Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
33 results about "Hormone Receptor Antagonists" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
For the use of hormone antagonists in cancer, see hormonal therapy (oncology) A hormone antagonist is a specific type of receptor antagonist which acts upon hormone receptors. Such pharmaceutical drugs are used in antihormone therapy.
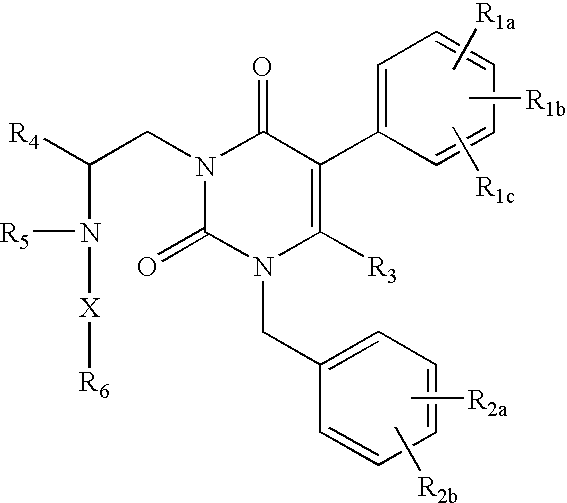
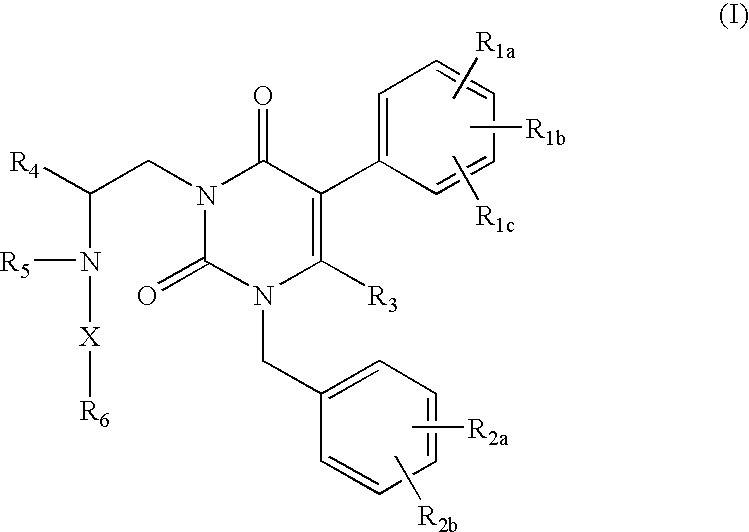
Gonadotropin-releasing hormone receptor antagonists and methods relating thereto
ActiveUS7056927B2Organic active ingredientsBiocideGonadotropin-releasing hormone receptorAntigonadotropin
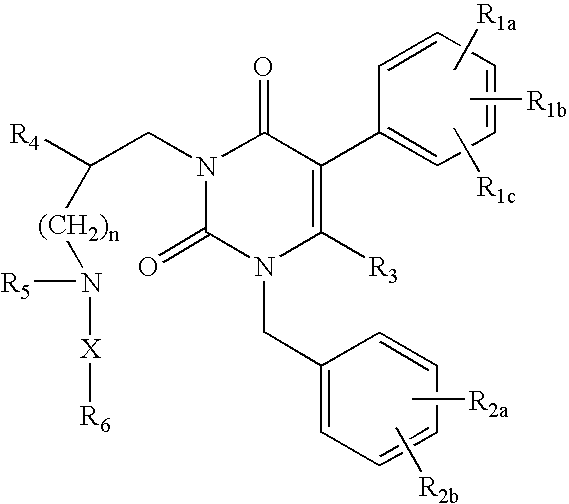
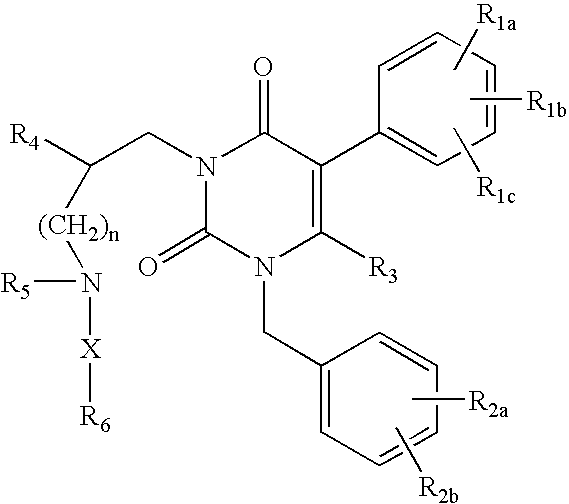
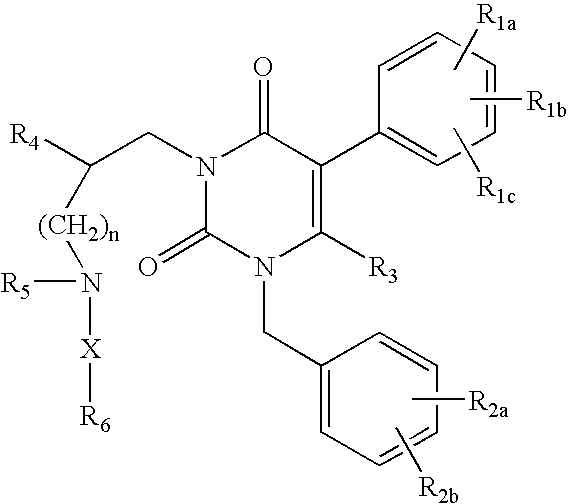
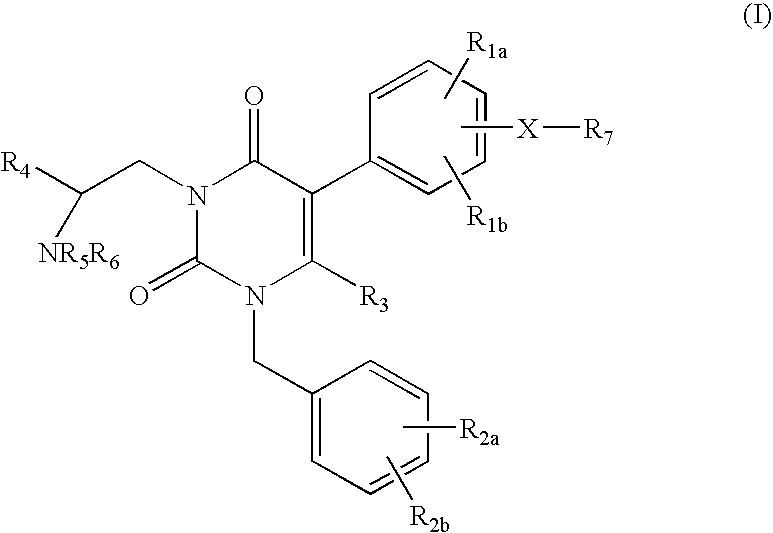
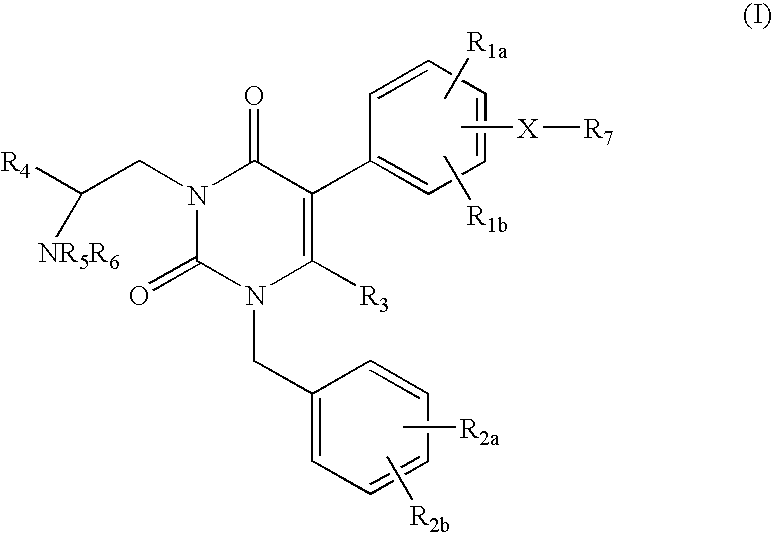
GnRH receptor antagonists are disclosed that have utility in the treatment of a variety of sex-hormone related conditions in both men and women. The compounds of this invention have the structure:wherein R1a, R1b, R1c, R2a, R2b, R3, R4, R5, R6 and X are as defined herein, including stereoisomers, prodrugs and pharmaceutically acceptable salts thereof. Also disclosed are compositions containing a compound of this invention in combination with a pharmaceutically acceptable carrier, as well as methods relating to the use thereof for antagonizing gonadotropin-releasing hormone in a subject in need thereof.
Owner:NEUROCRINE BIOSCI INC
Fused pyramidine derivative and use thereof
InactiveUS20070010537A1Excellent GnRH antagonizing activityHigh antagonistic activityBiocideOrganic active ingredientsLutenizing hormonePharmaceutical drug
There are provided a fused pyrimidine compound having antagonistic activity against luteinizing hormone releasing hormone, and a medicine containing the compound. A luteinizing hormone releasing hormone antagonist containing a compound represented by the formula: wherein R1a is a hydrocarbon group which may be substituted or a hydrogen atom, ring Aa is a 6-membered aromatic ring which may be further substituted, ring Ba is a homocyclic or heterocyclic ring which may be further substituted, Wa is an oxygen atom or a sulfur atom, Xa1 and Xa2, which may be identical or different, are each a hydrogen atom, a hydrocarbon group which may be substituted, or a heterocyclic group which may be substituted, or Xa1 and Xa2 together may form an oxygen atom, a sulfur atom or NR3a (wherein R3a is a hydrocarbon group which may be substituted or a hydrogen atom), and Ya is C1-6 alkylene which may be substituted or a bond, or a salt or prodrug thereof.
Owner:TAKEDA PHARMA CO LTD
Gonadotropin releasing hormone antagonists in gel-forming concentrations
InactiveUS20050245455A1Peptide/protein ingredientsLuteinising hormone-releasing hormoneActive agentGnRH Antagonist
Pharmaceutical compositions are provided for the treatment of steroid-dependent and other diseases. The compositions are solutions for subcutaneous or intramuscular injection, and the active agent is a GnRH antagonist peptide according to general formula (1): Ac-DNal-DCpa-DPal-Ser-Aph(X1)-DAph(X2)-Leu-Lys(iPr)-Pro-DAla-NH2 present at a concentration sufficient to from a gel following administration.
Owner:FERRING BV
Melanin-concentrating hormone antagonist
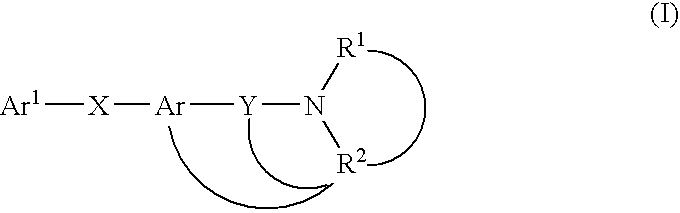
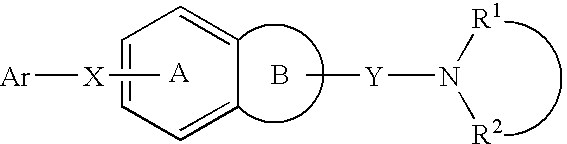
A melanin-concentrating hormone antagonist comprising a compound of the formula (I): wherein Ar1 is a cyclic group which may be substituted; X and Y are the same or different and are a spacer having a main chain of 1 to 6 atoms; Ar is a condensed polycyclic aromatic ring which may be substituted; R1 and R2 are the same or different and are hydrogen atom or a hydrocarbon group which may be substituted; or R1 and R2, together with the adjacent nitrogen atom, may form a nitrogen-containing heterocyclic ring which may be substituted; or R2, together with the adjacent nitrogen atom and Y, may form a nitrogen-containing heterocyclic ring which may be substituted; or R2, together with the adjacent nitrogen atom, Y and Ar, may form a condensed ring; or a salt thereof is useful as an agent for preventing or treating obesity, etc.
Owner:TAKEDA PHARMA CO LTD
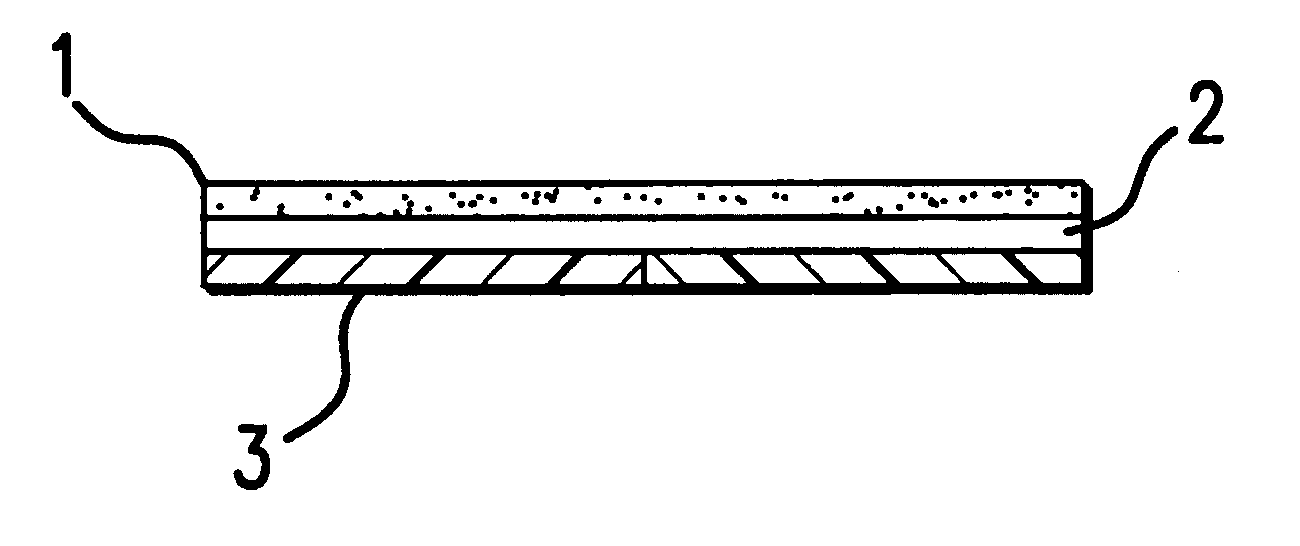
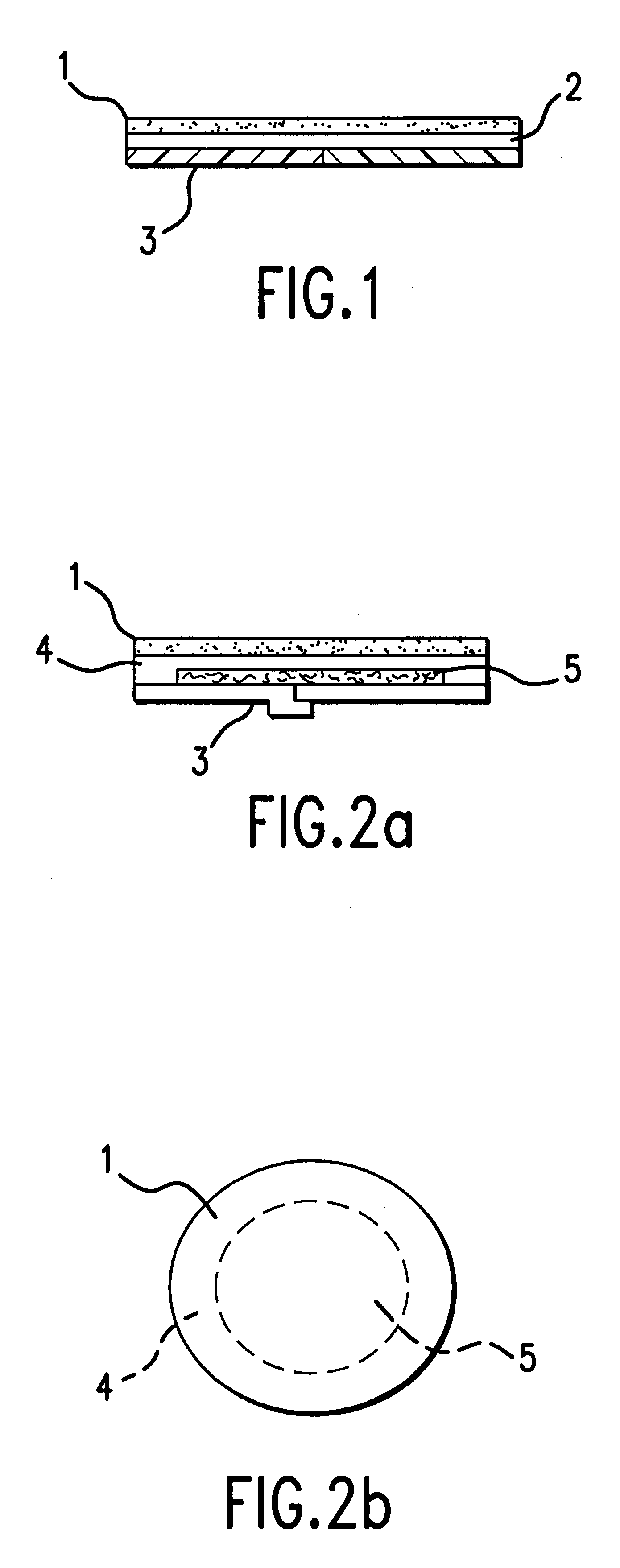
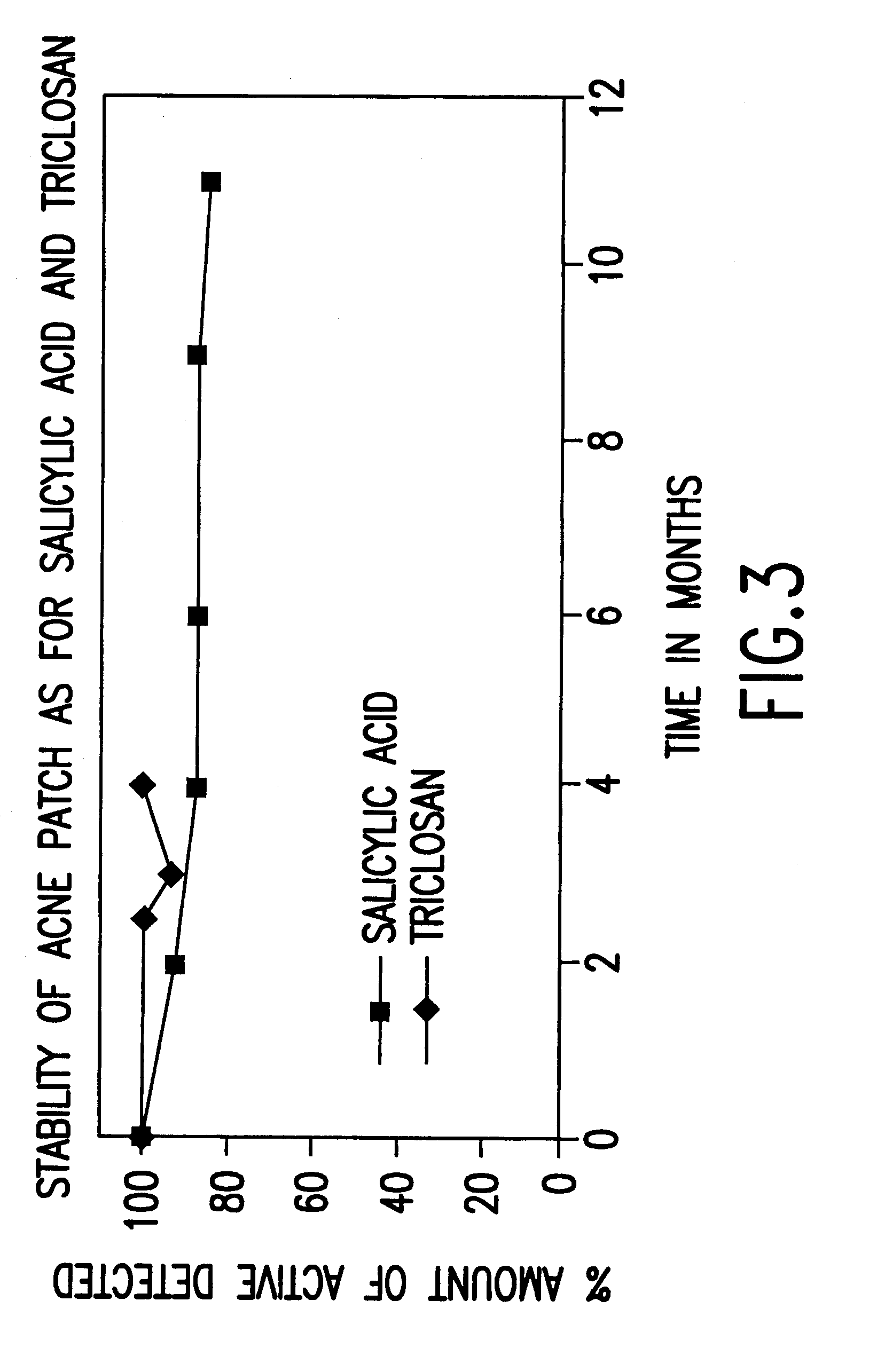
Device for topical treatment of acne and its method of manufacture
InactiveUS6280764B1Maximize efficacyReduce deliverySalicyclic acid active ingredientsBiocideTopical treatmentAgonist
A patch for topical application of an anti-acne formulation has in various embodiments a backing film, a release layer and at least one adhesive polymeric matrix layer located between the backing film and the release layer. The anti-acne formulation is uniformly distributed throughout one or more polymeric matrix layers and has an anti-acne effective amount of at least two agents selected from the group consisting of an anti-microbial, an antiseptic, an anti-irritant, a keratolytic agent, a hormone, a hormone agonist and a hormone antagonist.
Owner:THALLIUM HLDG CO LLC
Method for the treatment of fertility disorders
InactiveUS6319192B1Organic active ingredientsPeptide/protein ingredientsObstetricsHormones regulation
An improvement to the method of intrauterine insemination by the administration of luteinizing hormone-releasing hormone antagonists (LHRH antagonists).
Owner:ZENTARIS IVF
Gonadotropin-releasing hormone receptor antagonists and methods relating thereto
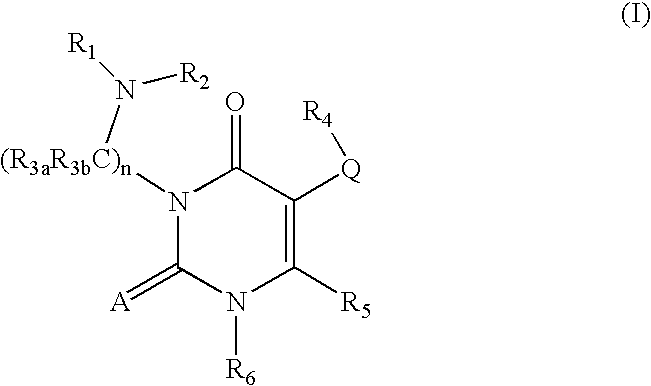
GnRH receptor antagonists are disclosed which have utility in the treatment of a variety of sex-hormone related conditions in both men and women. The compounds of this invention have the structure: wherein A, Q, R1, R2, R3a, R3b, R4, R5, R6 and n are as defined herein, including stereoisomers, prodrugs and pharmaceutically acceptable salts thereof. Also disclosed are compositions containing a compound of this invention in combination with a pharmaceutically acceptable carrier, as well as methods relating to the use thereof for antagonizing gonadotropin-releasing hormone in a subject in need thereof.
Owner:NEUROCRINE BIOSCI INC
Method of controlled ovarian hyperstimulation and pharmaceutical kit for use in such method
InactiveUS20050235374A1Easy to solveImprove developmentPeptide/protein ingredientsPeptide preparation methodsGanirelixCo administration
One aspect of the present invention is concerned with a method of controlled ovarian hyperstimulation in a mammalian female, said method comprising the co-administration to said female of a substance having follicle stimulating hormone activity (FSH substance) in an amount effective to stimulate multiple follicular development;—gonadotropin releasing hormone (GnRH) antagonist in an amount equivalent to a daily subcutaneous dose of at least 0.5 mg ganirelix to prevent a premature LH-surge; and—a LH substance in an amount effective to prevent or suppress symptoms of luteinising hormone (LH) deficiency resulting from the administration of the GnRH antagonist; followed by administering a meiosis and luteinisation inducing substance (ML substance) in an amount effective to stimulate resumption of meiosis and luteinisation, and wherein the LH substance is not obtained from the urine of human females. Another aspect of the to invention relates to a pharmaceutical kit for use in a method of controlled hyperstimulation, which kit comprises:—at least one parenteral or oral dosage unit containing one or more FSH substances in an amount equivalent to a subcutaneous dose of 50-1500 I.U. FSH;—at least one parenteral dosage unit containing one or more GnRH antagonists in an amount equivalent to a subcutaneous dose of 0.5-25 mg ganirelix;—at least one parenteral dosage unit containing one or more LH substances in an amount equivalent to a subcutaneous dose of 50-3000 I.U. recombinant LH; wherein the LH substance is not obtained from the urine of human females.
Owner:ZONE IND DE IOURIETTAZ
Pyrrolobenzodiazepine arylcarboxamides and derivatives thereof as follicle-stimulating hormone receptor antagonists
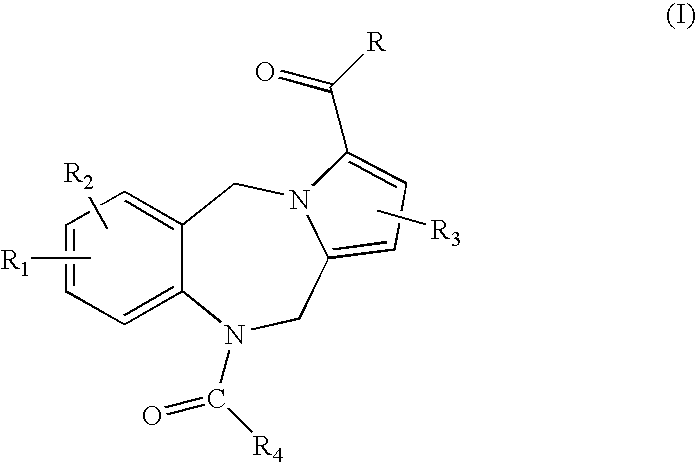
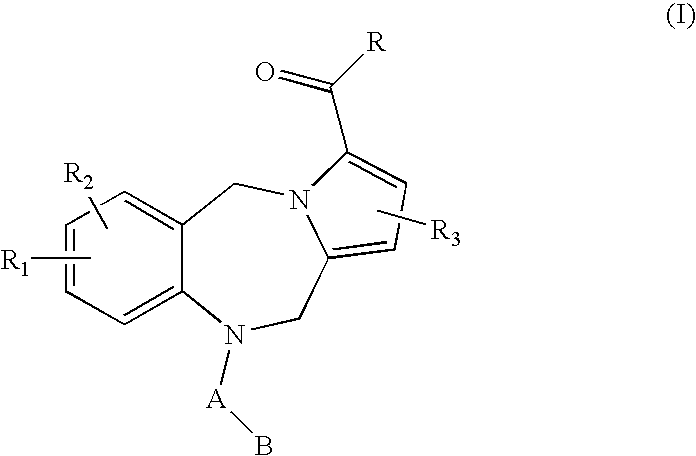
This invention provides pyrrolobenzodiazepine arylcarboxamides selected from those of Formula (1), which act as follicle stimulating hormone receptor antagonists, as well as pharmaceutical compositions and methods of treatment utilizing these compounds.
Owner:WYETH LLC
Gonadotropin-releasing hormone receptor antagonists and methods relating thereto
ActiveUS7015226B2Organic active ingredientsBiocideGonadotropin-releasing hormone receptorNK1 receptor antagonist
GnRH receptor antagonists are disclosed that have utility in the treatment of a variety of sex-hormone related conditions in both men and women. The compounds of this invention have the structure: wherein n, R1a, R1b, R1c, R2a, R2b, R3, R4, R5, R6 and X are as defined herein, including stereoisomers, prodrugs and pharmaceutically acceptable salts thereof. Also disclosed are compositions containing a compound of this invention in combination with a pharmaceutically acceptable carrier, as well as methods relating to the use thereof for antagonizing gonadotropin-releasing hormone in a subject in need thereof.
Owner:NEUROCRINE BIOSCI INC
Gonadotropin-releasing hormone receptor antagonists and methods relating thereto
GnRH receptor antagonists are disclosed which have utility in the treatment of a variety of sex-hormone related conditions in both men and women. The compounds of this invention have the structure, wherein R1a, R1b, R1c, R1d, R2, R2a, and A are as defined herein, including stereoisomers, esters, solvates and pharmaceutically acceptable salts thereof. Also disclosed are compositions containing a compound of this invention in combination with a pharmaceutically acceptable carrier, as well as methods relating to the use thereof for antagonizing gonadotropin-releasing hormone in a subject in need thereof.
Owner:NEUROCRINE BIOSCI INC
Melanin-concentrating hormone receptor antagonists containing piperidine derivatives as the active ingredient
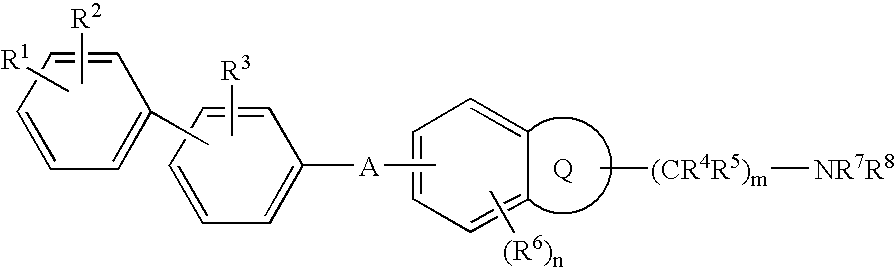
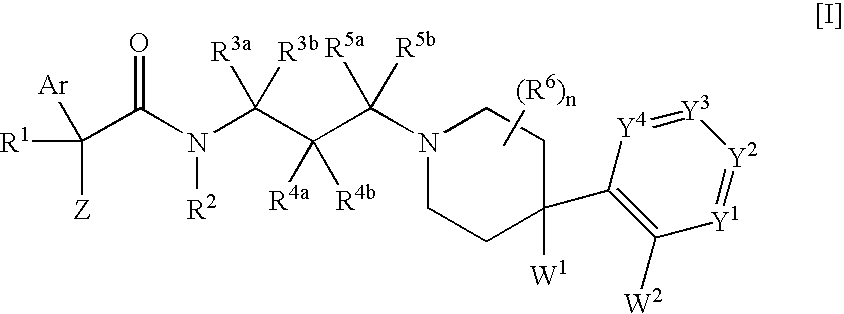
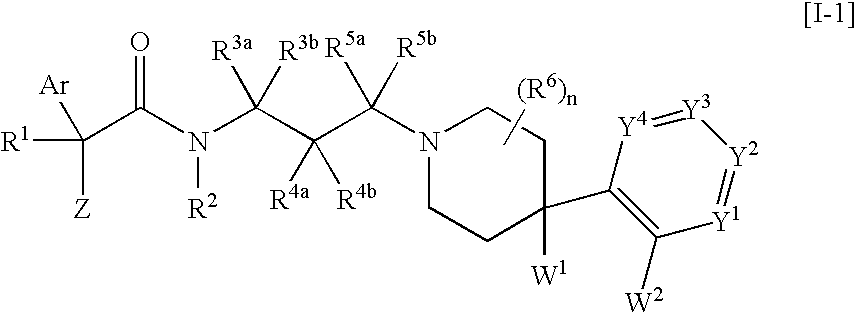
The invention provides melanin-concentrating hormone receptor antagonists containing as the active ingredient piperidine derivatives represented by the general formula [I]: [wherein R1 is hydrogen, hydroxyl, lower alkyl, or the like; R2, R3a, R3b, R4a, R4b, R5a, R5b and R6 each stands for hydrogen, halogen, or the like; W1 and W2 each independently stands for —O—, —CH2—, or the like; Y1, Y2, Y3 and Y4 stand for —CH—, —CF—, —N—, or the like; Z stands for lower alkyl, an aliphatic heterocyclic group, or the like; Ar is a mono- or bi-cyclic aliphatic heterocycle or an aromatic heterocycle; and n is an integer of 1 to 8]. The compounds act as antagonist against melanin-concentrating hormone receptor and are useful as drugs for central diseases, circulatory diseases, or metabolic diseases.
Owner:MSD KK
GnRh antagonists being modified in positions 5 and 6
InactiveCN1259959APeptide/protein ingredientsLuteinising hormone-releasing hormoneSide chainGnRH Antagonist
Peptides are provided which have improved duration of GnRH antagonistic properties. These antagonists may be used to regulate fertility and to treat steroid-dependent tumors and for other short-term and long-term treatment indications. These antagonists have a derivative of aminoPhe or its equivalent in the 5- or the 5- and 6-positions. This derivative is modified so as to contain a carbamoyl group or heterocycle, including a urea moiety, in its side chain. Decapeptides having the formula:wherein Q2 is Cbm or MeCbm and Xaa10 is D-Ala-ol or Ala-ol are particularly effective and continue to exhibit very substantial suppression of LH secretion at 96 hours following injection.
Owner:FERRING BV
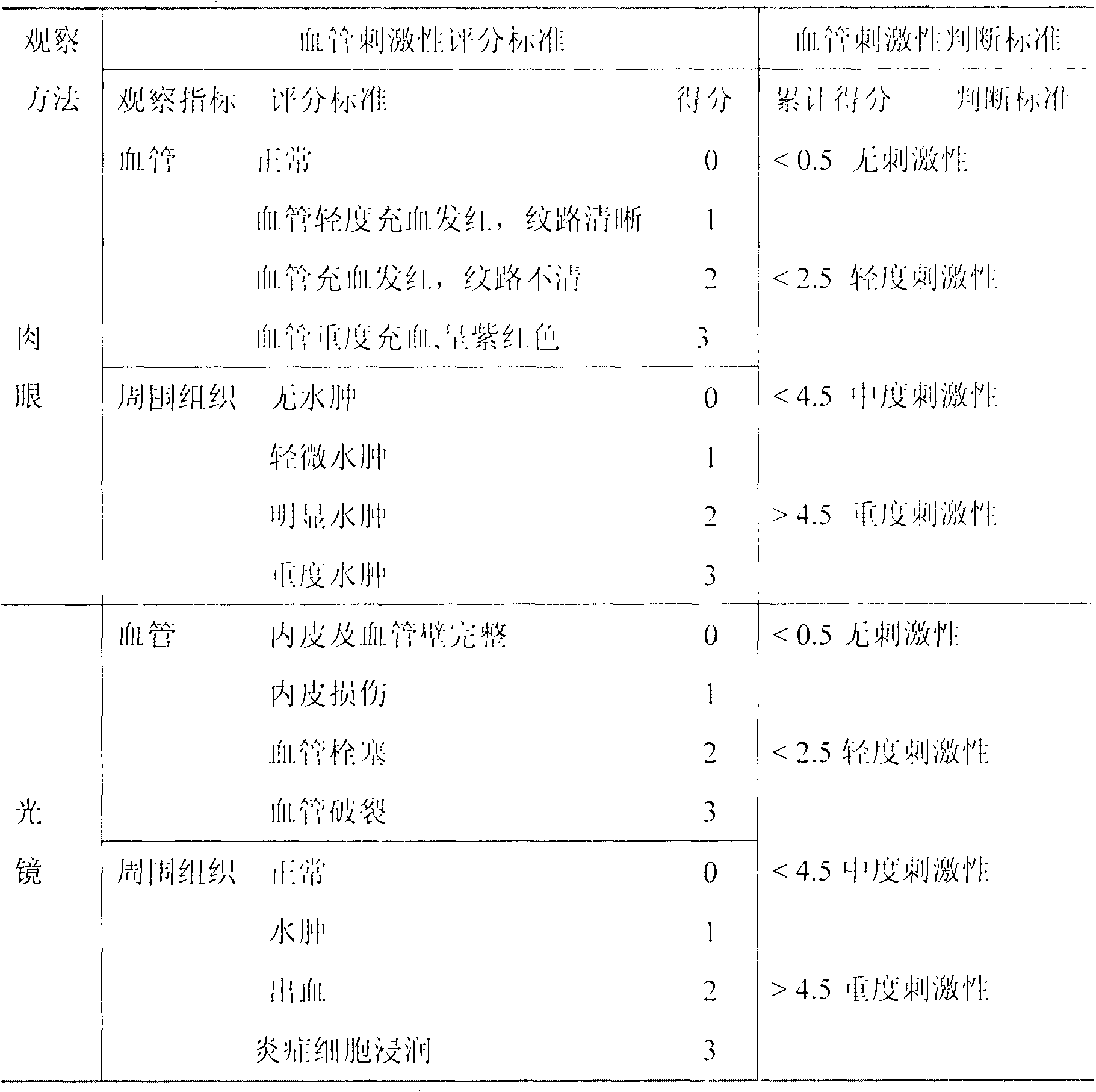
LHRH (luteinizing hormone-releasing hormone antagonist) lyophilized powder injection with improved stability
ActiveCN102144980AQuality improvementDefinite curative effectPowder deliveryPeptide/protein ingredientsPatient complianceAntioxidant
The invention relates to an LHRH (luteinizing hormone-releasing hormone antagonist) lyophilized powder injection with improved stability. The preparation mainly comprises cetrorelix as an active ingredient, an excipient, a pH regulator, an antioxidant and a local analgesic agent, wherein the addition of the antioxidant can improve the stability of the preparation, and the addition of the local analgesic agent can relieve the pains of patients while having no impact on the active ingredient. The preparation process of the preparation mainly comprises weighing, dissolving, adsorbing pyrogen with activated carbon, filtering, final filtering, subpackaging, vacuum drying, plugging, and covering. The LHRH lyophilized powder injection provided by the invention can control the stimulatory function of an ovary, prevent early release of immature ovarian follicles, and help become pregnant. The LHRH lyophilized powder injection has the advantages of stable quality, definite therapeutic effect, and good patient compliance.
Owner:深圳市健翔生物制药有限公司
Spiroindoline derivatives as gonadotropin- releasing hormone receptor antagonists
Spiroindoline derivatives, process for their preparation and pharmaceutical compositions thereof, their use for the treatment and / or prophylaxis of diseases, and their use for the manufacture of medicaments for the treatment and / or prophylaxis of diseases, especially sex-hormone-related diseases in both men and women, in particularly those selected from the group of endometriosis, uterine fibroids, polycystic ovarian disease, hirsutism, precocious puberty, gonadal steroid-dependent neoplasia such as cancers of the prostate, breast and ovary, gonadotrope pituitary adenomas, sleep apnea, irritable bowel syndrome, premenstrual syndrome, benign prostatic hypertrophy, contraception and infertility (e.g., assisted reproductive therapy such as in vitro fertilization). The present application relates in particular to spiroindoline derivatives as gonadotropin-releasing hormone (GnRH) receptor antagonists.
Owner:BAYER INTELLECTUAL PROPERTY GMBH
Production method of cocoon silk of extra fine size
InactiveCN101422141AExcellent finenessHigh-quality finenessAnimal feeding stuffAccessory food factorsDisease ratesZoology
The invention discloses a manufacture method for ultrathin size cocoon filaments, which comprises the steps of: breeding a trimolter variety which can be stably genetic to a newly molted second larva or a newly molted third larva stage according to a normal breeding method; using an antijuvenile hormone antagonist to induce the trimolter variety in the newly molted second larva or newly molted third larva stage into a dimolter; and then carrying out normal mulberry leaf feeding, frame mounting, cocoon drying, cocoon cooking and silk reeling in turn. The manufacture method can obtain the top-grade ultra-thin size cocoon filaments with average size of 0.7 to 0.9 dtex; the thinnest can achieve 0.4dtex. The manufactured cocoon filaments have the advantages of excellent solving, thin size, small size error, good purity, high mightiness, good cohesive force, and the like. Simultaneously the method can effectively shorten the sericiculture period, which has extremely large promoting effects on adjusting the labors and improving the production efficiency of the sericiculture; besides, the method can reduce the disease rate of silkworms, reduce the harm to the production of the silkworms in summer and autumn and is beneficial to improving the safety.
Owner:THE INST OF BIOTECHNOLOGY OF THE CHINESE ACAD OF AGRI SCI
Gonadotropin-releasing hormone receptor antagonists and methods relating thereto
ActiveUS7329669B2BiocideOrganic active ingredientsGonadotropin-releasing hormone receptorNK1 receptor antagonist
GnRH receptor antagonists are disclosed that have utility in the treatment of a variety of sex-hormone related conditions in both men and women. The compounds of this invention have the structure:wherein R1a, R1b, R2a, R2b, R3, R4, R5, R6, R7 and X are as defined herein, including stereoisomers, prodrugs and pharmaceutically acceptable salts thereof. Also disclosed are compositions containing a compound of this invention in combination with a pharmaceutically acceptable carrier, as well as methods relating to the use thereof for antagonizing gonadotropin-releasing hormone in a subject in need thereof.
Owner:NEUROCRINE BIOSCIENCES INC
Spiroindoline derivatives as gonadotropin-releasing hormone receptor antagonists
InactiveCN104169287AOrganic active ingredientsOrganic chemistryGonadotropin-releasing hormone receptorDisease
Owner:BAYER IP GMBH
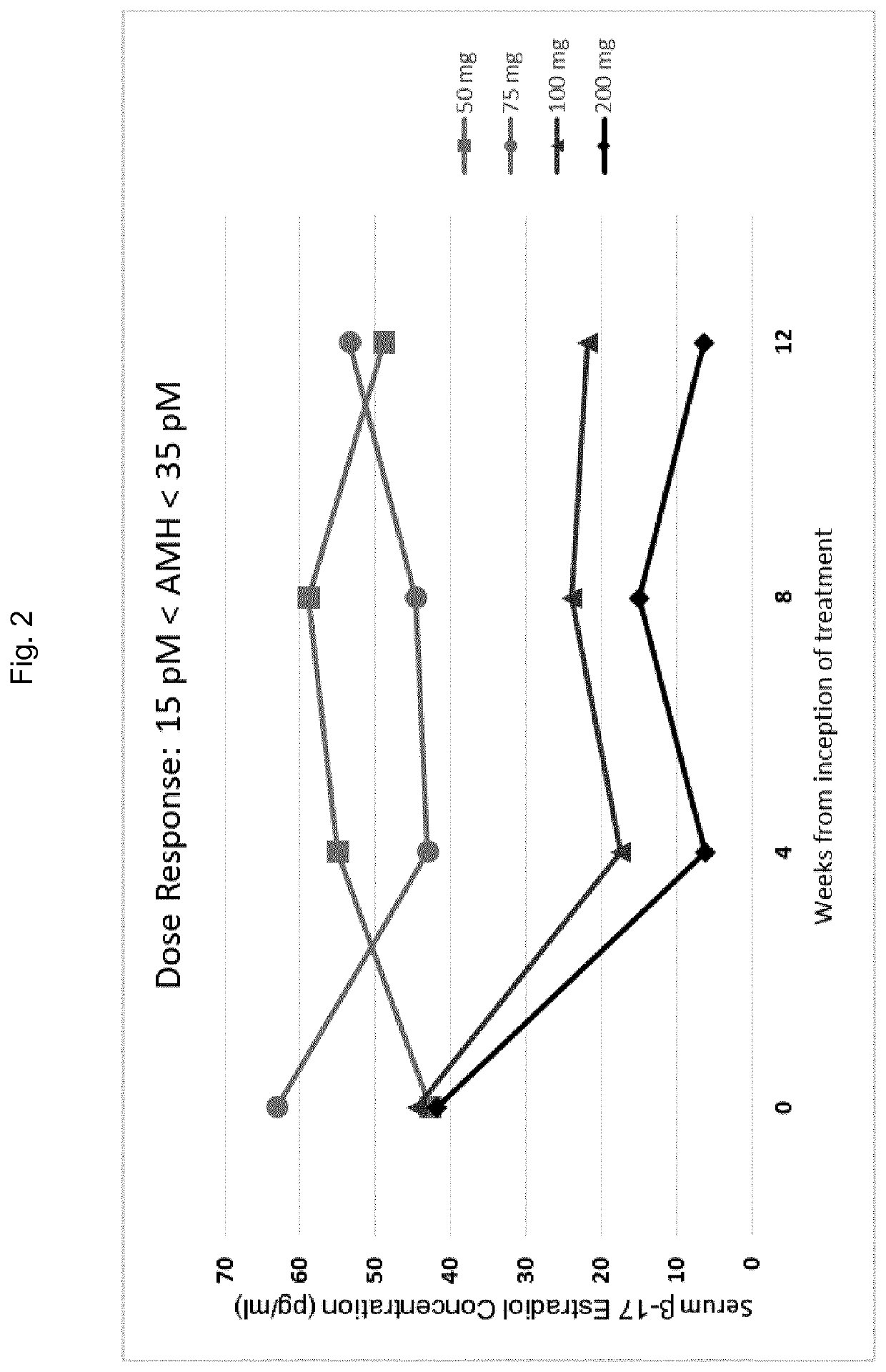
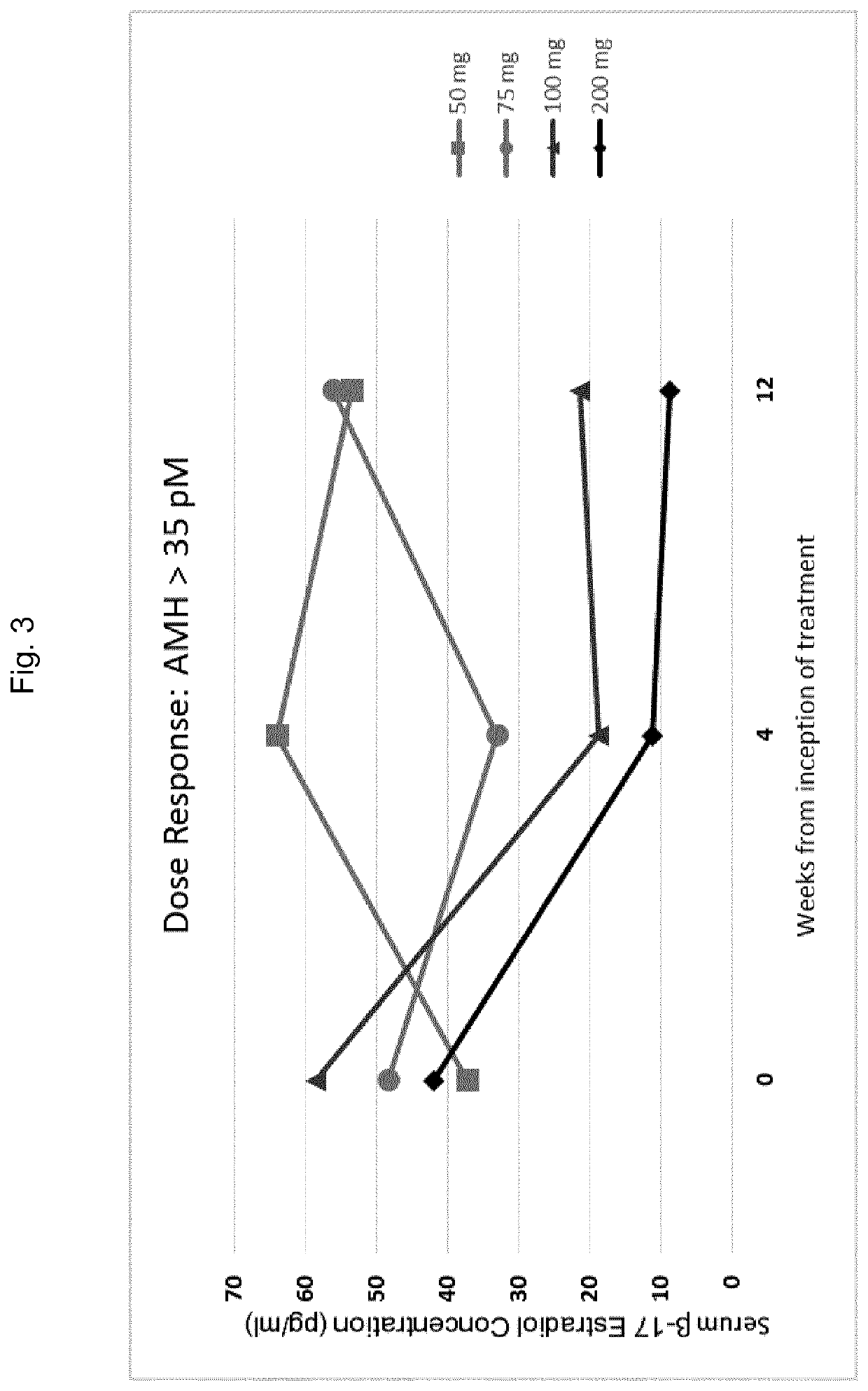
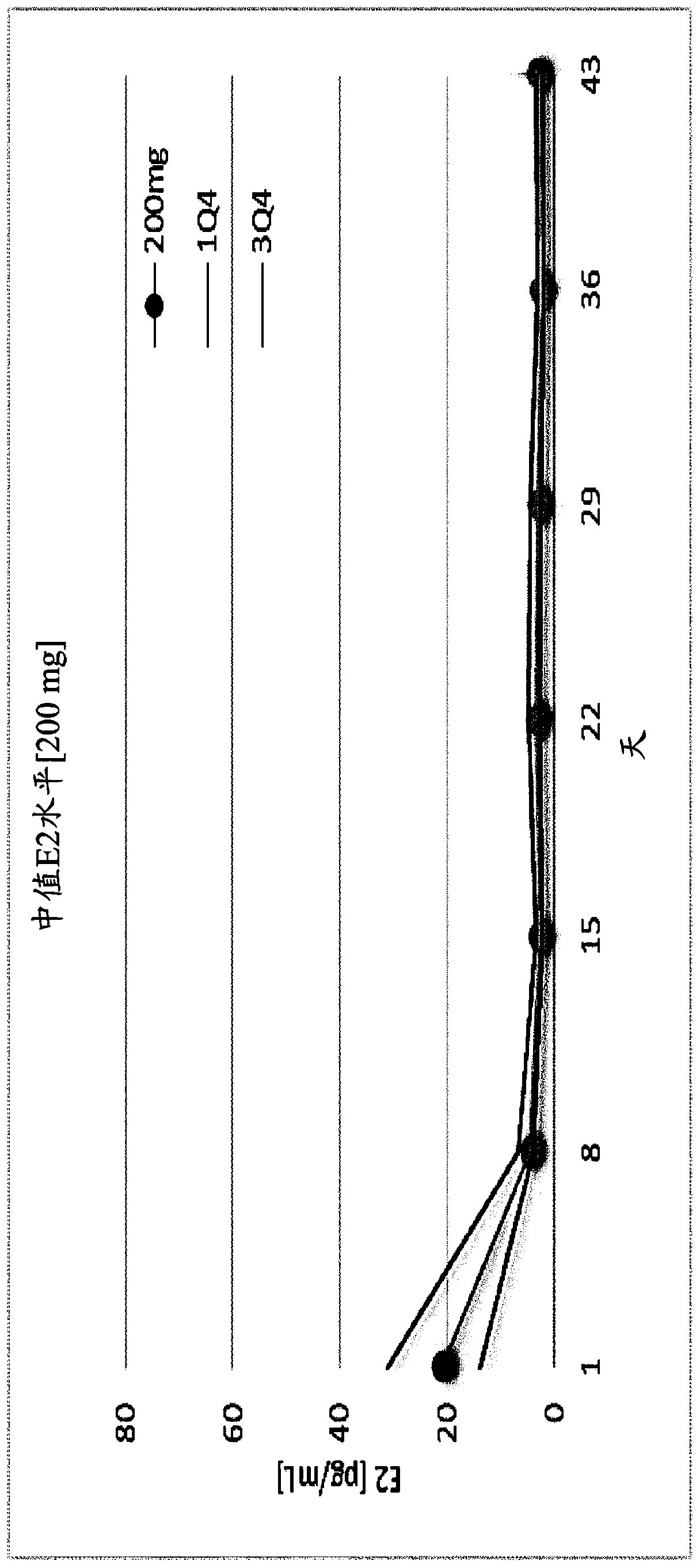
Gonadotropin-releasing hormone antagonist dosing regimens for the treatment of endometriosis
PendingUS20200138819A1Organic active ingredientsPeptide/protein ingredientsDosing regimenGonadotropin-releasing hormone antagonist
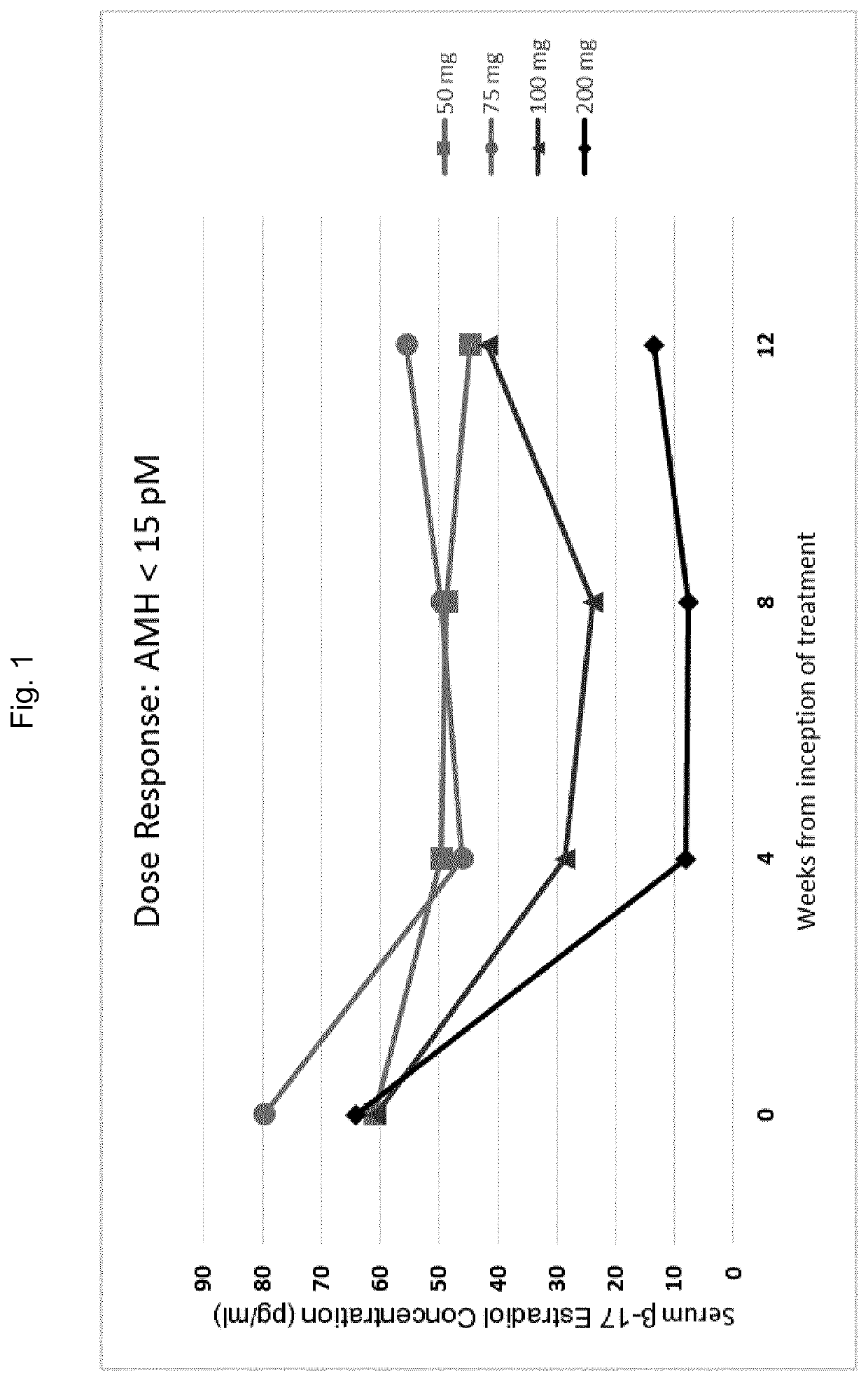
The invention provides methods of treating endometriosis in a patient by administration of a gonadotropin-releasing hormone (GnRH) antagonist, for instance, according to dosing regimens predicated on the patient's level of anti-Müllerian hormone (AMH) or β17-estradiol (E2).
Owner:KISSEI PHARMA
GnRh antagonists being modified in positions 5 and 6
InactiveCN1230442CPeptide/protein ingredientsLuteinising hormone-releasing hormoneSide chainGnRH Antagonist
Owner:FERRING BV
Hormone releasing hormone receptor antagonists and uses thereof
ActiveCN113527213AOrganic active ingredientsNervous disorderPharmaceutical medicineMedicinal chemistry
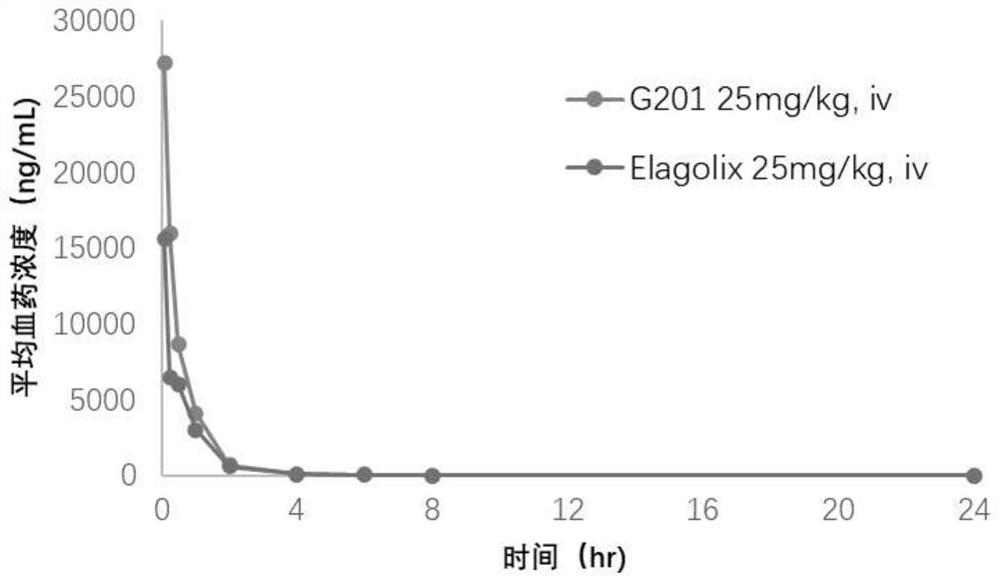
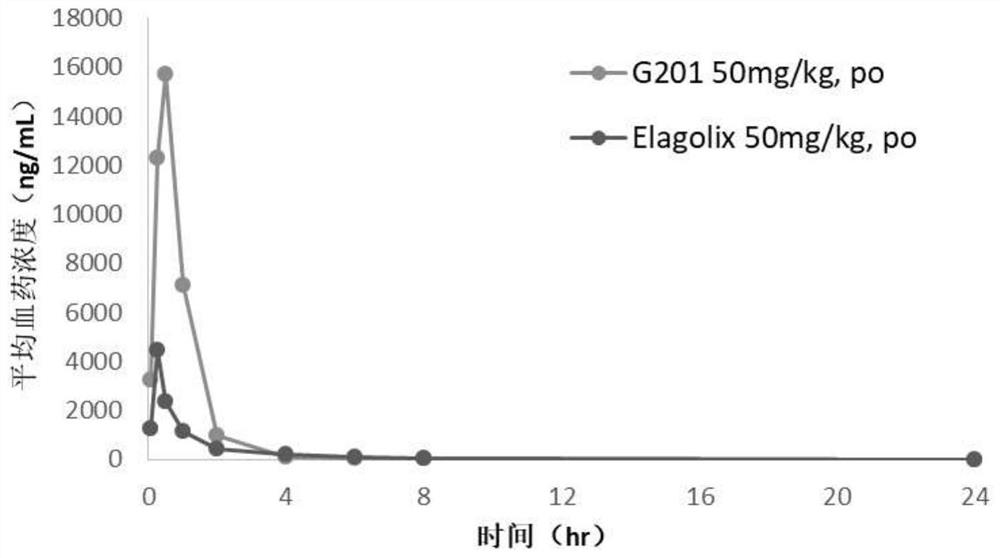
The invention relates to a hormone releasing hormone receptor antagonists and uses thereof. The invention relates to the field of organic chemistry, in particular to a compound or pharmaceutically acceptable salt, isomer, prodrug, polymorphic substance or solvate thereof as well as a preparation method and application of same. The invention provides a compound or a pharmaceutically acceptable salt, an isomer, a prodrug, a polymorphic substance or a solvate thereof. The chemical structural formula of the compound is as shown in formula I in the specification. The compound provided by the invention is used as a GnRHR antagonist in a calcium flow detection experiment, the activity of the compound is equivalent to or better than that of Elagolix, and the compound has better pharmacokinetic characteristics.
Owner:SHIJIAZHUANG YILING PHARMA
Amorphous solid dispersion of an orally available gonadotropin-releasing hormone receptor antagonist
ActiveUS10966979B2Prevent deliquescenceEasy to useOrganic active ingredientsOrganic chemistryPharmaceutical medicineExcipient
The invention relates to an amorphous solid dispersion comprising elagolix sodium and at least one silicon-based inorganic compound and to a process for preparing the same. Furthermore, it relates to a pharmaceutical composition comprising said solid dispersion and one or more additional pharmaceutical acceptable excipient(s), wherein the pharmaceutical composition can be used as a medicament, in particular for the treatment of endometriosis and uterine fibroids.
Owner:SANDOZ AG
Gonadotropin-releasing hormone antagonist dosing regimens for treating uterine fibroids and reducing menstrual blood loss
PendingCN110996957AReduce concentrationDecreased menstrual blood lossOrganic active ingredientsSexual disorderObstetricsRegimen
The invention provides compositions and methods for reducing the volume of menstrual blood loss in a patient, such as a human patient, for instance, that has uterine fibroids, by administration of a gonadotropin-releasing hormone (GnRH) antagonist. Suitable GnRH antagonists useful in conjunction with the compositions and methods described herein include thieno[3,4d]pyrimidine derivatives, such as3-[2-fluoro-5-(2,3-difluoro-6-methoxybenzyloxy)-4-methoxypheny I]-2,4-dioxo-1,2,3,4-tetrahydrothieno[3,4d]pyrimidine-5-carboxylic acid and the choline salt thereof.
Owner:KISSEI PHARMA
Gonadotropin-releasing hormone receptor antagonists and methods relating thereto
InactiveUS20050234082A1BiocideNervous disorderGonadotropin-releasing hormone receptorAntigonadotropin
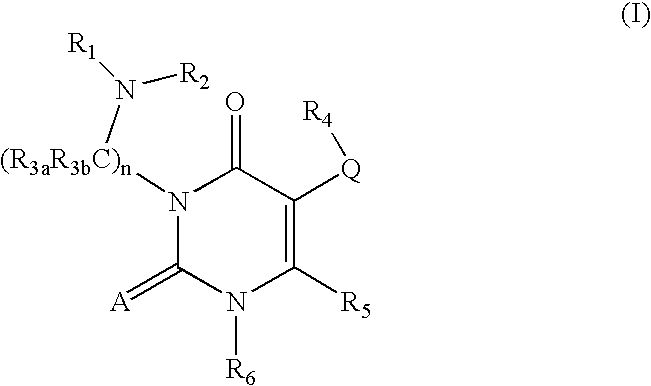
GnRH receptor antagonists are disclosed that have utility in the treatment of a variety of sex-hormone related conditions in both men and women. The compounds of this invention have the structure: wherein A, Q, R1, R2, R3a, R3b, R4, R5, R6 and n are as defined herein, including stereoisomers, prodrugs and pharmaceutically acceptable salts thereof. Also disclosed are compositions containing a compound of this invention in combination with a pharmaceutically acceptable carrier, as well as methods relating to the use thereof for antagonizing gonadotropin-releasing hormone in a subject in need thereof.
Owner:NEUROCRINE BIOSCI INC
Insect pharyngeal voxin antagonist and use thereof
Owner:SHANGHAI INST OF TECH
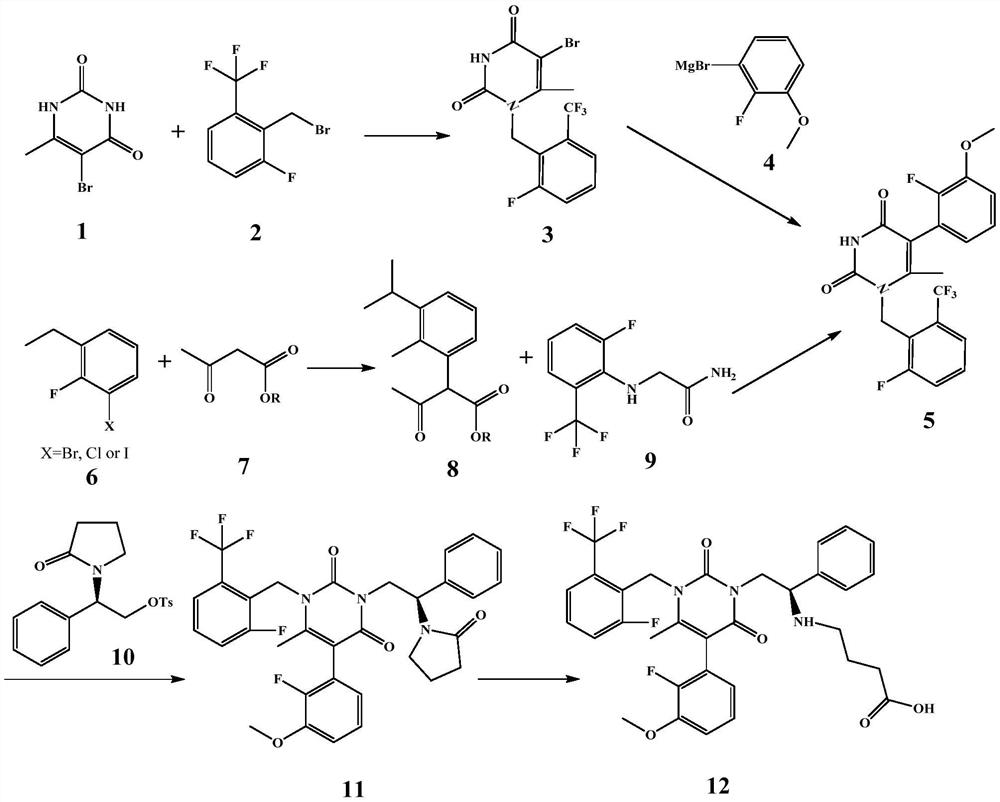
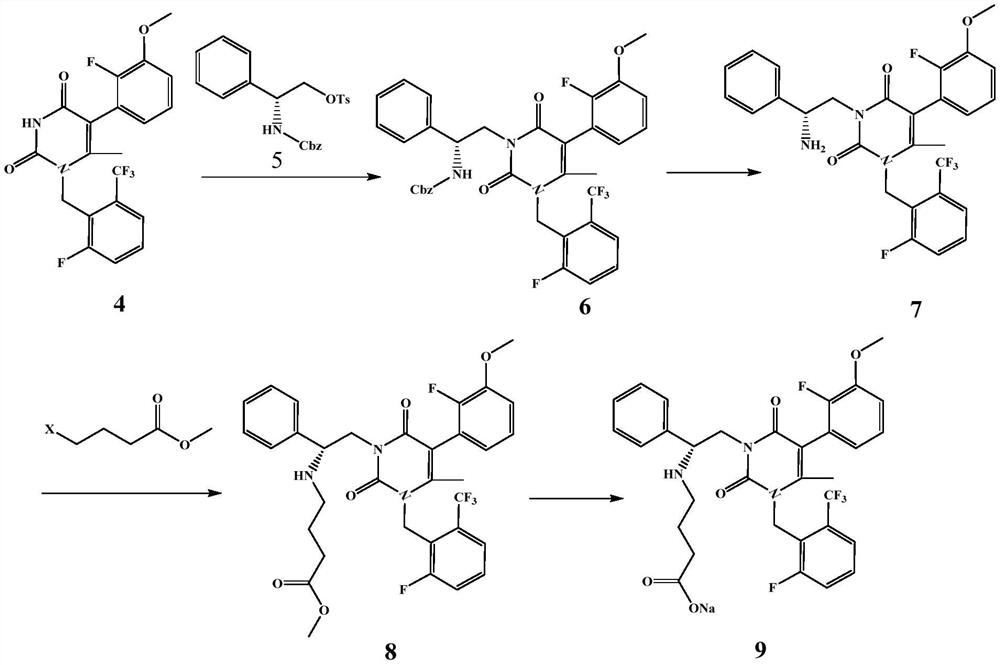
Preparation method of gonadotropin-releasing hormone antagonist intermediate and antagonist sodium
The invention discloses a preparation method of a gonadotropin-releasing hormone antagonist intermediate and antagonist sodium. An organic zinc reagent is obtained by reacting compound 1 with active zinc in an aprotonated solvent, and then performing Negishi coupling reaction to obtain the target intermediate body, and then synthesized to obtain gonadotropin-releasing hormone antagonist sodium. The invention has mild reaction conditions, simple operation, less side reactions, and reduces the production quality risk of preparing GnRHR medicine. The intermediate compound can be prepared directly in a "one-pot method", the intermediate is easy to separate and purify, the operation is simple and reproducible, and the product purity and yield are higher than the prior art. At the same time, the raw materials of the present invention are safe and low in cost, and are environmentally friendly, which is beneficial to cost control and environmental protection.
Owner:LIVZON NEW NORTH RIVER PHARMA
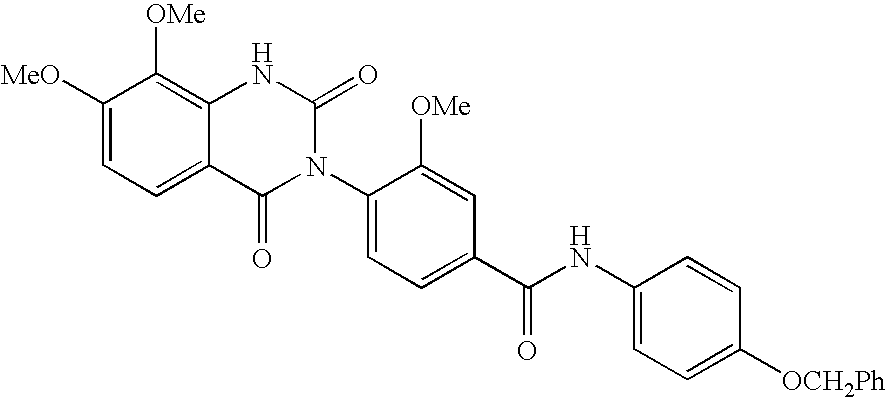
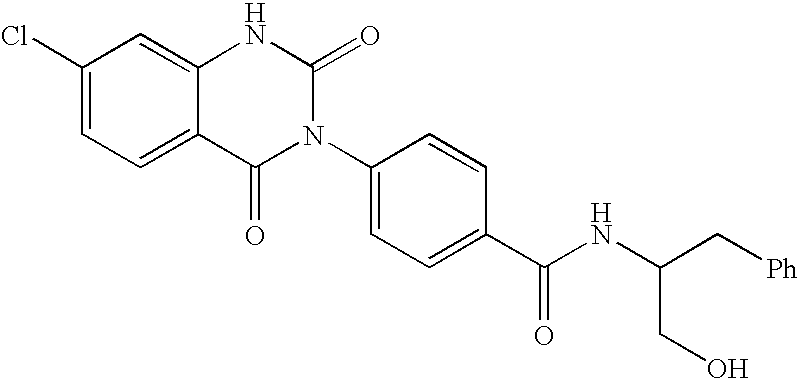
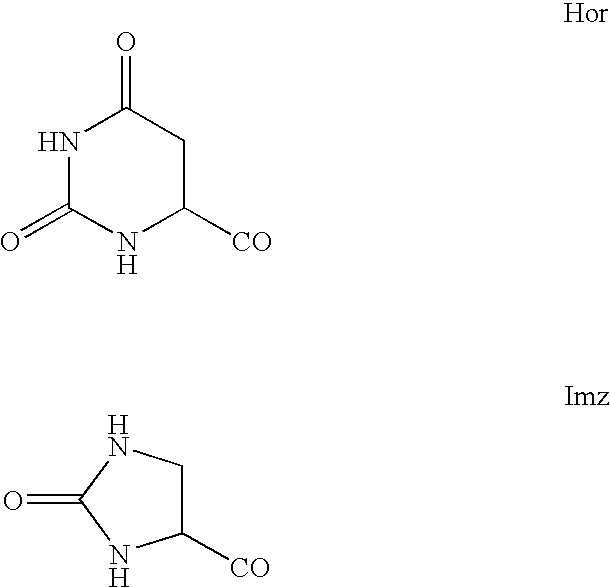
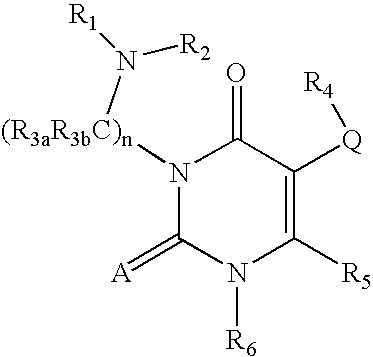
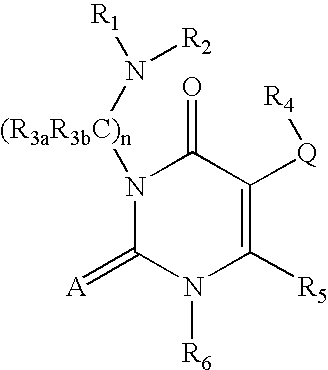
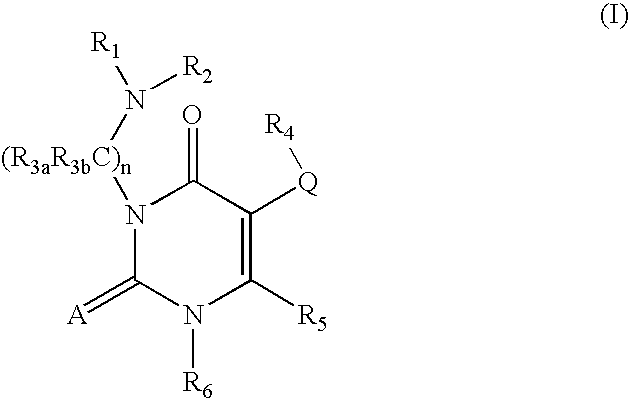
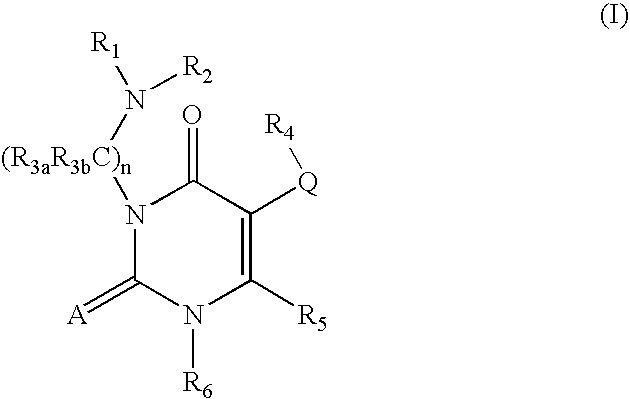
7-heterocyclyl quinoline and thieno[2,3,-b] pyridine derivatives useful as antagonists of gonadotropin releasing hormone
The present invention is directed to novel 7-heterocyclyl quinoline and thieno[2,3-b]pyridine derivatives of the general formula (I) or (II), wherein all variables are as herein defined, pharmaceutical compositions containing them and their use in the treatment of disorders and conditions associated with gonadotropin releasing hormone (GnRH). The compounds of the invention are antagonists of GnRH, useful in the treatment of the infertility, prostate cancer, benign prostate hyperplasia (BPH) and as contraceptives.
Owner:ORTHO MCNEIL PHARM INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com






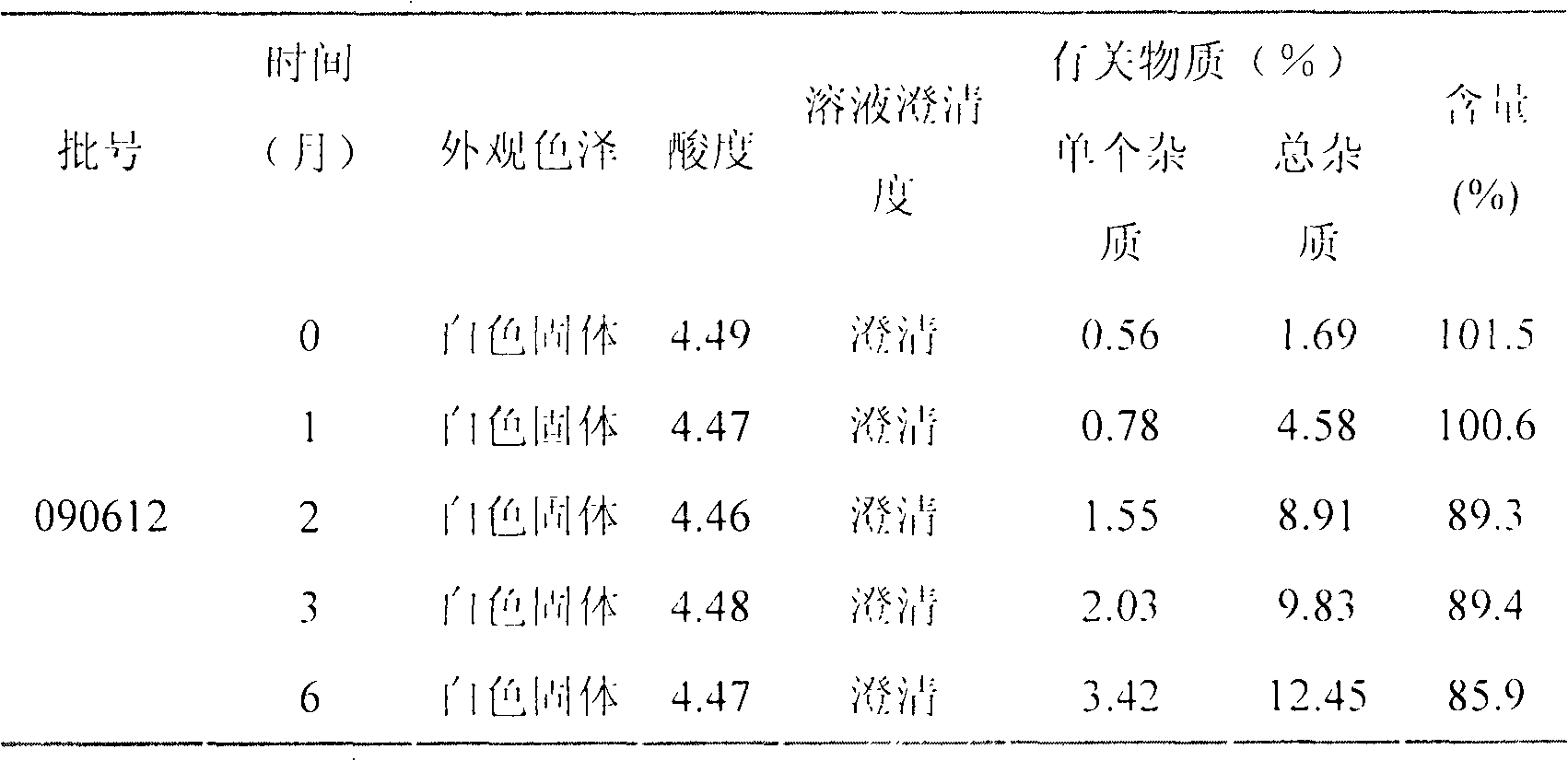
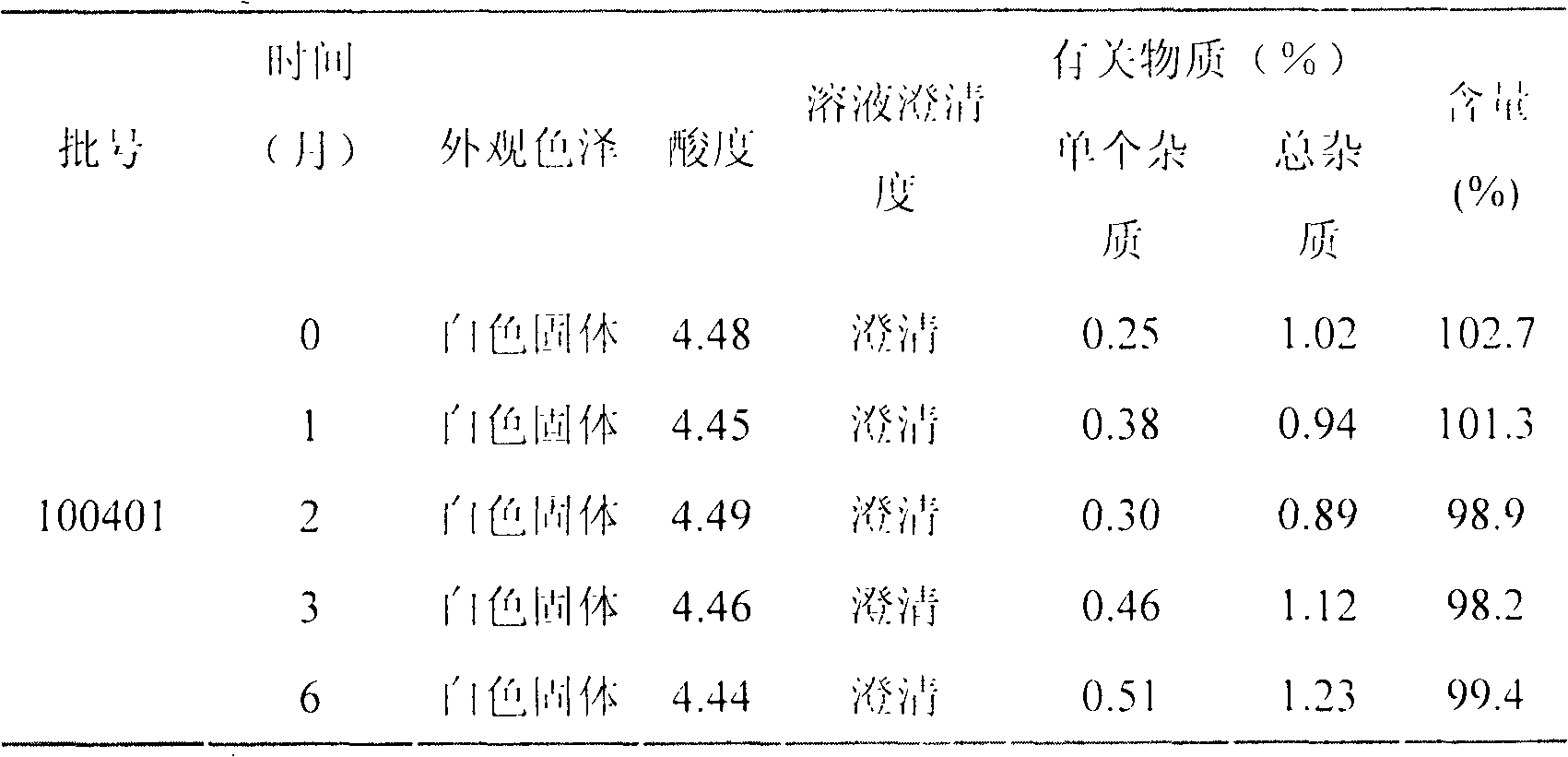




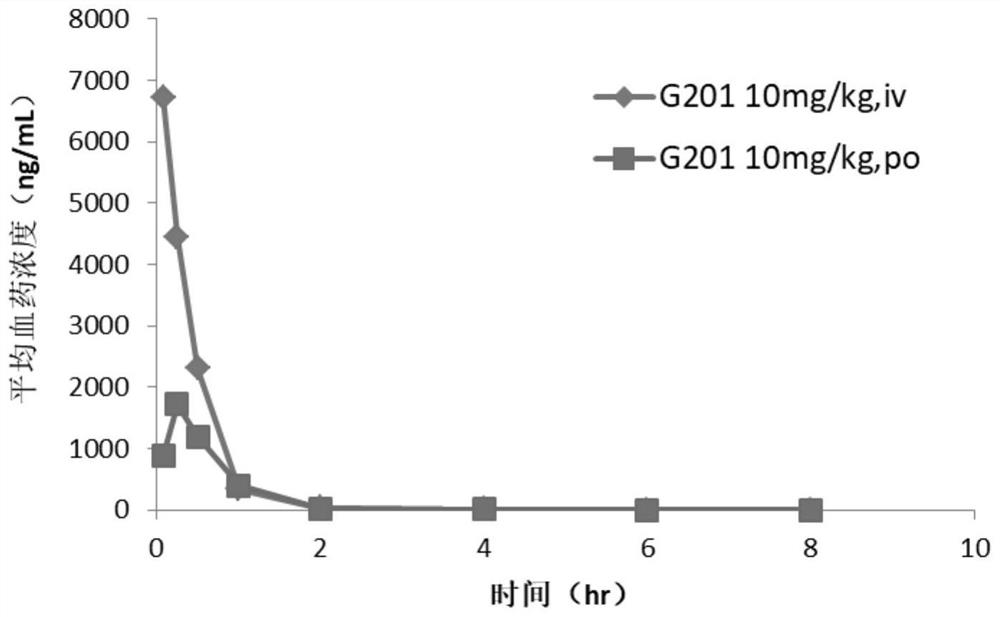
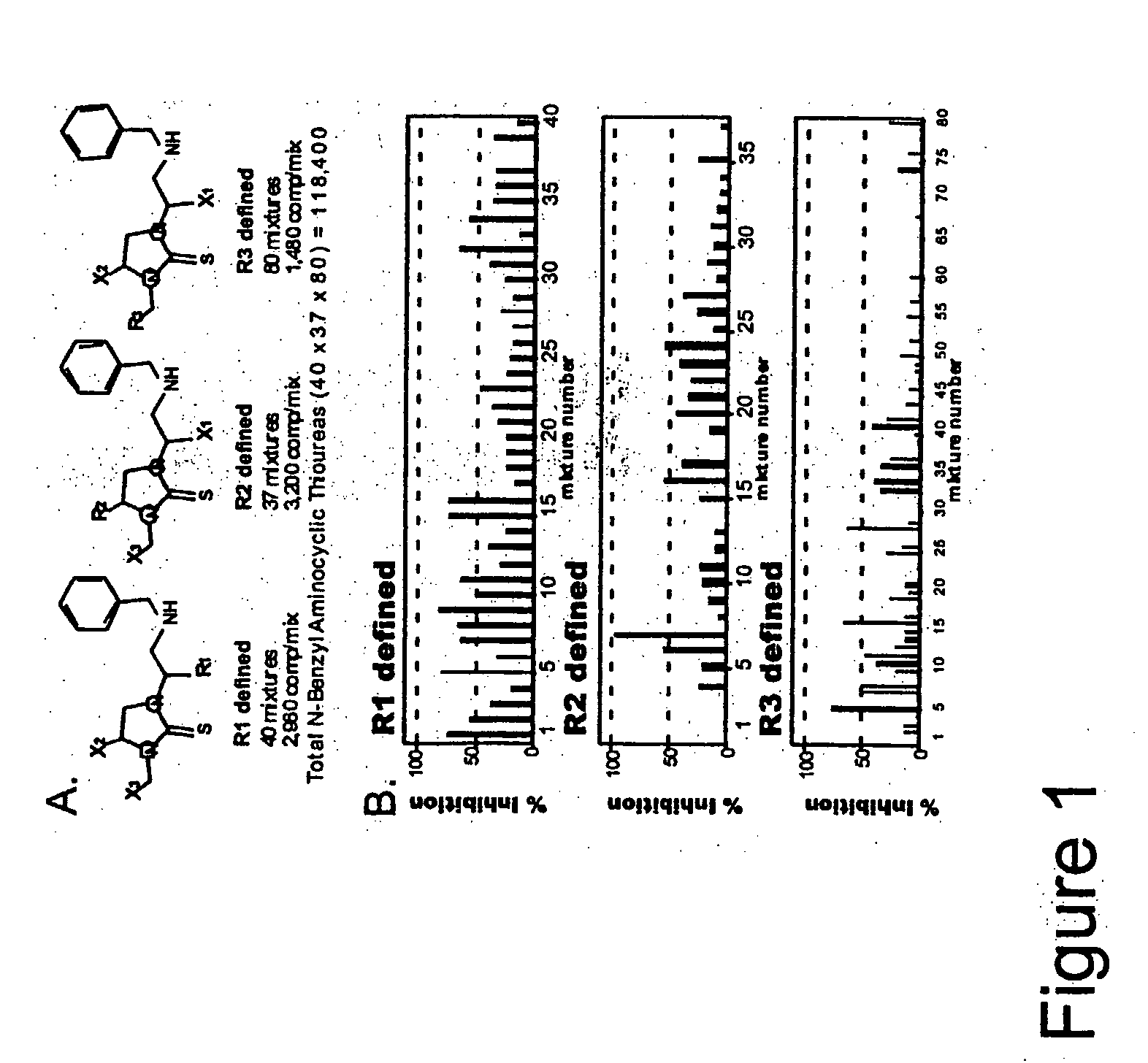
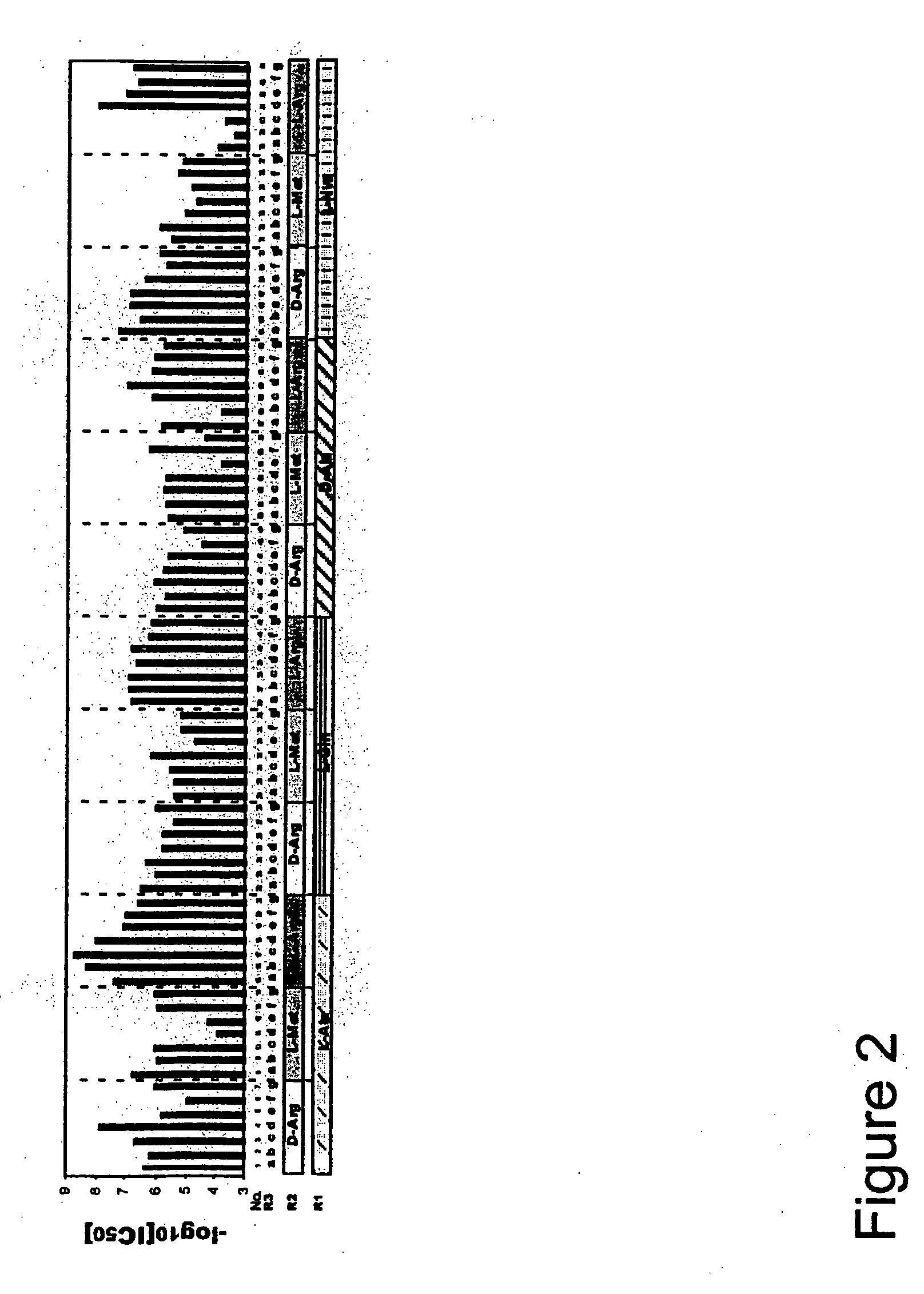

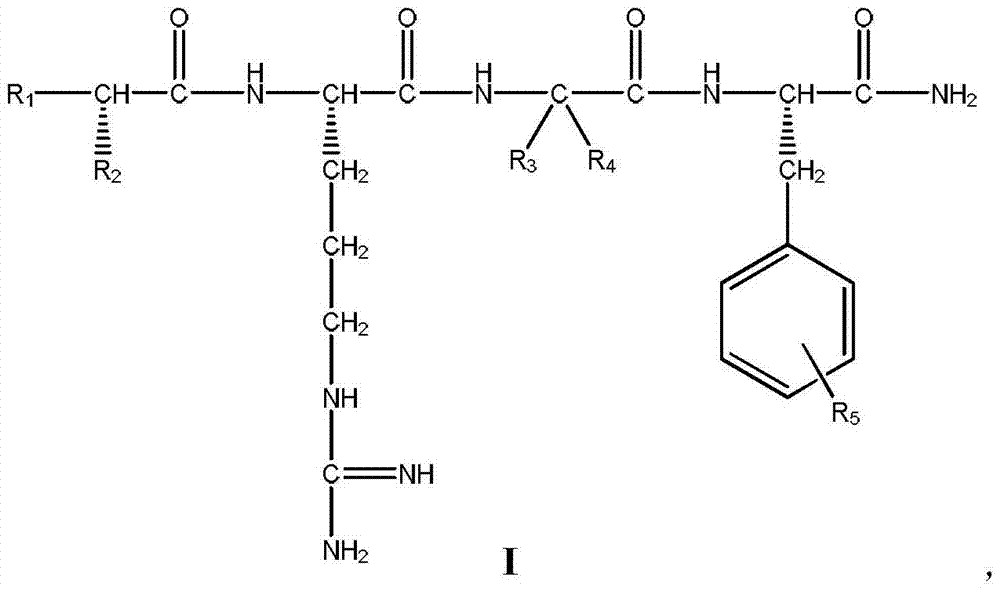
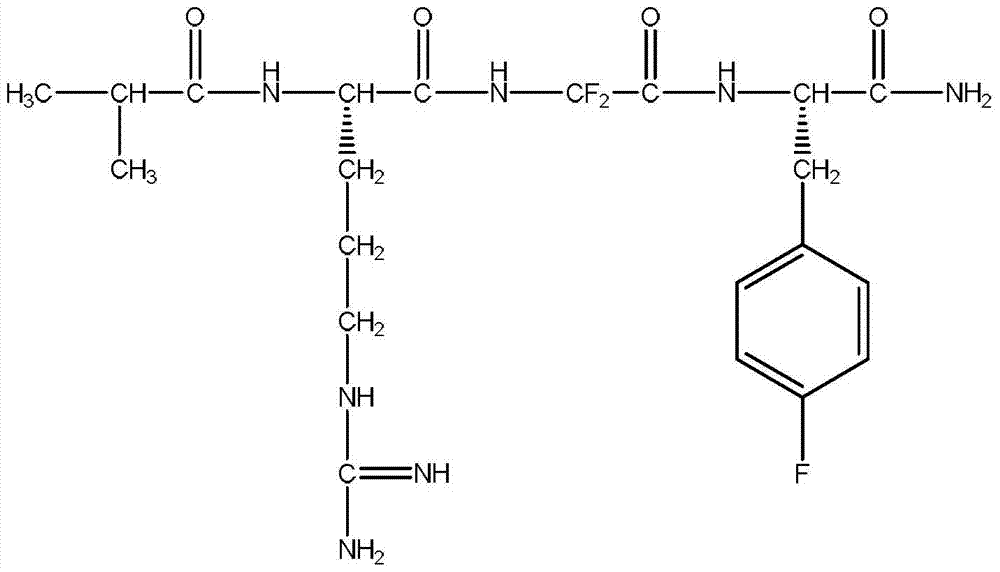
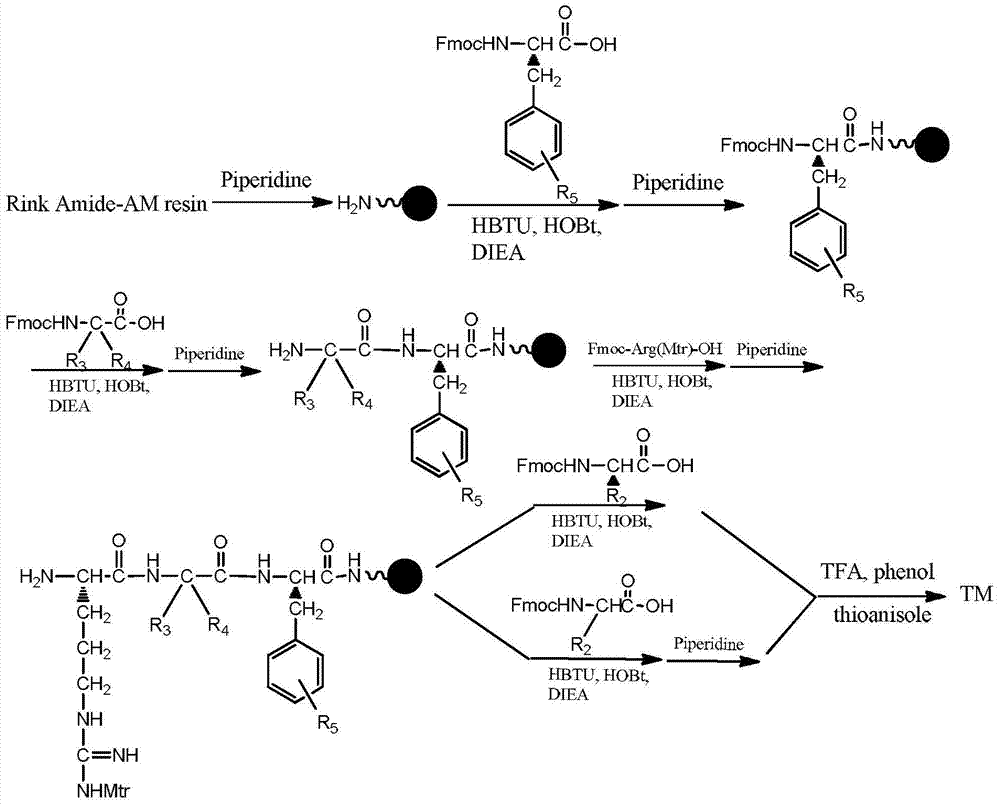
![7-heterocyclyl quinoline and thieno[2,3,-b] pyridine derivatives useful as antagonists of gonadotropin releasing hormone 7-heterocyclyl quinoline and thieno[2,3,-b] pyridine derivatives useful as antagonists of gonadotropin releasing hormone](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/dd659e67-22a5-4eca-a4ba-ed1a9e78f017/01822598.PNG)
![7-heterocyclyl quinoline and thieno[2,3,-b] pyridine derivatives useful as antagonists of gonadotropin releasing hormone 7-heterocyclyl quinoline and thieno[2,3,-b] pyridine derivatives useful as antagonists of gonadotropin releasing hormone](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/dd659e67-22a5-4eca-a4ba-ed1a9e78f017/A0182259800021.PNG)
![7-heterocyclyl quinoline and thieno[2,3,-b] pyridine derivatives useful as antagonists of gonadotropin releasing hormone 7-heterocyclyl quinoline and thieno[2,3,-b] pyridine derivatives useful as antagonists of gonadotropin releasing hormone](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/dd659e67-22a5-4eca-a4ba-ed1a9e78f017/A0182259800051.PNG)