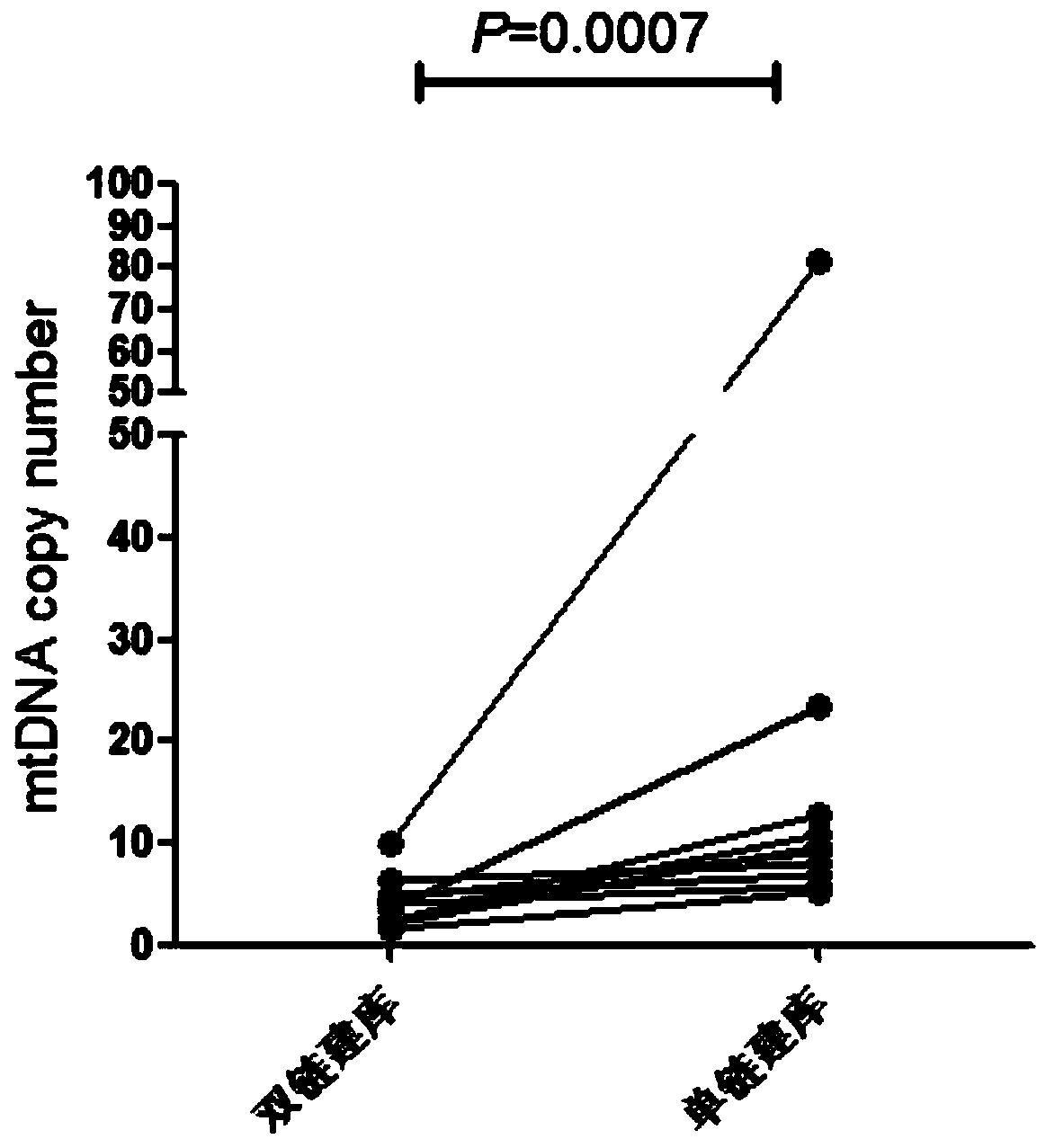
PendingCN110305945AAvoiding the False Positive ProblemImprove the comparison rateMicrobiological testing/measurementSequence analysisMutation detectionMutation frequency
The invention discloses a free mitochondrial DNA mutation detection technique based on a bibasic sequencing technique. The free mitochondrial DNA mutation detection technique mainly comprises the following steps of (1) preparing a cfDNA single-chain sequencing library; (2) performing mtDNA capturing; and (3) performing data analysis. Compared with the prior art, the free mitochondrial DNA mutationdetection technique has the advantages that in accordance with the characteristics of high gragmentation degree and low mutation frequency of free mtDNA, free mtDNA sequence information is enriched in NGS, so that the low-frequency mutation detection false positive problem is solved, interference of NUMT to mutation detection is overcome, and pinpoint detection of free mitochondrial DNA mutationis realized.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
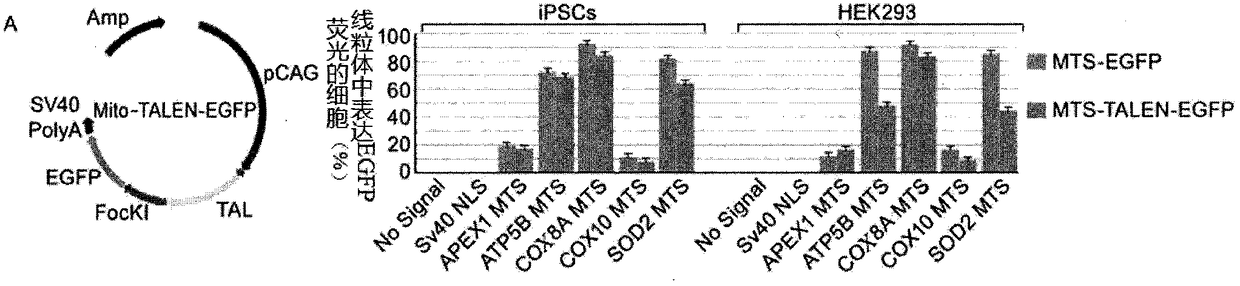
Repair method of iPSCs mitochondrial DNA mutation sites based on mitoTALENs
InactiveCN109504707AImprove modification efficiencyRetouching work is convenientGenetically modified cellsArtificially induced pluripotent cellsLactic acidosisMitochondrial myopathy
The invention discloses a repair method of iPSCs mitochondrial DNA mutation sites based on mitoTALENs. The method disclosed by the invention has the advantages of high selectivity, high repair efficiency and low miss rate; the repair method can be successfully applied to induced pluripotent stem cells MiPSCs of specific sources of MELAS (mitochondrial myopathy, encephalopathy, lactic acidosis andstroke-like episodes) patients.
Owner:THE THIRD AFFILIATED HOSPITAL OF GUANGZHOU MEDICAL UNIVERSITY
Fluorescent quantitative PCR (Polymerase Chain Reaction) detection kit for mutation T12201C of mitochondrial deafness and application of fluorescent quantitative PCR detection kit
PendingCN107287302AAccurate detectionAccurate diagnosisMicrobiological testing/measurementPositive controlFluorescence
The invention provides a fluorescent quantitative PCR (Polymerase Chain Reaction) detection kit for common mutation T12201C of mitochondrial deafness. The kit comprises quantitative PCR reaction solutions, a first positive control, a second positive control, a negative control, a description and a box body, wherein the quantitative PCR reaction solutions contain a PCR buffer solution, MgCl2, dNTPs, heat-resistant DNA polymerase, an upstream amplification primer, a downstream amplification primer, a first fluorescent probe and a second fluorescent probe. According to the kit provided by the invention, two MGB probes are designed for T>C mono-base mutation of a 12201th locus of mtDNA, and whether the locus is subjected to T>C mutation or not is determined through detecting 12201th nucleotide of a mitochondrion correlated to deafness by fluorescent quantitative PCR, so that the kit has the advantages of simplicity, convenience, rapidness, accuracy and the like, can be applied to the clinical diagnosis and control of deafness related to mitochondrial mutation T12201C and can also be used for providing a foundation for preventing and treating the mitochondrial deafness induced by the mutation T12201C.
Owner:ZHEJIANG UNIV
Primer pair probe combination product and its kit and detection method for detecting mitochondrial 3243a>g mutation
ActiveCN114085903BStrong specificityIncreased sensitivityMicrobiological testing/measurementDNA/RNA fragmentationGeneticsGene technology
Owner:GUANGZHOU JIAJIAN MEDICAL TESTING CO LTD
A detection primer, kit and detection method for mitochondrial dna gene mutation of leber hereditary optic neuropathy
ActiveCN106755335BSensitiveImprove stabilityMicrobiological testing/measurementDNA/RNA fragmentationGenes mutationDisease
The invention relates to a detection primer, a kit and a detection method for the mutation of the mitochondrial DNA gene of Leber's hereditary optic neuropathy. Based on the principle of Sanger sequencing, the present invention has the characteristics of high sensitivity, stability and accuracy, can simultaneously detect the four most common pathogenic mutations of LHON mtDNA, and solves the low sensitivity and the The problems of insufficient specificity, easy pollution and high cost provide LHON with an accurate genetic diagnosis method, which is of great significance for genetic counseling and gene therapy of the disease.
Owner:ZHONGSHAN OPHTHALMIC CENT SUN YAT SEN UNIV
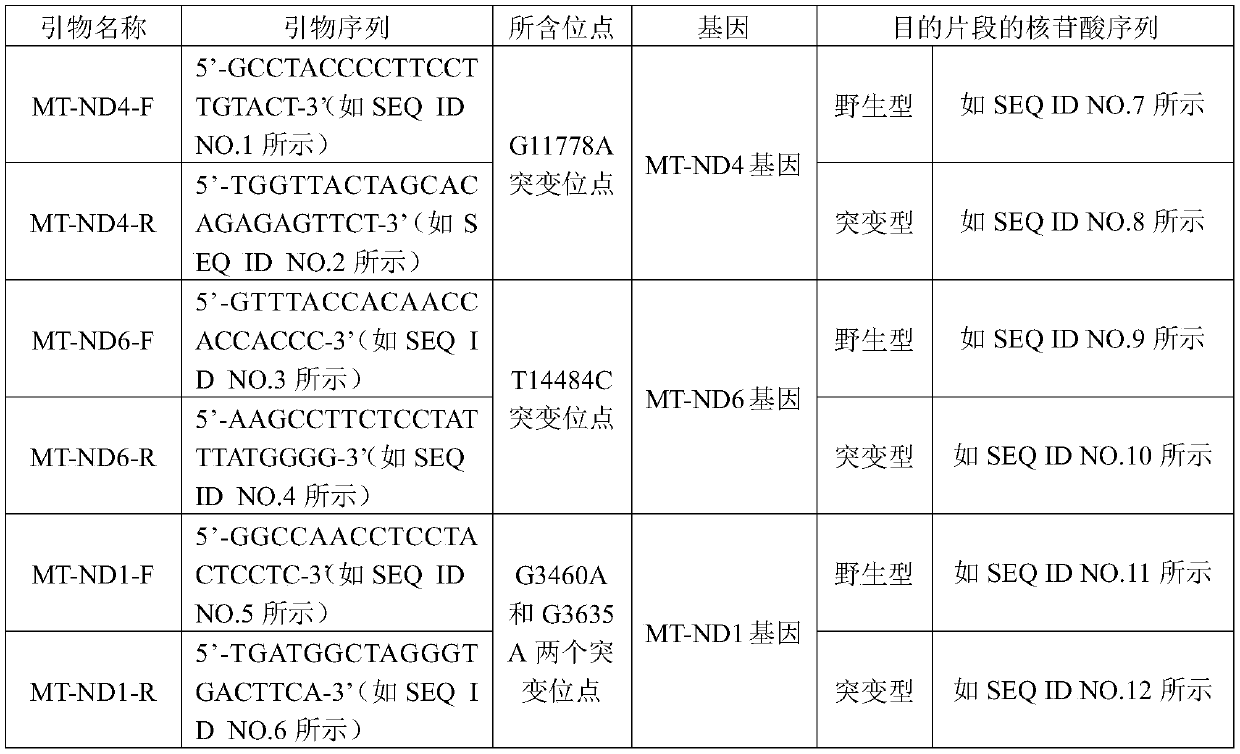
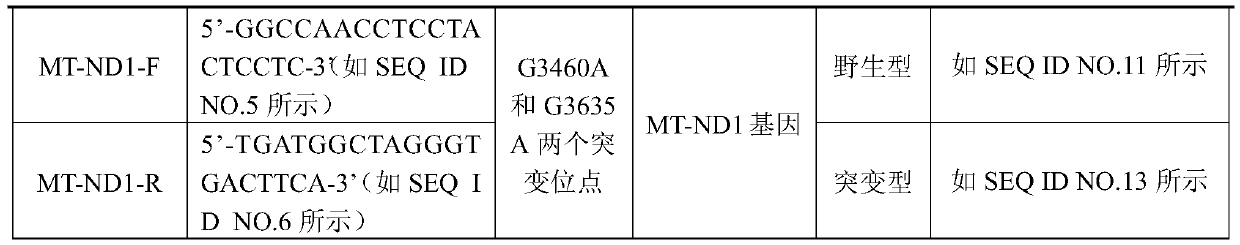
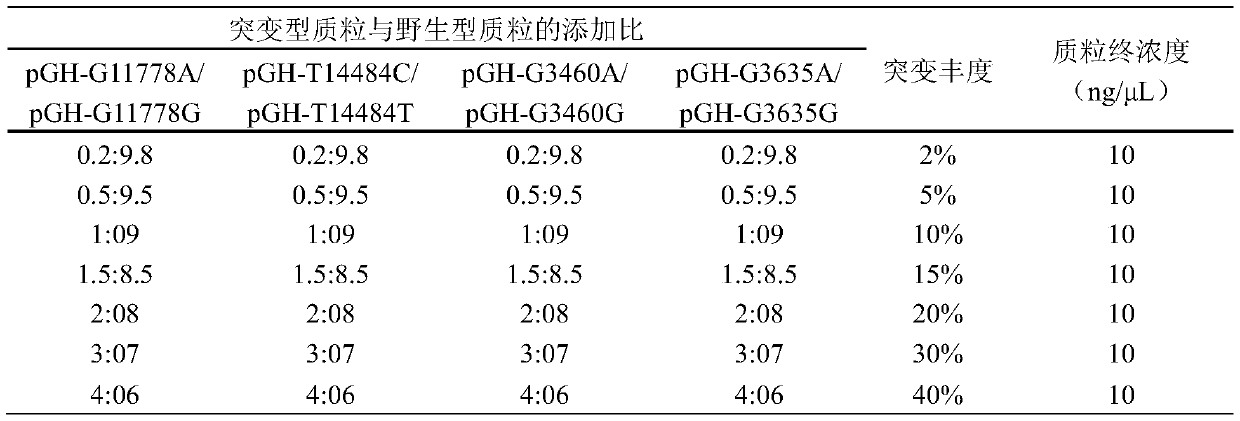
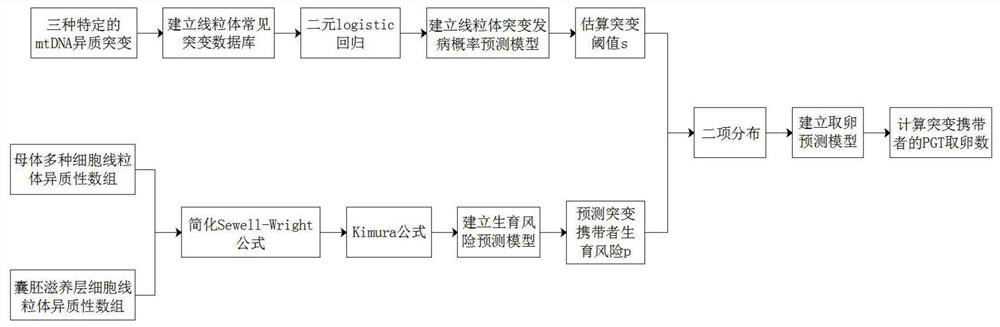
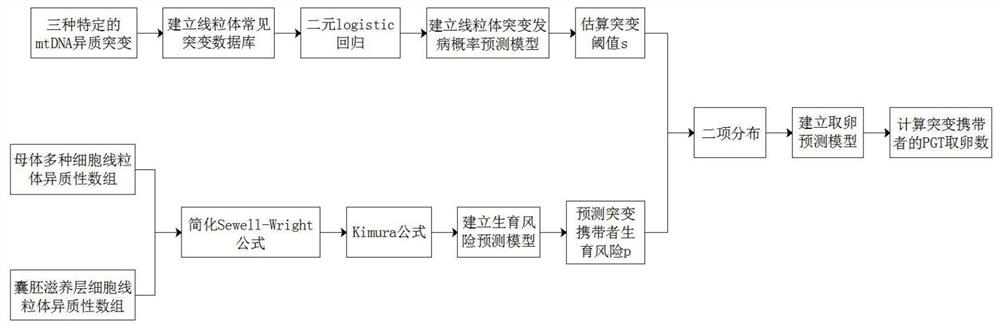
Methods for Predicting Mitochondrial DNA Mutation Threshold, Fertility Risk, and Oocyte Retrieval
ActiveCN114023382BAids in genetic managementHealth-index calculationBiostatisticsMutation CarrierPhysiology
The present invention publicly predicts the method of mitochondrial DNA mutation threshold, fertility risk and egg retrieval, including the following steps: first establish a mitochondrial mutant probability predictive model and estimate the mutation threshold S, then establish a maternity risk prediction model, and predict the maternity risk of mutant carrier carrier.P, then use two distributions to establish an egg mother cell extraction predictive model and calculate the number of egg retrieval of the mutant carrier; the prediction model of the incidence of the present invention predicts the threshold of common MTDNA mutations, and predicts the mother's fertility risk and PGT egg retrieval numberOn the basis of the establishment of a maternity risk prediction model and an egg retrieval prediction model, you only need to know that the mitochondrial DNA mutation ratio of the mutant carrier can calculate the minimum PGT egg collection required by the healthy child generation.Help clinicians to conduct genetic management of families carrying MTDNA heterogeneous mutations and provide genetic consultation, and provide standard guidelines for PGT.
Owner:THE FIRST AFFILIATED HOSPITAL OF ANHUI MEDICAL UNIV
Multiplex Taqman fluorescence detection kit for diagnosis of mitochondria mutational deafness
ActiveCN105506120AImprove accuracyCutting costsMicrobiological testing/measurementDNA/RNA fragmentationFluorescenceRefrigerated temperature
The invention discloses a multiplex Taqman fluorescence detection kit for diagnosis of mitochondria mutational deafness. The kit contains primers as shown in SEQ ID NO:1-2, probes as shown in SEQ ID NO:3-6, DNA polymerase, negative reference substances and the like. At present, clinical diagnosis of deafness is mainly conducted by means of a Jingxin nine deafness gene detection chip produced by the CapitalBio Corporation, and the detection cost of each site is 100 yuan; the requirement for the chip storage condition is high, high-end equipment is adopted, the detection process is complicated, and time consumption is high. The multiplex Taqman fluorescence detection kit has the advantages of being simple, efficient, low in cost and high in accuracy; no high-end equipment is needed, and all reagents can be stored in a refrigerator which is at 4 DEG C; two sites can be detected at the same time through a single hole, a result can be obtained through one step of PCR, and the detection cost of two sites is about 40 yuan. Therefore, the kit and detection method make detection quick, accurate and low in cost, and popularization of deafness screening is facilitated.
Owner:石玉玲
Method for predicting mitochondrial DNA mutation threshold, fertility risk and egg taking number
ActiveCN114023382AAids in genetic managementHealth-index calculationBiostatisticsMutation CarrierEgg count
The invention discloses a method for predicting a mitochondrial DNA mutation threshold value, a fertility risk and an egg taking number. The method comprises the following steps: firstly, establishing a mitochondrial mutation incidence probability prediction model and estimating a mutation threshold value s, then establishing a fertility risk prediction model and predicting a fertility risk p of a mutation carrier, and then, establishing an oocyte extraction prediction model by utilizing binomial distribution, and calculating the PGT egg taking number of a mutation carrier. According to the method, the disease probability prediction model is used for estimating the threshold value of common mtDNA mutation, and the fertility risk and the PGT egg taking number of the mother are predicted. On the basis of the established fertility risk prediction model and an egg taking prediction model, the reproduction risk and the minimum PGT egg taking number required by reproduction of a healthy offspring can be calculated only by knowing the mitochondrial DNA mutation proportion of a mutation carrier, so that clinical doctors can carry out genetic management on families carrying mtDNA heteromutation and provide genetic counseling, and a standard guide of PGT is provided.
Owner:THE FIRST AFFILIATED HOSPITAL OF ANHUI MEDICAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com