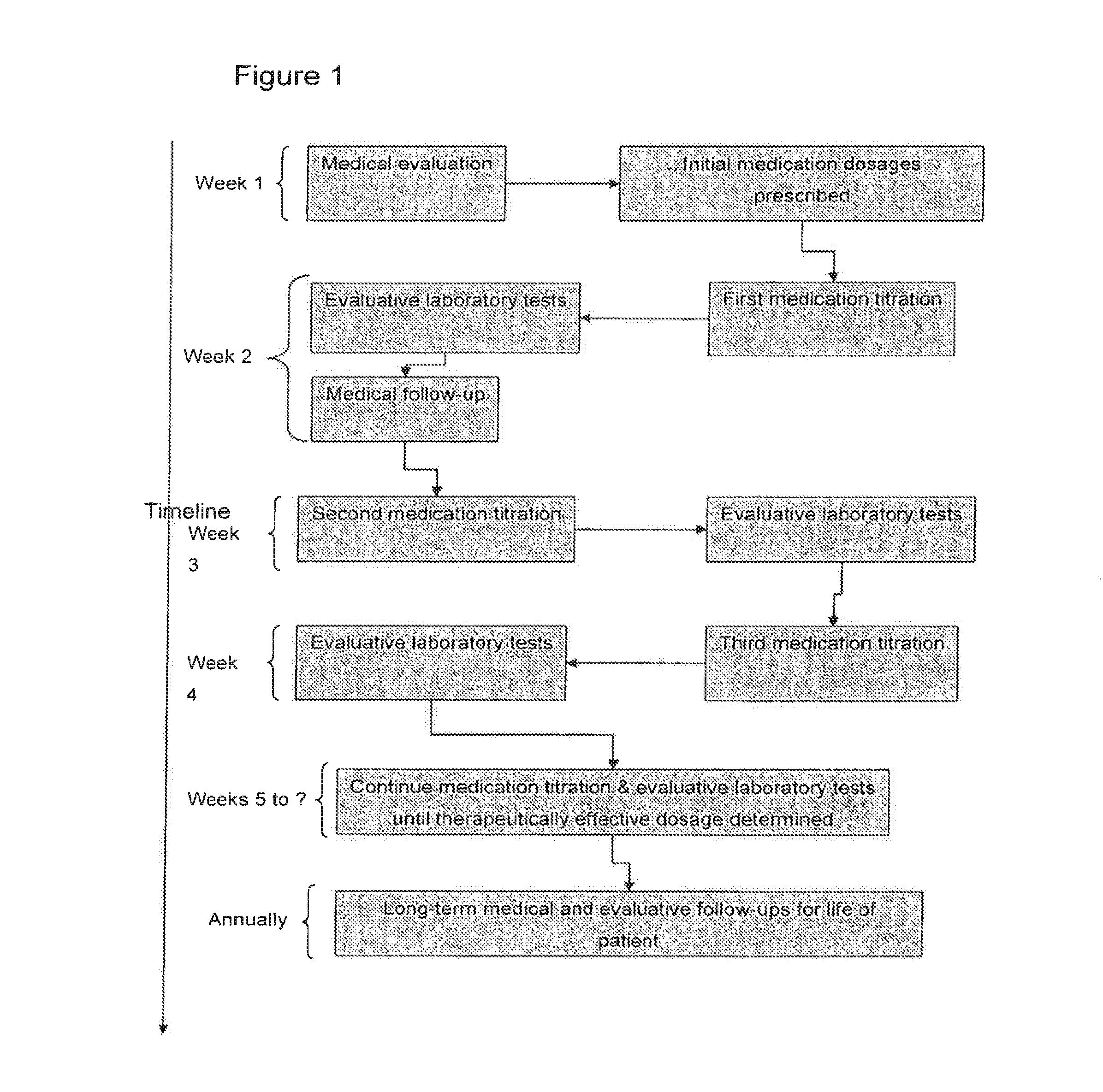
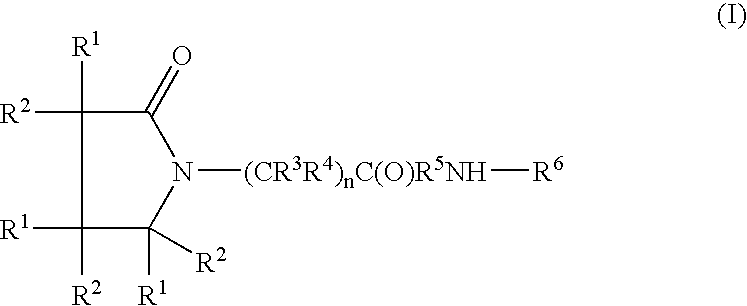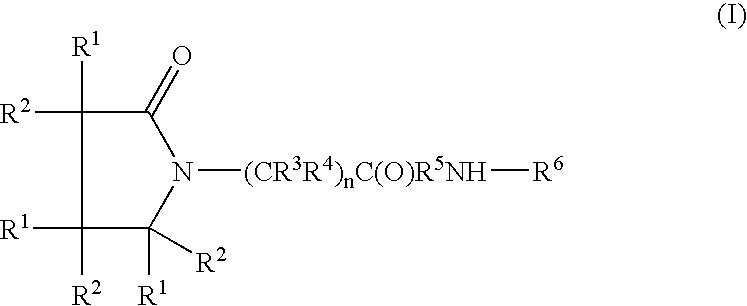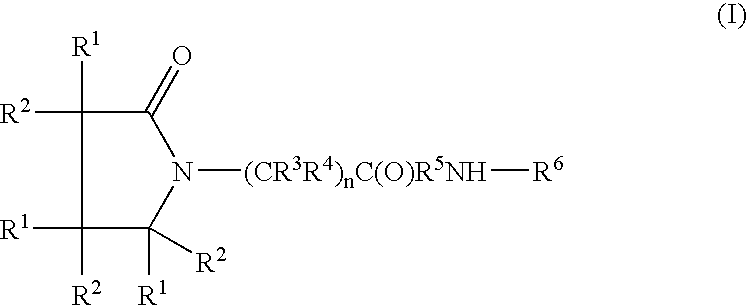Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
136 results about "Dense Core Vesicles" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Neurons very often make both a conventional neurotransmitter (such as glutamate, GABA or dopamine) and one or more neuropeptides. Peptides are generally packaged in large dense-core vesicles, and the co-existing neurotransmitters in small synaptic vesicles.
Neuropeptide Y receptor antagonists
Compounds of the structuresare useful for treating conditions related to an excess of neuropeptide Y including obesity and circulatory disorders.
Owner:PFIZER INC +1
Compound and method of treating neurogenic conditions using non-steroidal anti-inflammatory drug complexes
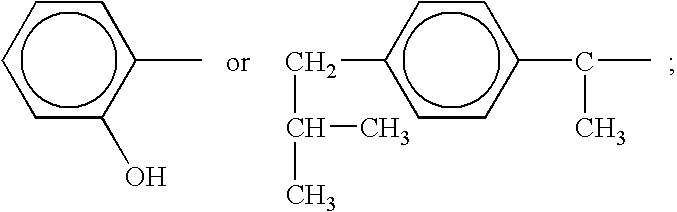
A complex is provided for the treatment of neurogenic conditions having the formula: where R1 is M is a metal ion Ca(II), Mg(II), Cu(II) or Ni(II); n is an integer 1 or 2; R is BBB peptide, transferrin, membrane transporter peptide, TAT peptide, bradykinin, beta-endorphin, bombesin, calcitonin, cholecystokinin, an enkephalin, dynorphin, insulin, gastrin, substance P, neurotensin, glucagon, secretin, somatostatin, motilin, vasopressin, oxytocin, prolactin, thyrotropin, an angiotensin, galanin, neuropeptide Y, thyrotropin-releasing hormone, gonadotropnin-releasing hormone, growth hormone-releasing hormone, luteinizing hormone, vasoactive intestinal peptidegluconate, L-lactate, L-leucine, L-tryptophan, and L-glutamate; and R is coupled to M through a carboxylate moiety. Magnesium (II) represents the preferred metal ion as magnesium is known to have neuroprotective effects. The metal ion is in part chelated by a non-steroidal anti-inflammatory drug that does not inhibit platelet activity and includes salicylate and ibuprofenate. The complex also includes a ligand operative in transport across the blood brain barrier. A process for making an inventive complex includes the stoichiometric addition of ligands containing carboxylate groups to a solution of the metal ion. In instances where the metal ion is magnesium (II), a stoichiometric ratio of 1:1:1 is found between the non-steroidal anti-inflammatory ligand:magnesium (II):transporter ligand.
Owner:MILLER LANDON C G
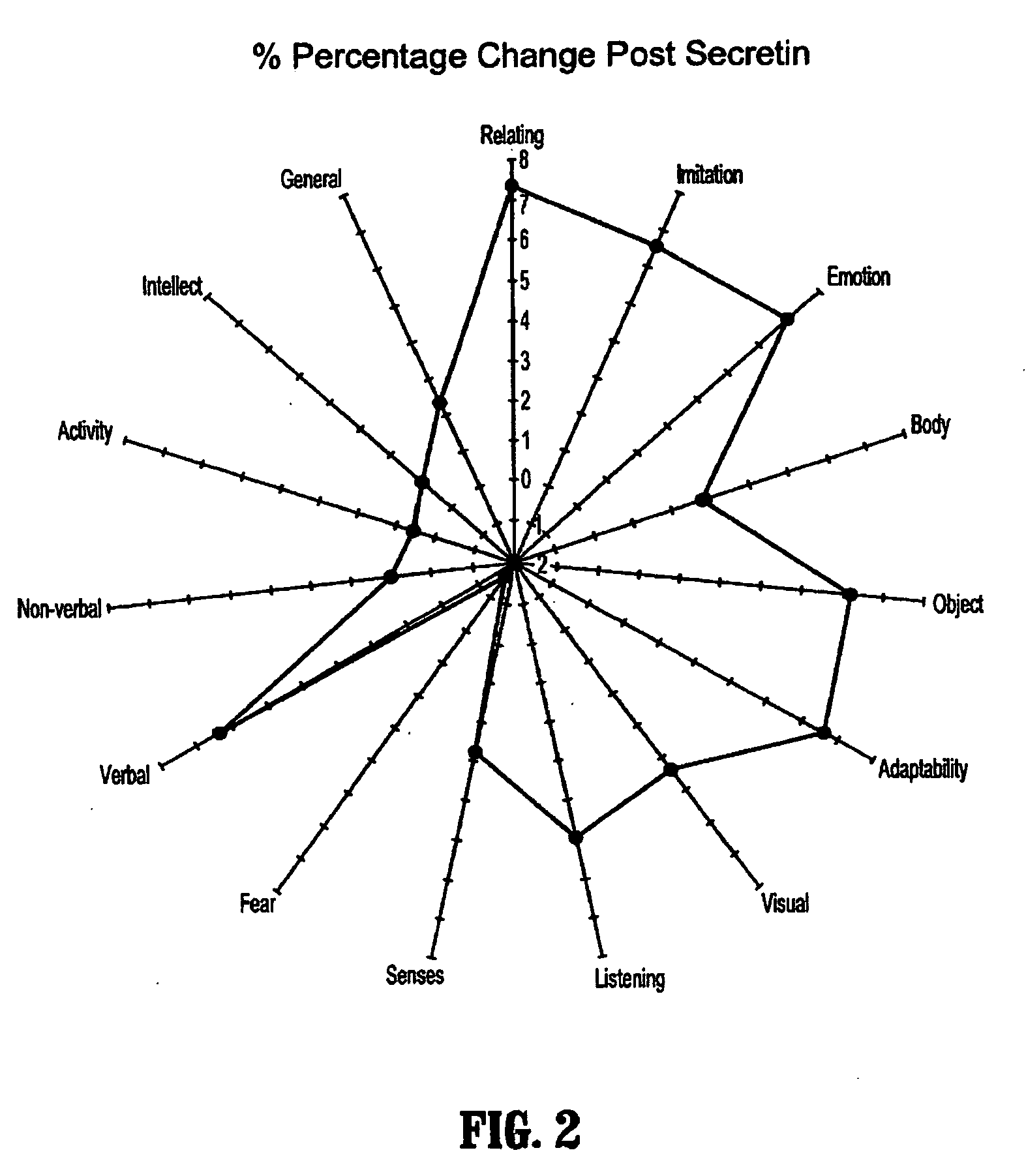
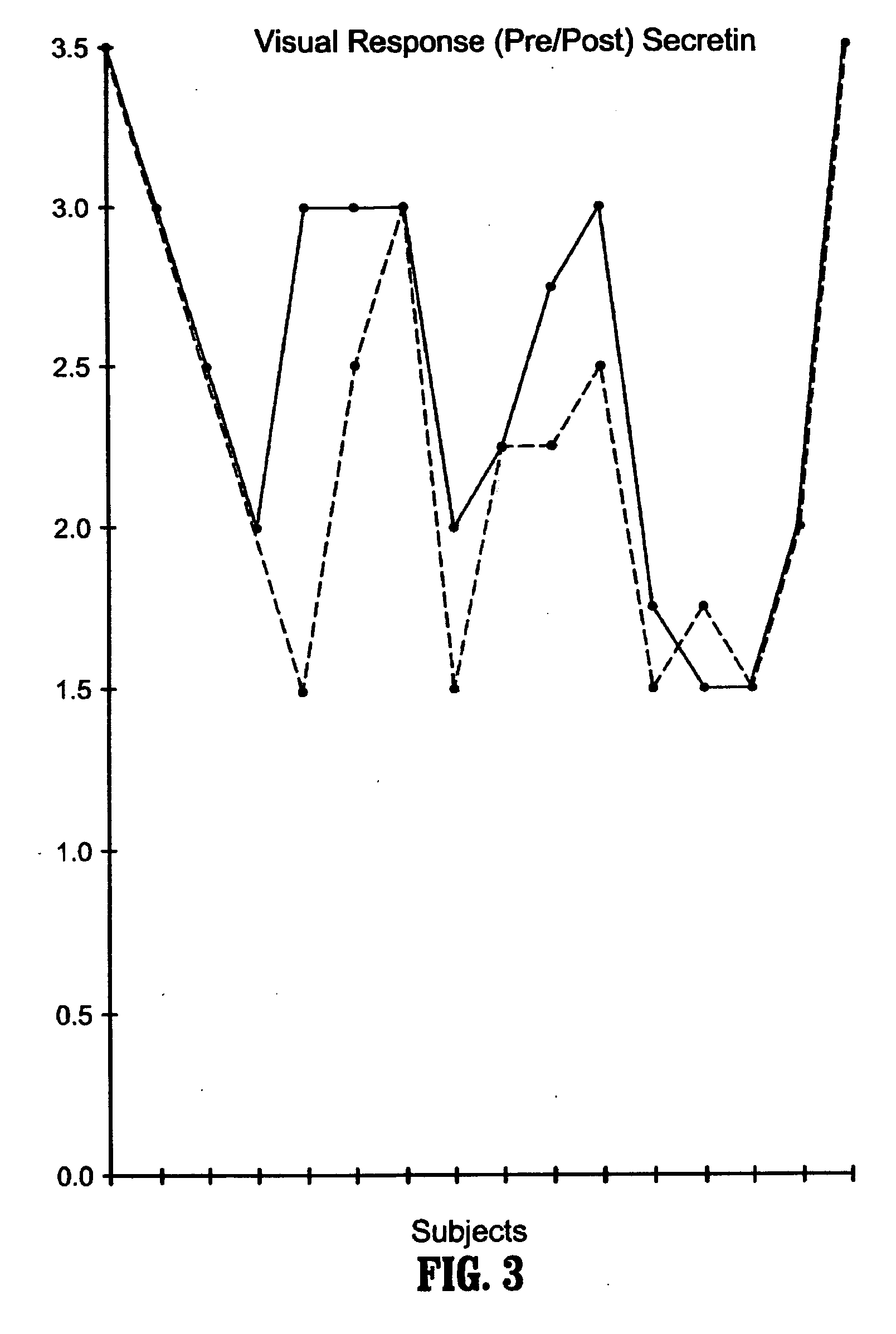
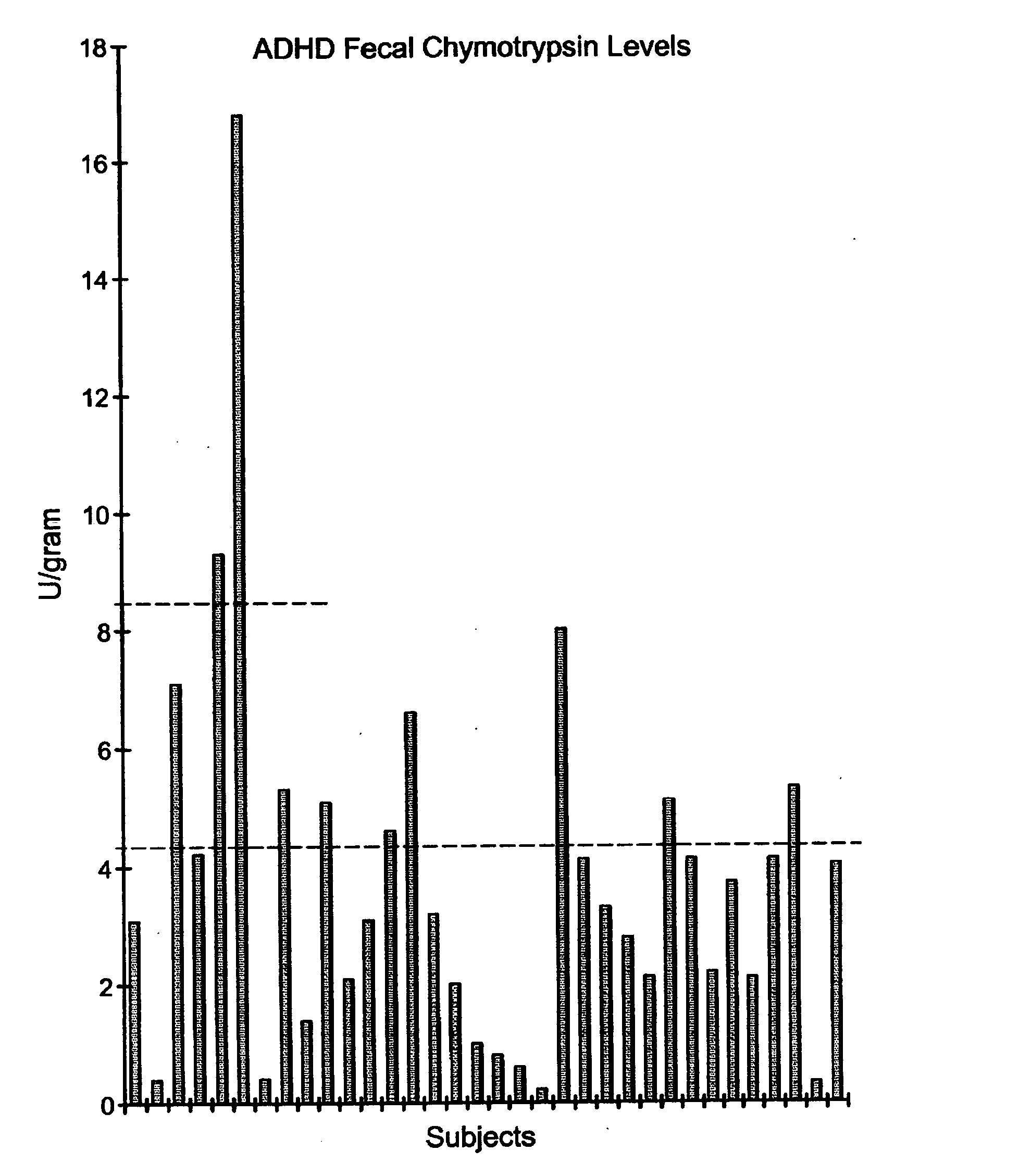
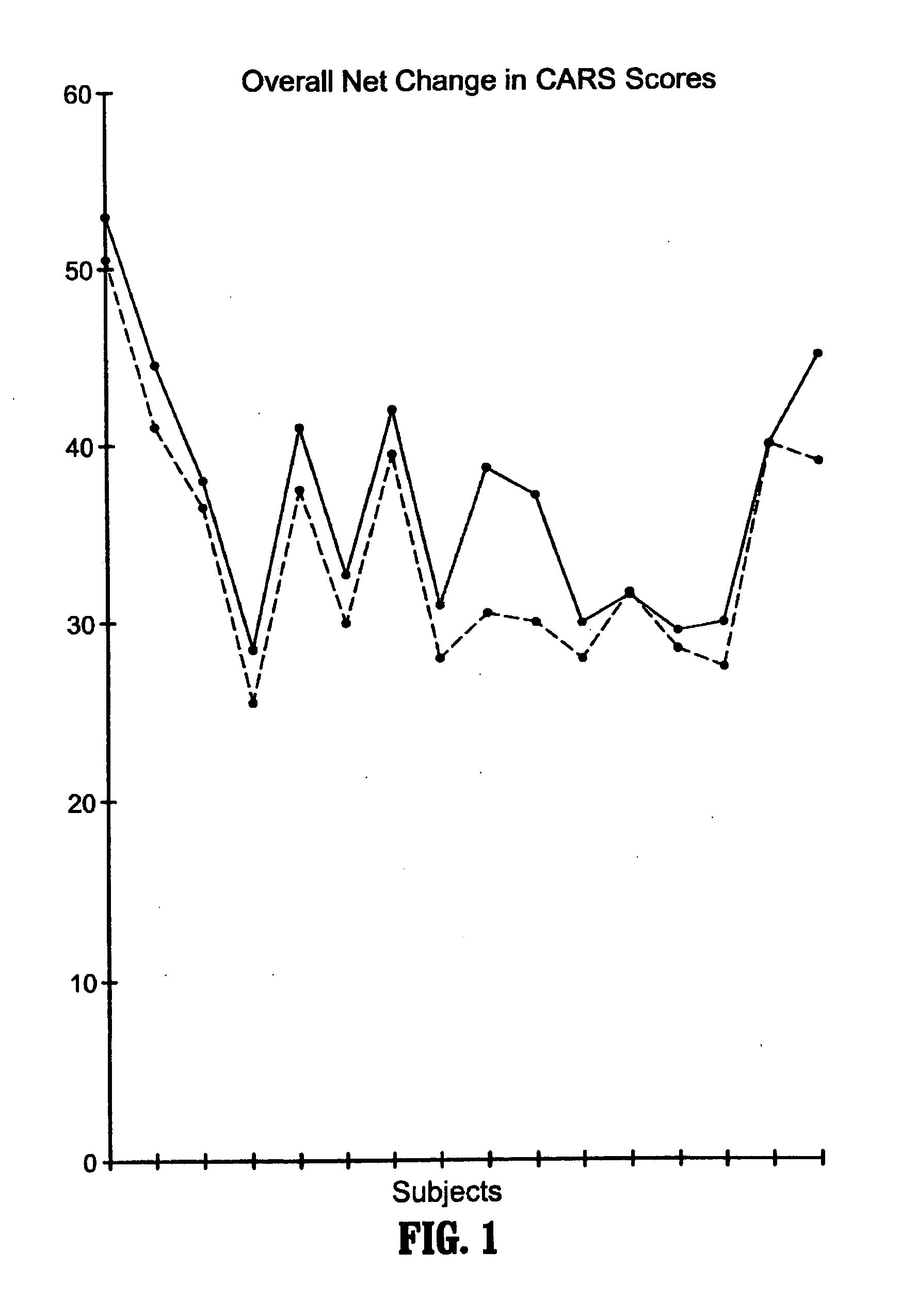
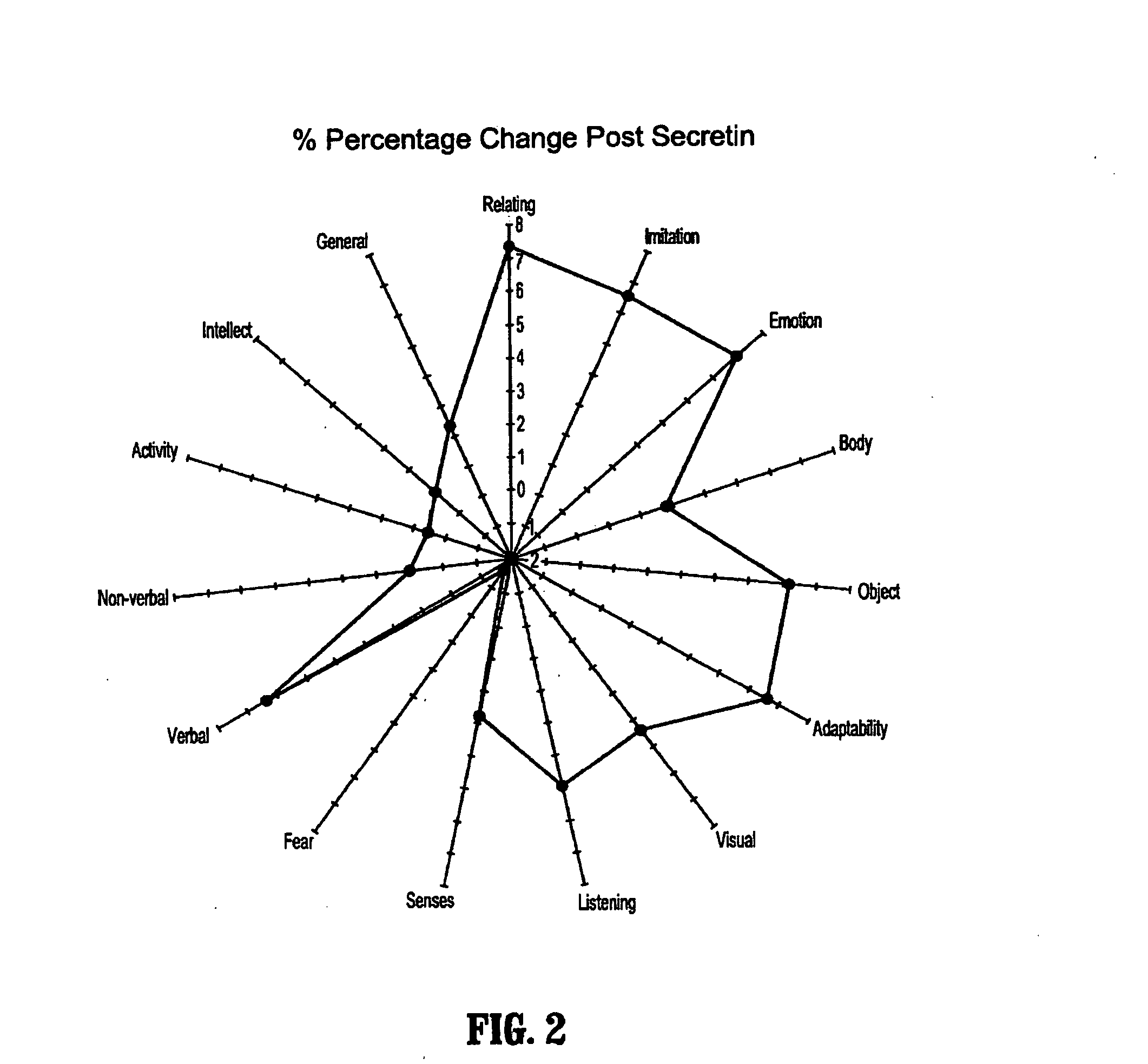
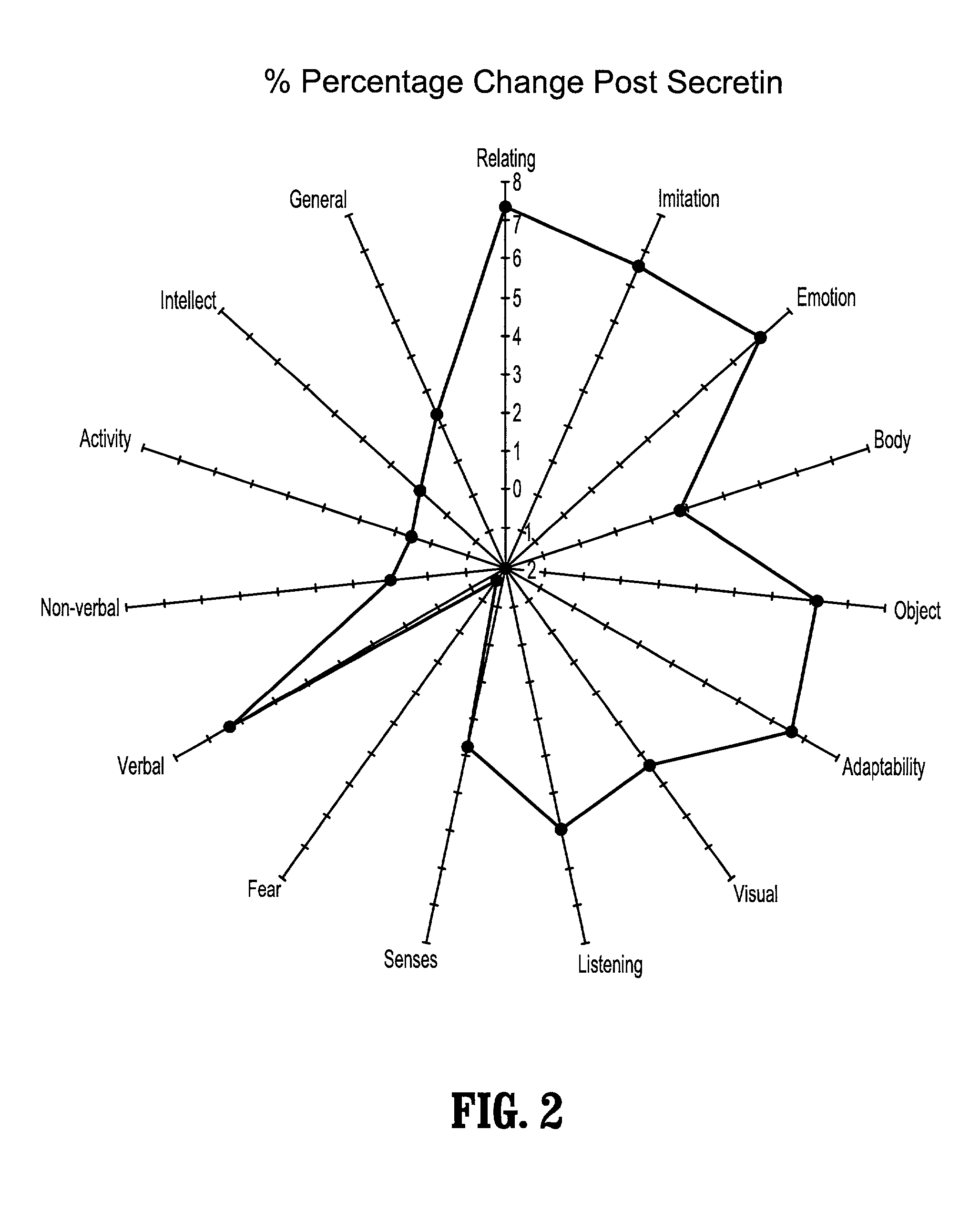
Methods for treating pervasive development disorders
InactiveUS20060183180A1Symptoms improvedPromote digestionNervous disorderPeptide/protein ingredientsDiseasePervasive developmental disorder
A method of utilizing the chymotrypsin level of an individual as a measure of the success of secretin, other neuropeptides, and peptides or digestive enzyme administration to such individuals, and in particular, as a prognosticative of potential secretin, other neuropeptides, peptides, and digestive enzyme administration for persons having ADHD, Autism and other PDD related disorders. In one aspect, a method for determining the efficacy of secretin, other neuropeptides, peptides, or digestive enzymes for the treatment of an individual diagnosed with a pervasive developmental disorder (PDD) comprises obtaining a sample of feces from an individual, determining a quantitative level of chymotrypsin present in the sample, and correlating the quantitative level of chymotrypsin determined to be present in the sample with the PDD to determine the efficacy of treating the individual with secretin, other neuropeptides, peptides, or digestive enzyme administration. In another aspect, a therapeutic method for treating an individual diagnosed with i PDD pervasive developmental disorder comprises determining the efficacy of secretin, other neuropeptides, peptides, and digestive enzyme administration for the treatment of the individual based on a measure of the individual's chymotrypsin level, and administering secretin, other neuropeptides, peptides, or digestive enzymes to the individual based on the determination of the measure of the individual's chymotrypsin level.
Owner:CUREMARK
Methods for treating pervasive development disorders
InactiveUS20060182728A1Symptoms improvedPromote digestionNervous disorderPeptide/protein ingredientsDiseasePervasive developmental disorder
A method of utilizing the chymotrypsin level of an individual as a measure of the success of secretin, other neuropeptides, and peptides or digestive enzyme administration to such individuals, and in particular, as a prognosticative of potential secretin, other neuropeptides, peptides, and digestive enzyme administration for persons having ADHD, Autism and other PDD related disorders. In one aspect, a method for determining the efficacy of secretin, other neuropeptides, peptides, or digestive enzymes for the treatment of an individual diagnosed with a pervasive developmental disorder (PDD) comprises obtaining a sample of feces from an individual, determining a quantitative level of chymotrypsin present in the sample, and correlating the quantitative level of chymotrypsin determined to be present in the sample with the PDD to determine the efficacy of treating the individual with secretin, other neuropeptides, peptides, or digestive enzyme administration. In another aspect, a therapeutic method for treating an individual diagnosed with i PDD pervasive developmental disorder comprises determining the efficacy of secretin, other neuropeptides, peptides, and digestive enzyme administration for the treatment of the individual based on a measure of the individual's chymotrypsin level, and administering secretin, other neuropeptides, peptides, or digestive enzymes to the individual based on the determination of the measure of the individual's chymotrypsin level.
Owner:CUREMARK
Methods of treating pervasive development disorders
InactiveUS20020090653A1Symptoms improvedPromote digestionPeptide/protein ingredientsMicrobiological testing/measurementDiseaseFeces
A method of utilizing the chymotrypsin level of an individual as a measure of the success of secretin, other neuropeptides, and peptides or digestive enzyme administration to such individuals, and in particular, as a prognosticative of potential secretin, other neuropeptides, peptides, and digestive enzyme administration for persons having ADD, ADHD, Autism and other PDD related disorders. In one aspect, a method for determining the efficacy of secretin, other neuropeptides, peptides, or digestive enzymes for the treatment of an individual diagnosed with a pervasive developmental disorder (PDD) comprises obtaining a sample of feces from an individual, determining a quantitative level of chymotrypsin present in the sample, and correlating the quantitative level of chymotrypsin determined to be present in the sample with the PDD to determine the efficacy of treating the individual with secretin, other neuropeptides, peptides, or digestive enzyme administration. In another aspect, a therapeutic method for treating an individual diagnosed with a PDD pervasive developmental disorder comprises determining the efficacy of secretin, other neuropeptides, peptides, and digestive enzyme administration for the treatment of the individual based on a measure of the individual's chymotrypsin level, and administering secretin, other neuropeptides, peptides, or digestive enzymes to the individual based on the determination of the measure of the individual's chymotrypsin level.
Owner:CUREMARK
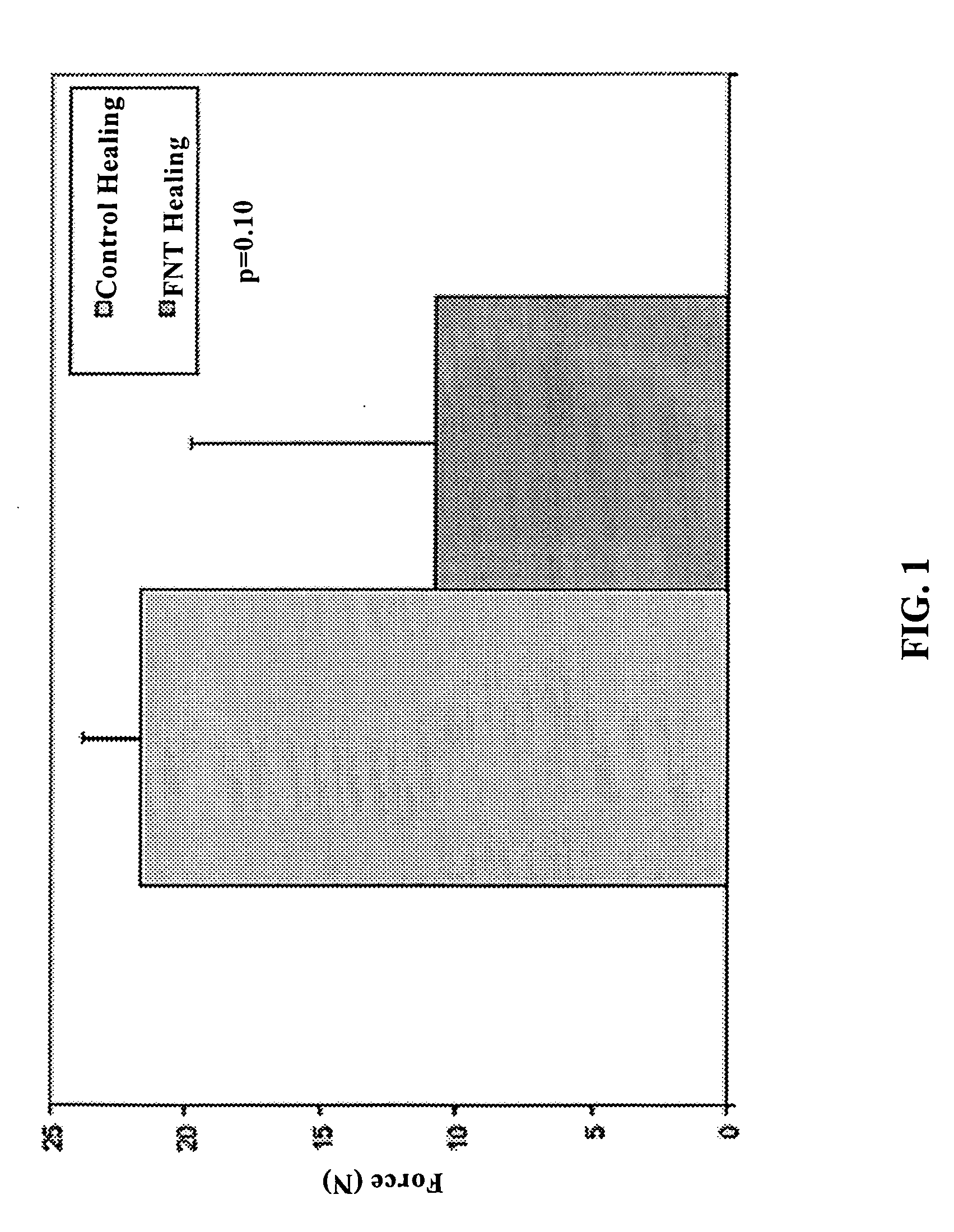
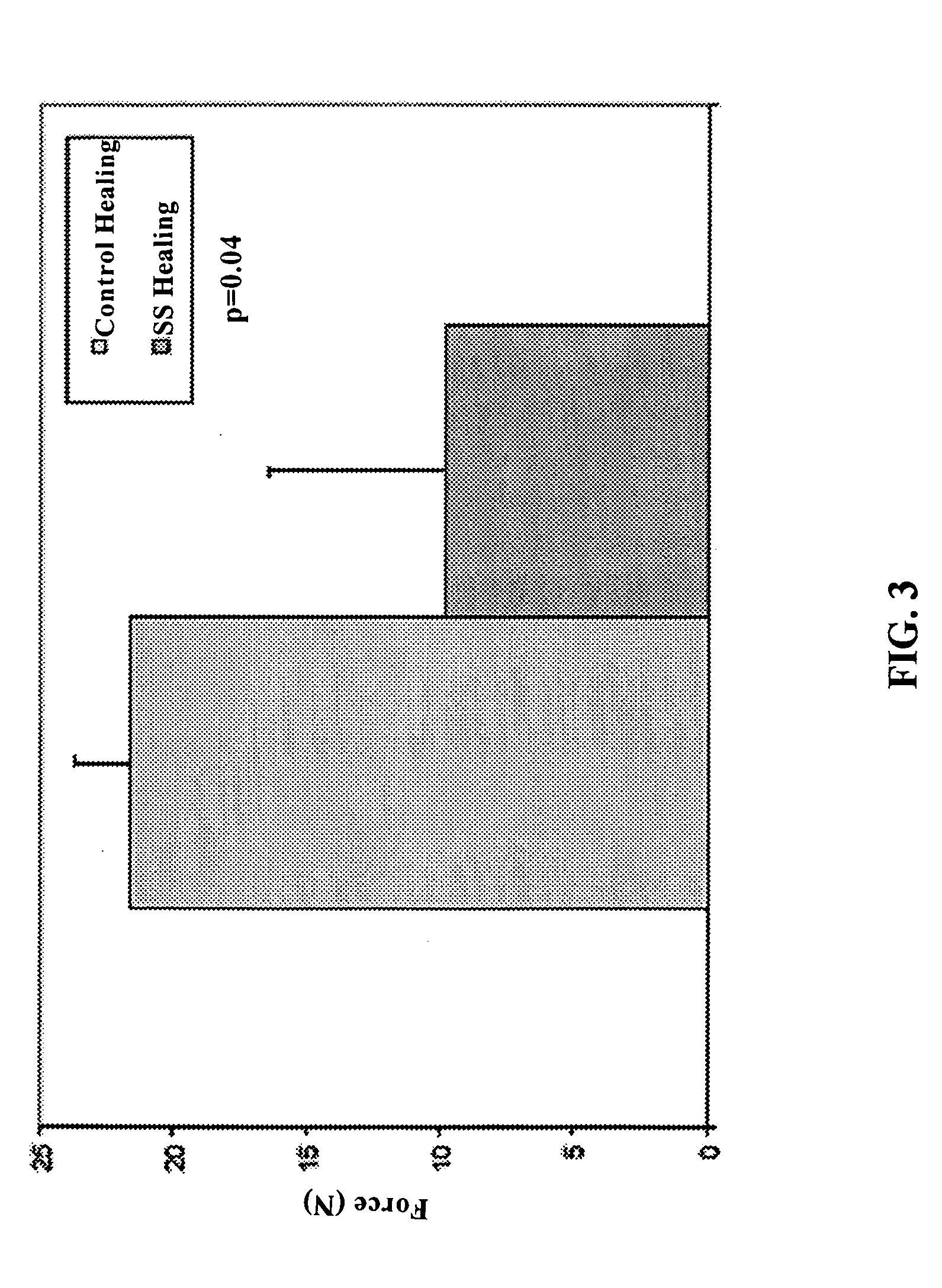
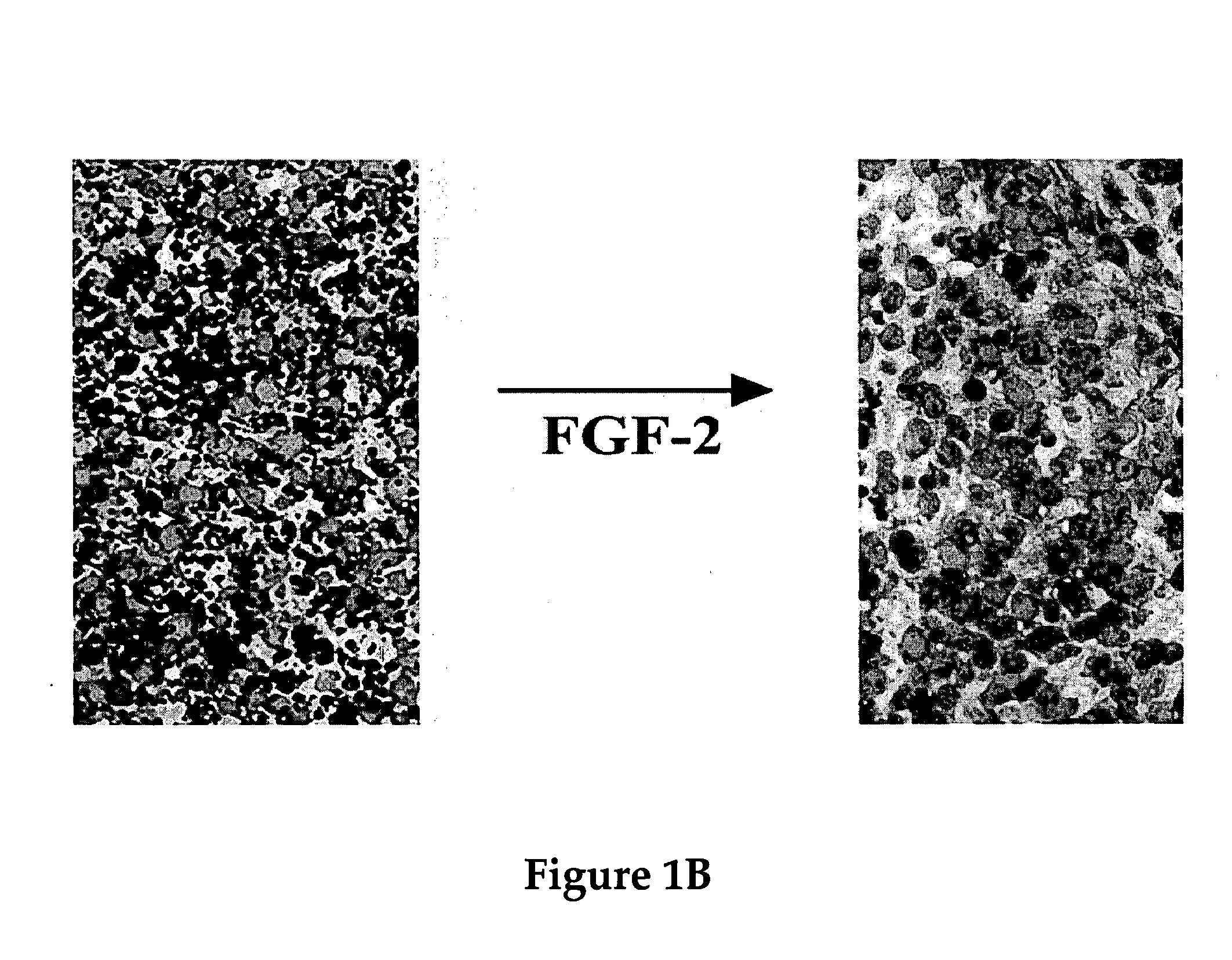
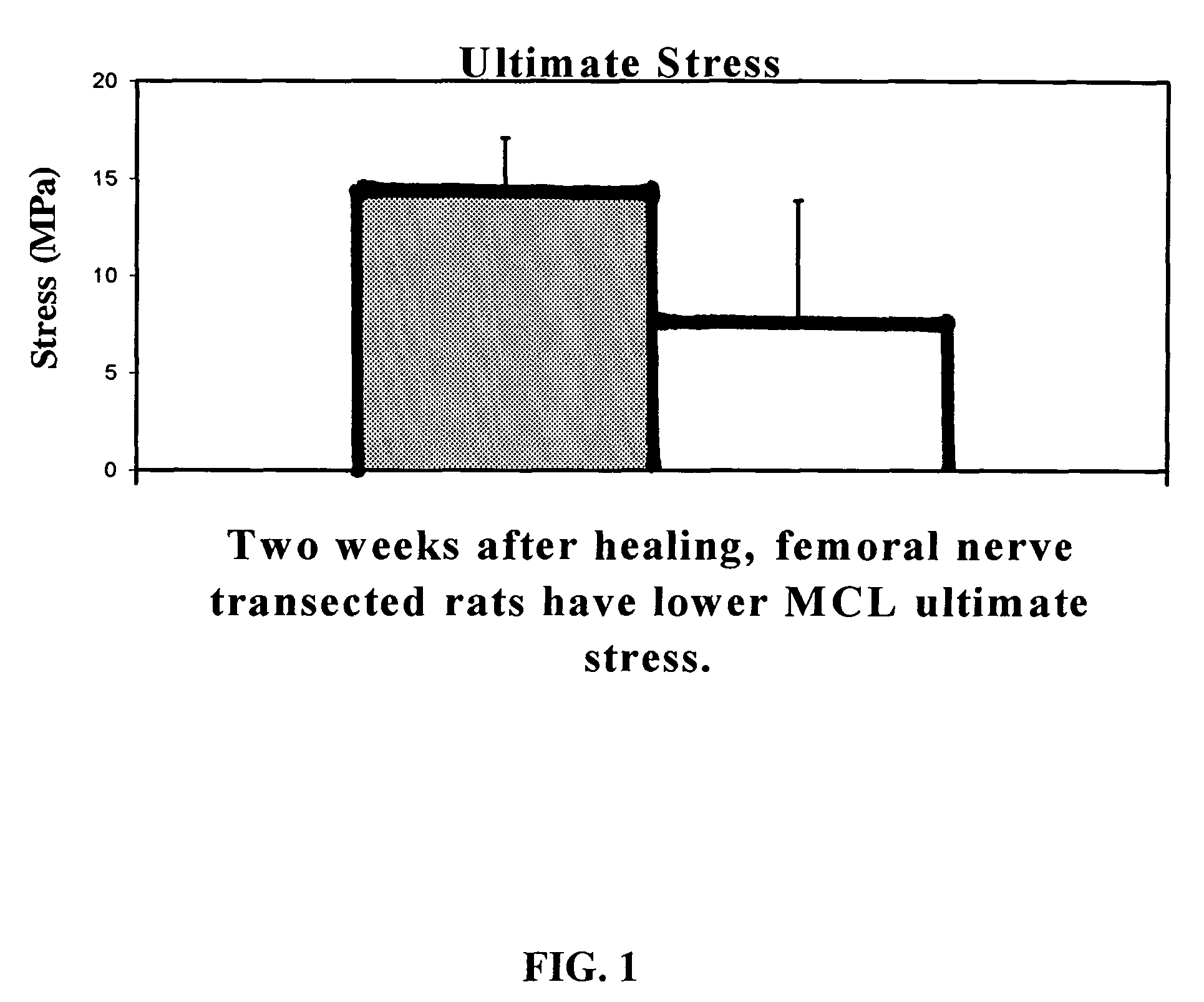
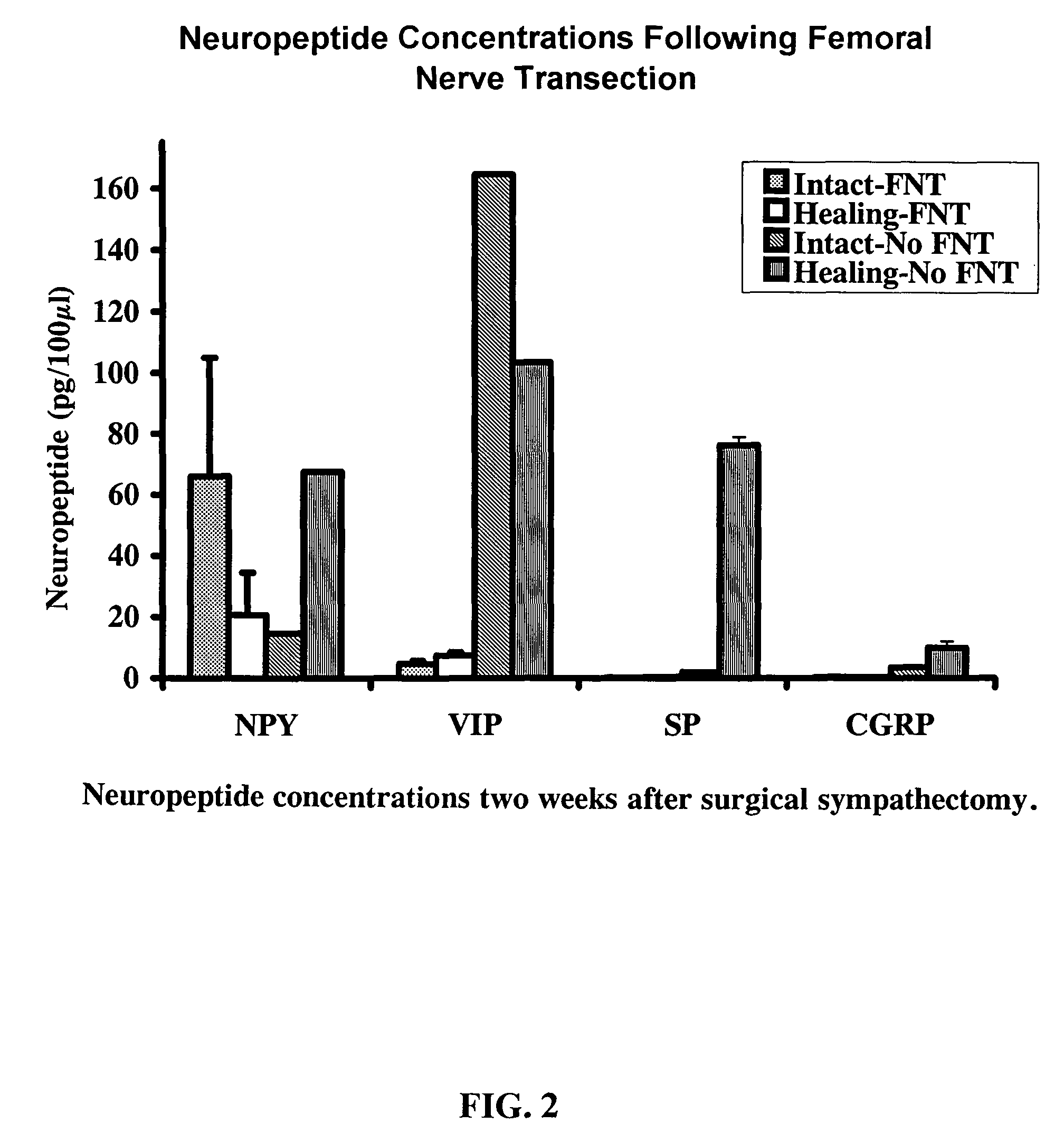
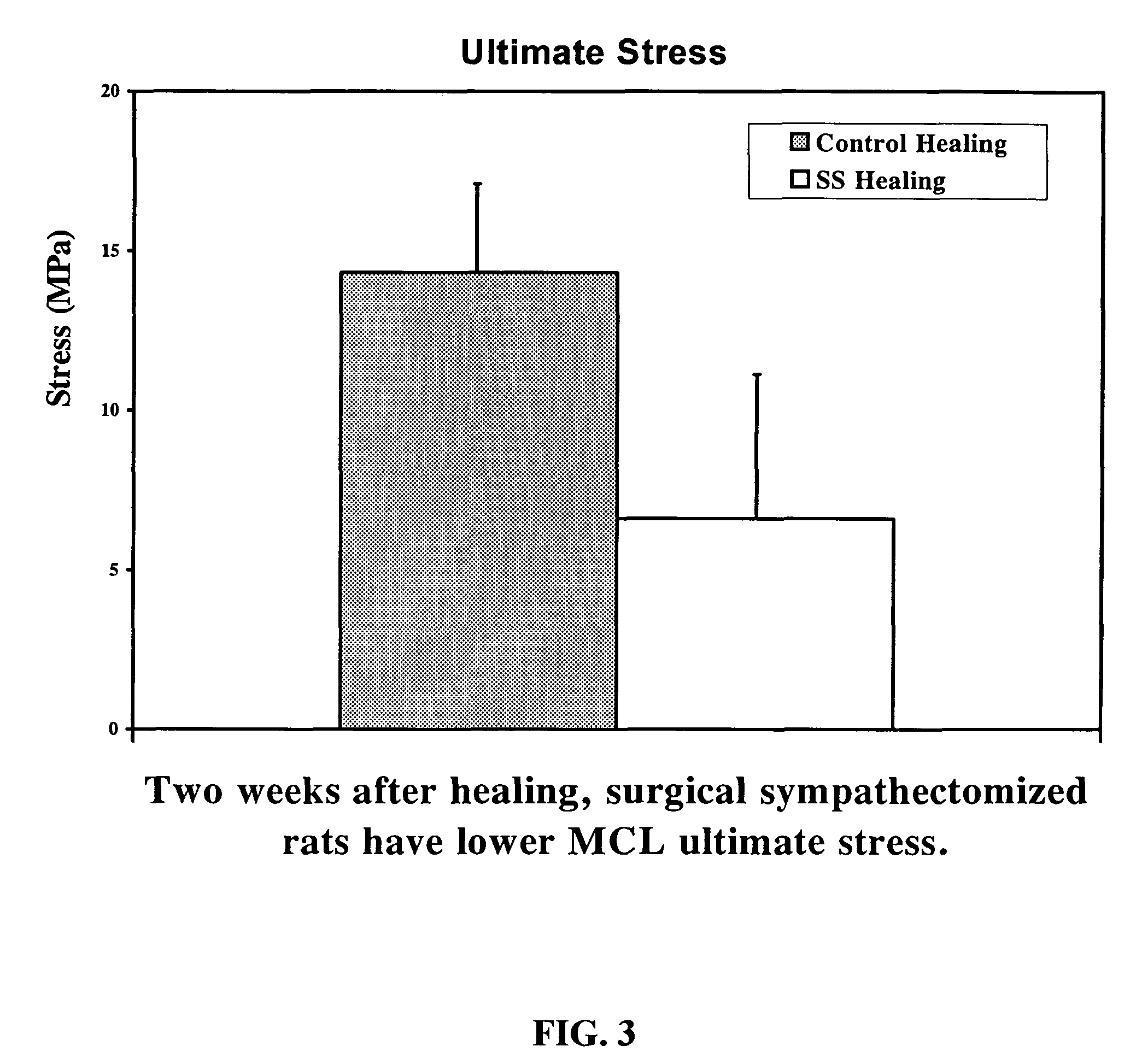
Use of neuropeptides for ligament, cartilage, and bone healing
Disclosed are a method and a corresponding pharmaceutical composition for treating damaged cartilage and subchondral bone. Neurogenic compounds in general and neuropeptides in particular have been found to be highly effective in stimulated repair of cartilage and bone damaged due to traumatic injury, ligament disease, and disuse. Preferred active ingredients for use in the method and corresponding pharmaceutical composition include calcitonin gene-related peptide (CGRP), cholecystokinin (CCK), dynorphin, enkephalin, galanin, neuropeptide Y (NPY), neurotensin, somatostatin, substance P (SP), thyrotropin-releasing hormone (TRH), vasoactive intestinal peptide (VIP).
Owner:WISCONSIN ALUMNI RES FOUND
Aminobutyramide conjugate and a pharmaceutical composition for treatment of neuronal disorders
ActiveUS20060058219A1Improve efficiencyEliminate side effectsBiocidePeptide/protein ingredientsTryptophanSaccharin
A compound is provided that has the formula NH2CH2CH2CH2C(O)N—R (I) where R is a moiety capable of crossing the blood brain barrier and is as a free compound serotonin, dopamine blood brain barrier (BBB) peptide, membrane translocating protein, TAT peptides, bradykinin, beta-endorphin, bombesin, calcitonin, cholecystokinin, an enkephalin, dynorphin, insulin, gastrin, substance P, neurotensin, glucagon, secretin, somatostatin, motilin, vasopressin, oxytocin, prolactin, thyrotropin, an angiotensin, galanin, neuropeptide Y, thyrotropin-releasing hormone, gonadotropnin-releasing hormone, growth hormone-releasing hormone, luteinizing hormone, vasoactive intestinal peptidetransferrin, glucosylamnine, amino saccharin, lactylamine, leucine, tryptophan, glutamate and amino cholines.
Owner:MILLER LANDON C G
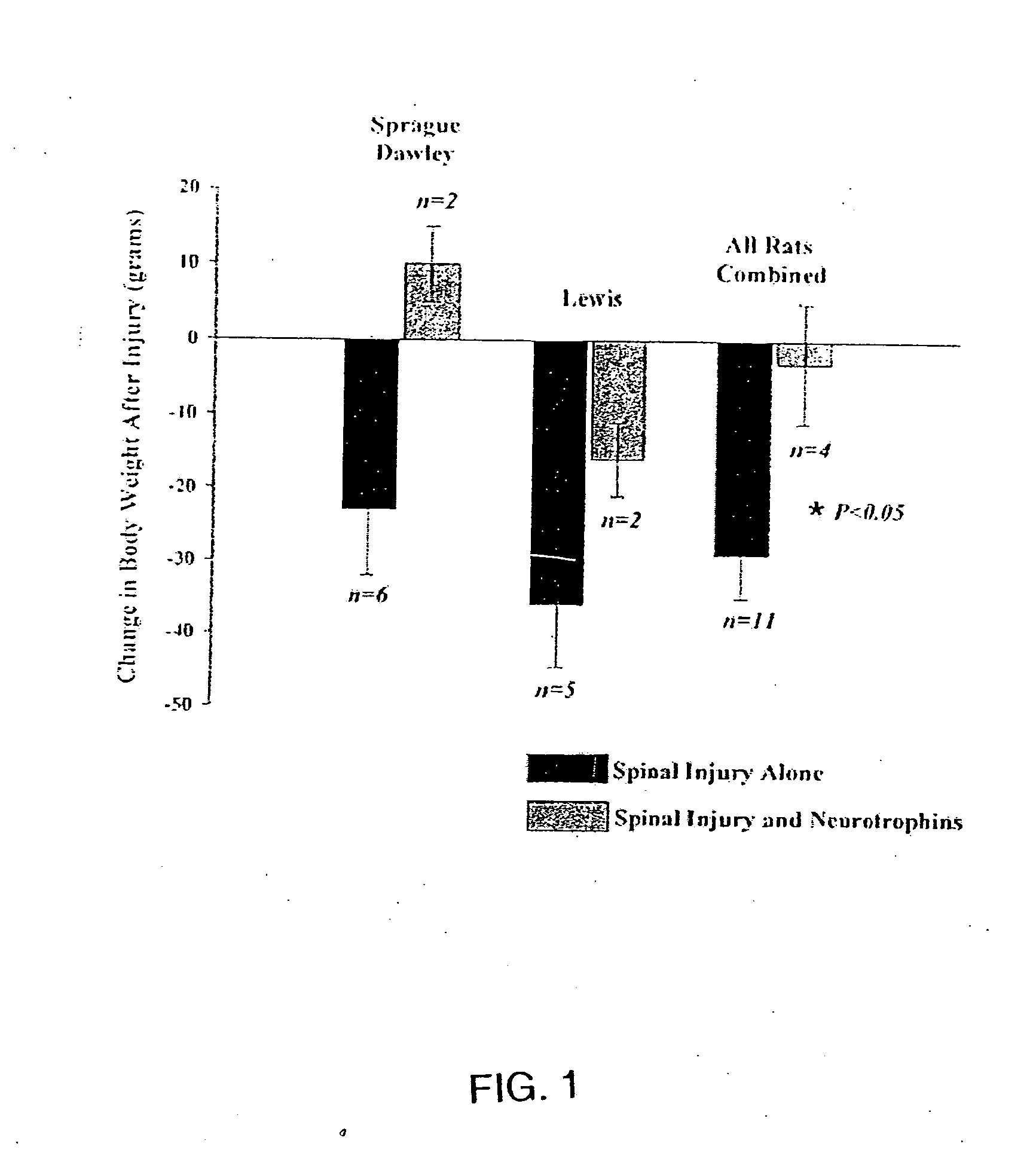
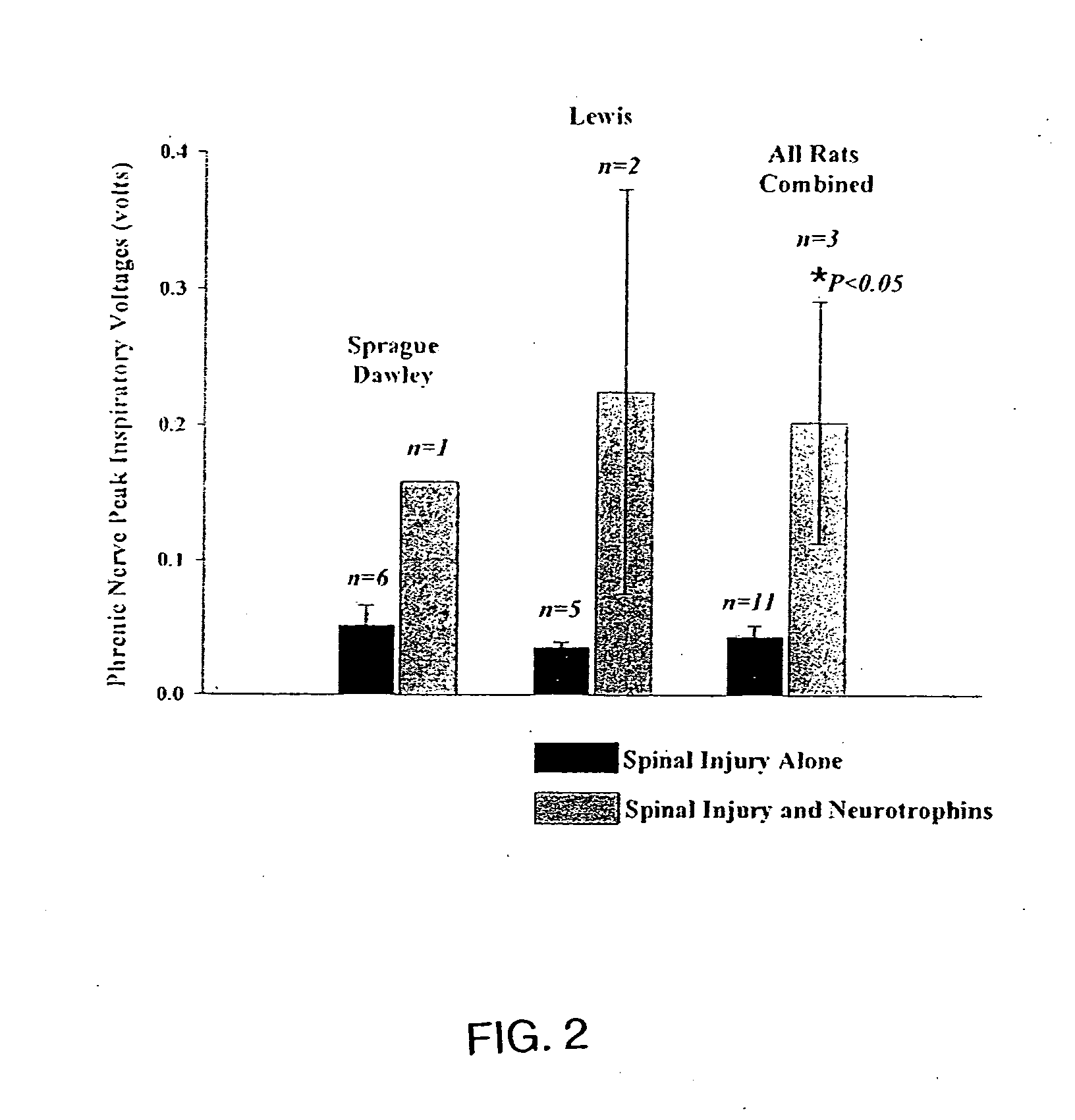
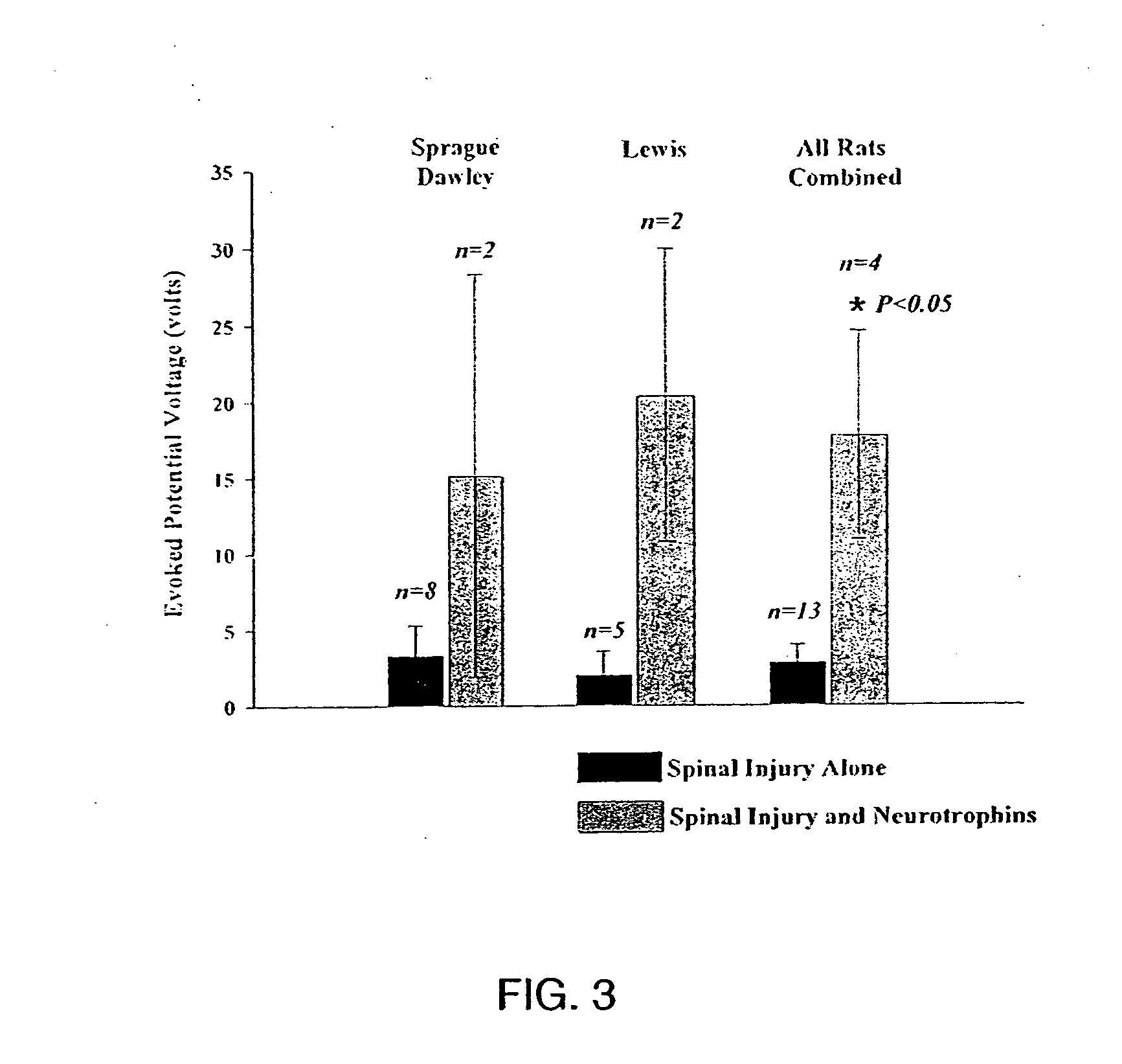
Trophic factor combinations for nervous system treatment
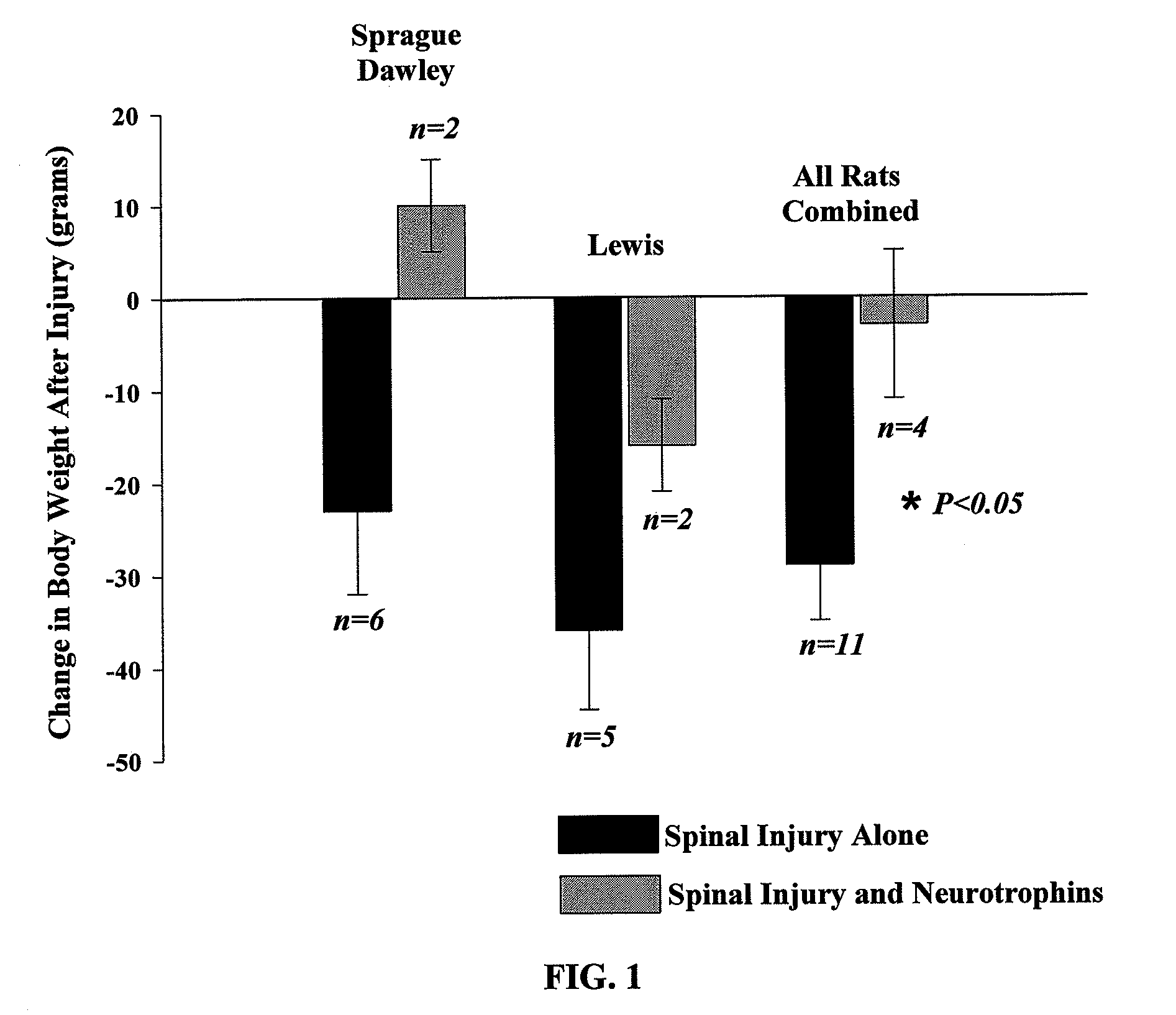
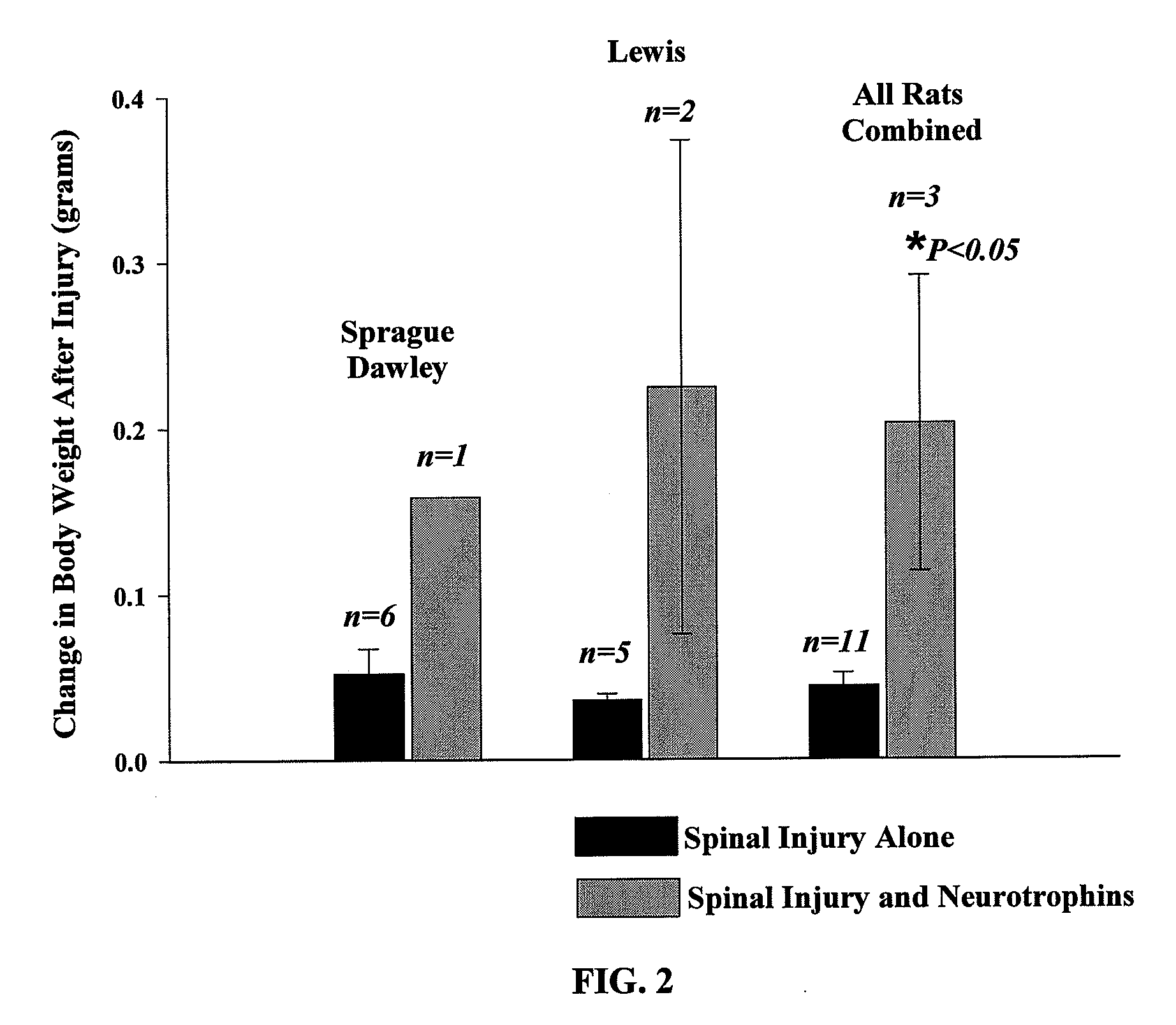
The present invention relates to a composition including an effective amount of at least one of an antimicrobial peptide and a substance having an antimicrobial peptide effect and an effective amount of a neurotrophin. The composition can also include an effective amount of at least one of a growth factor and a neuropeptide. The present invention also relates a method of treating an injury to a nervous system of an animal that includes the steps of identifying the injury to the nervous system and applying to the injury an effective amount of at least one of antimicrobial peptide and a substance having an antimicrobial peptide effect. The method can also include applying an effective amount of one or more trophic factors selected from the group consisting of a growth factor, a neurotrophin, and a neuropeptide to the injury.
Owner:WISCONSIN ALUMNI RES FOUND
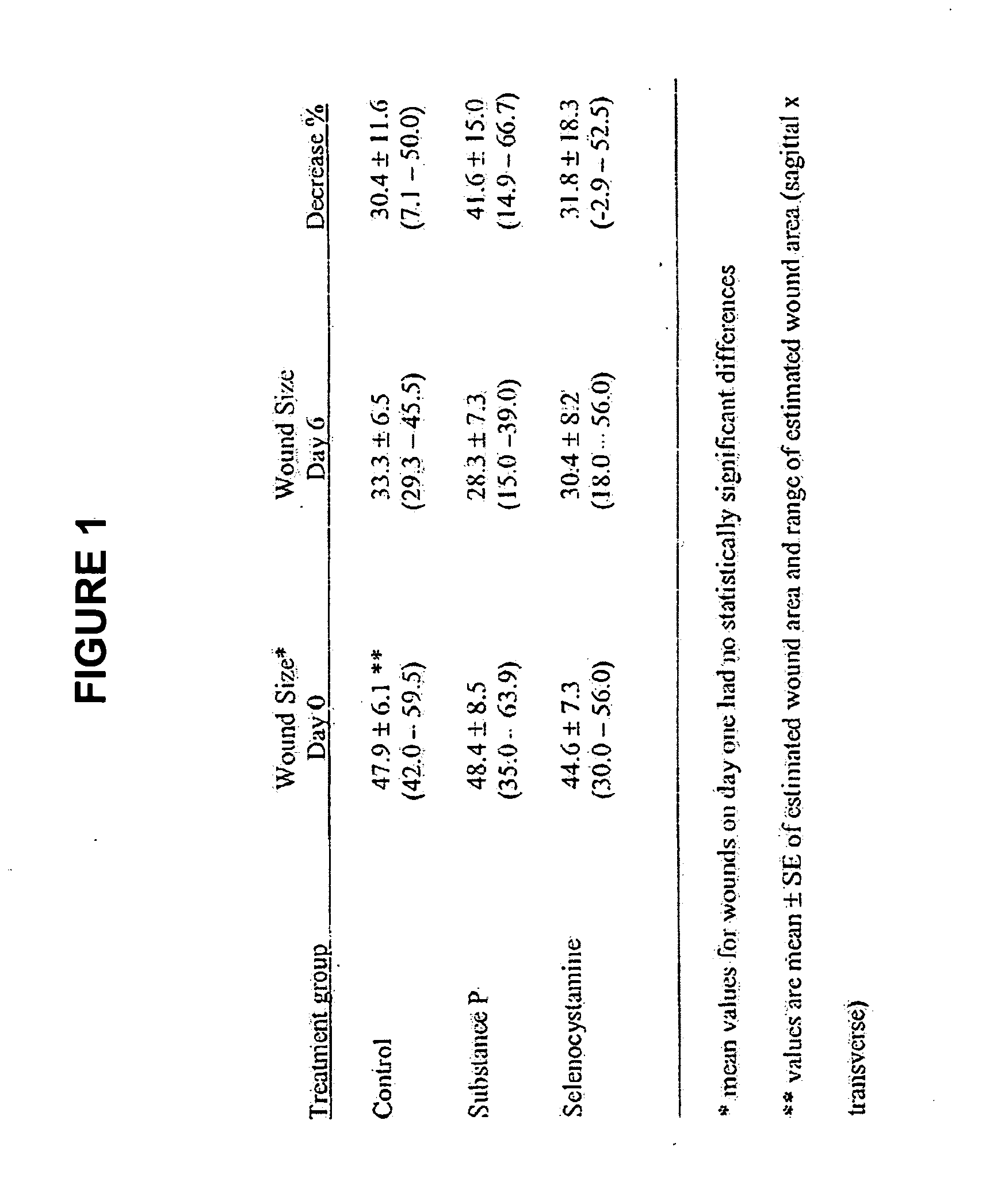
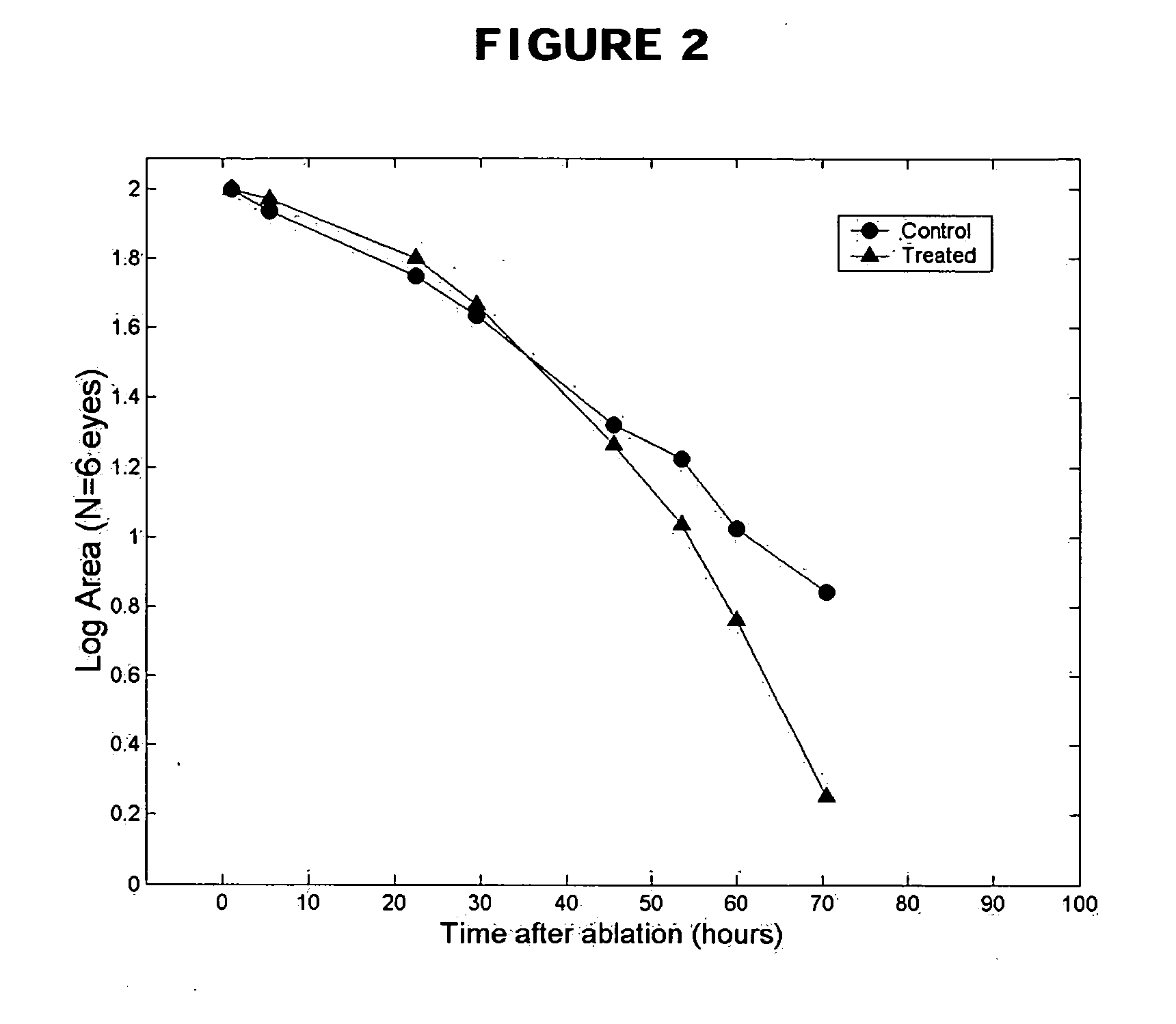
Methods and compositions using Substance P to promote wound healing
InactiveUS20070154448A1Promote healingGrowth promoting activityOrganic active ingredientsBiocideSubstance KMammalian tissue
Healing of wounds in mammalian tissue may be enhanced by the application of certain neuropeptides, optionally in combination with known growth promoting hormones. Exemplary neuropeptides include tachykinins, such as Substance P, Substance K, and the like, as well as calcitonin gene-related peptides. The compositions may further include a polymeric delivery carrier and are utilized by applying to the site of the wound. Wounds may be vascular or avascular wounds. The compositions promote elaboration of cellular matrices and development of cellular attachment mechanisms in addition to stimulating cellular proliferation.
Owner:AUXANO BIOLOGICS
Treatment of male sexual dysfunction
InactiveUS20030119714A1Reducing and eliminating prospectDrop in blood pressureBiocideDisease diagnosisNPY-Y1 receptorMale genitalia
The use of an inhibitor of a neuropeptide Y (NPY), preferably of a NPY Y1 receptor, which inhibitor is selective for an NPY or NPY Y1 receptor associated with male genitalia, in the preparation / manufacture of a medicament for the treatment or prevention of male erectile dysfunction (MED).
Owner:PFIZER INC
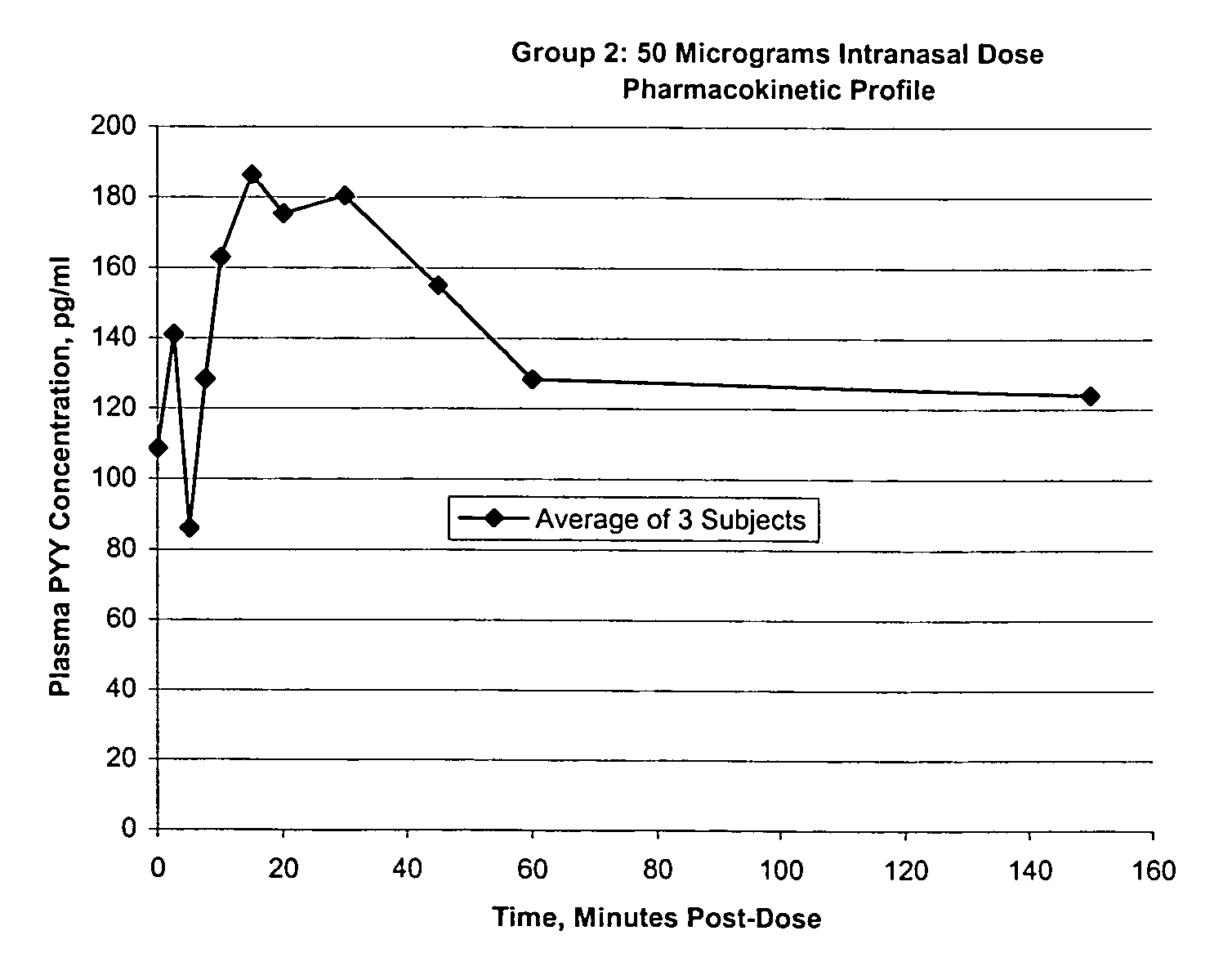
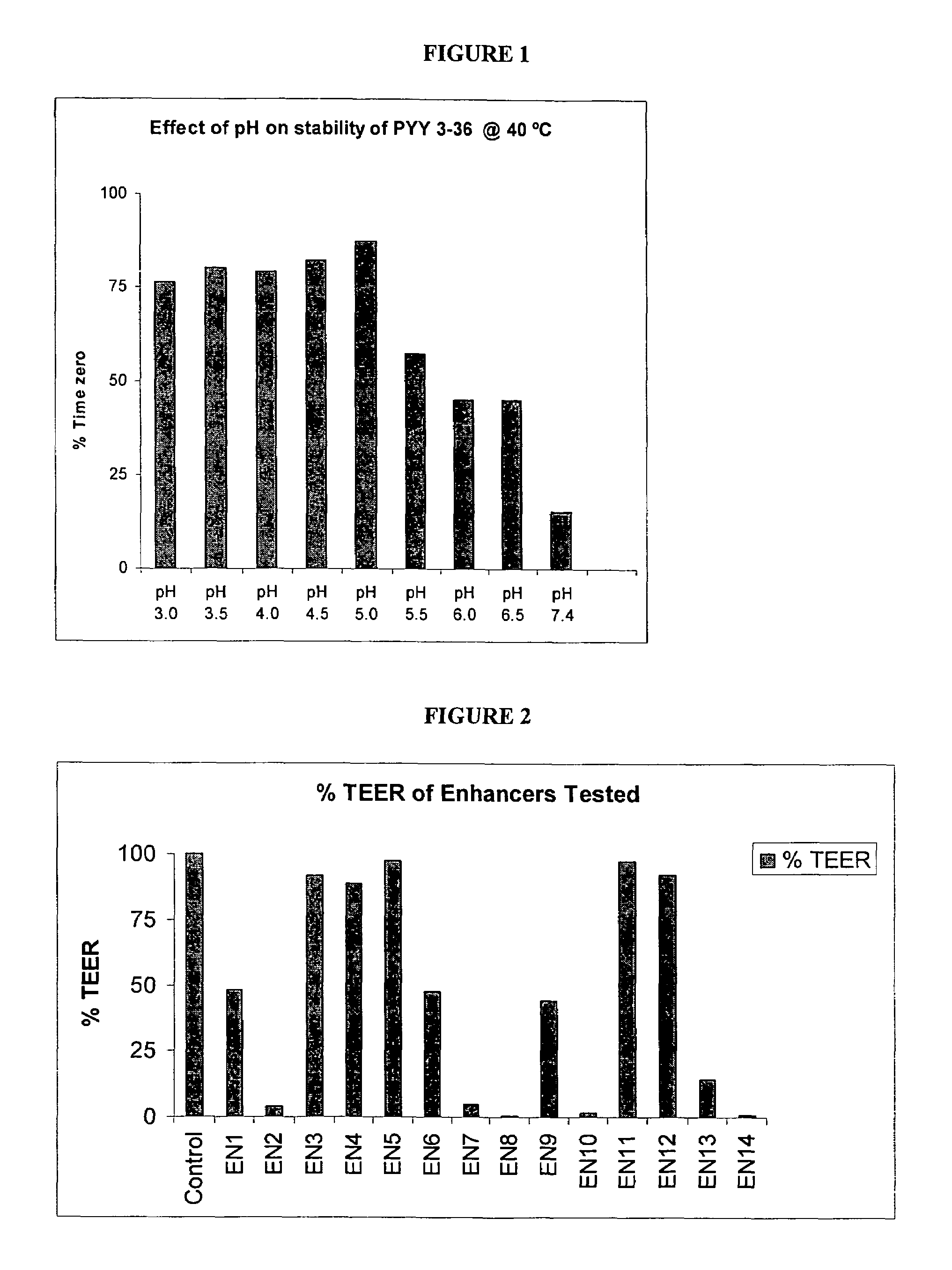
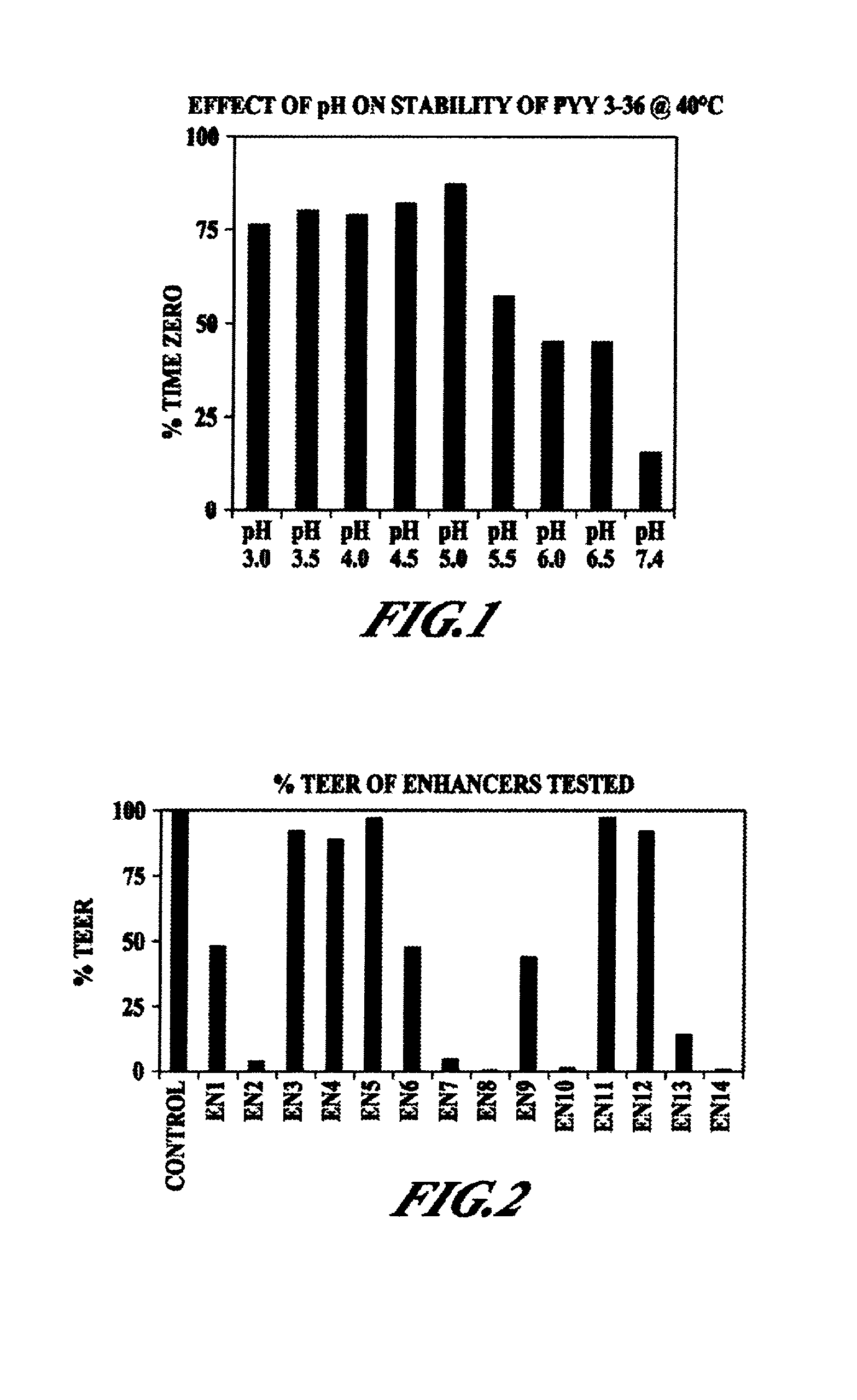
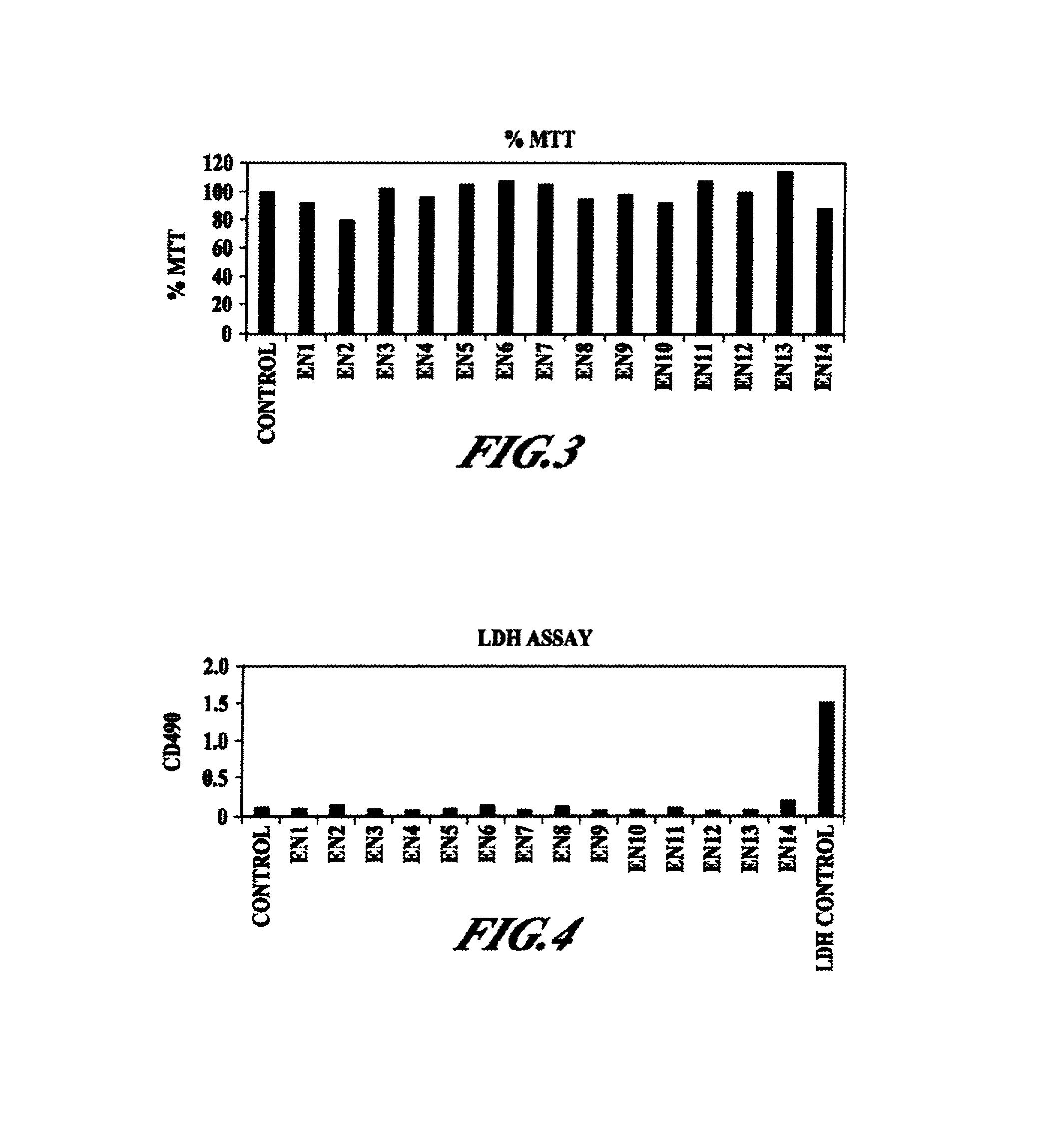
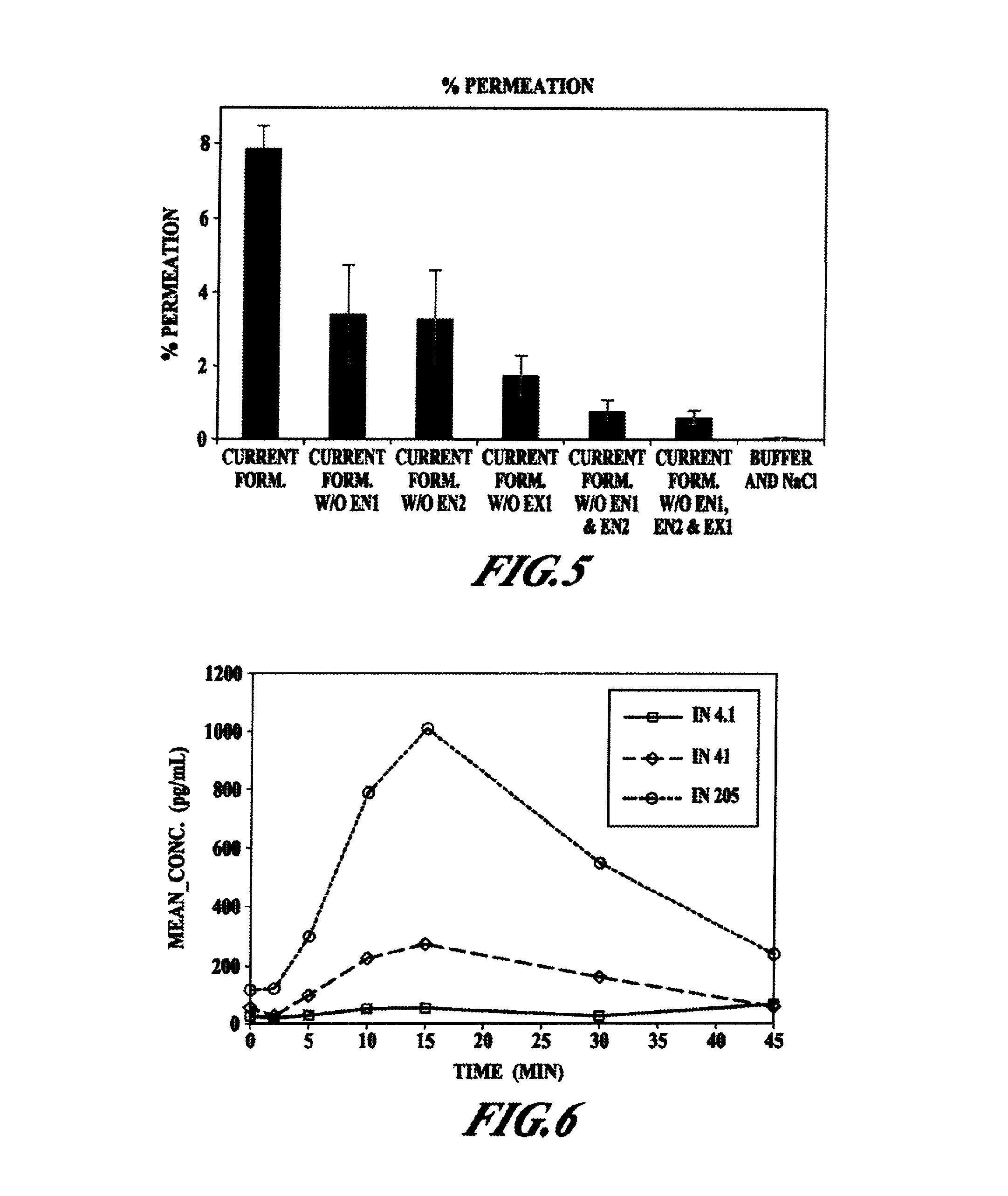
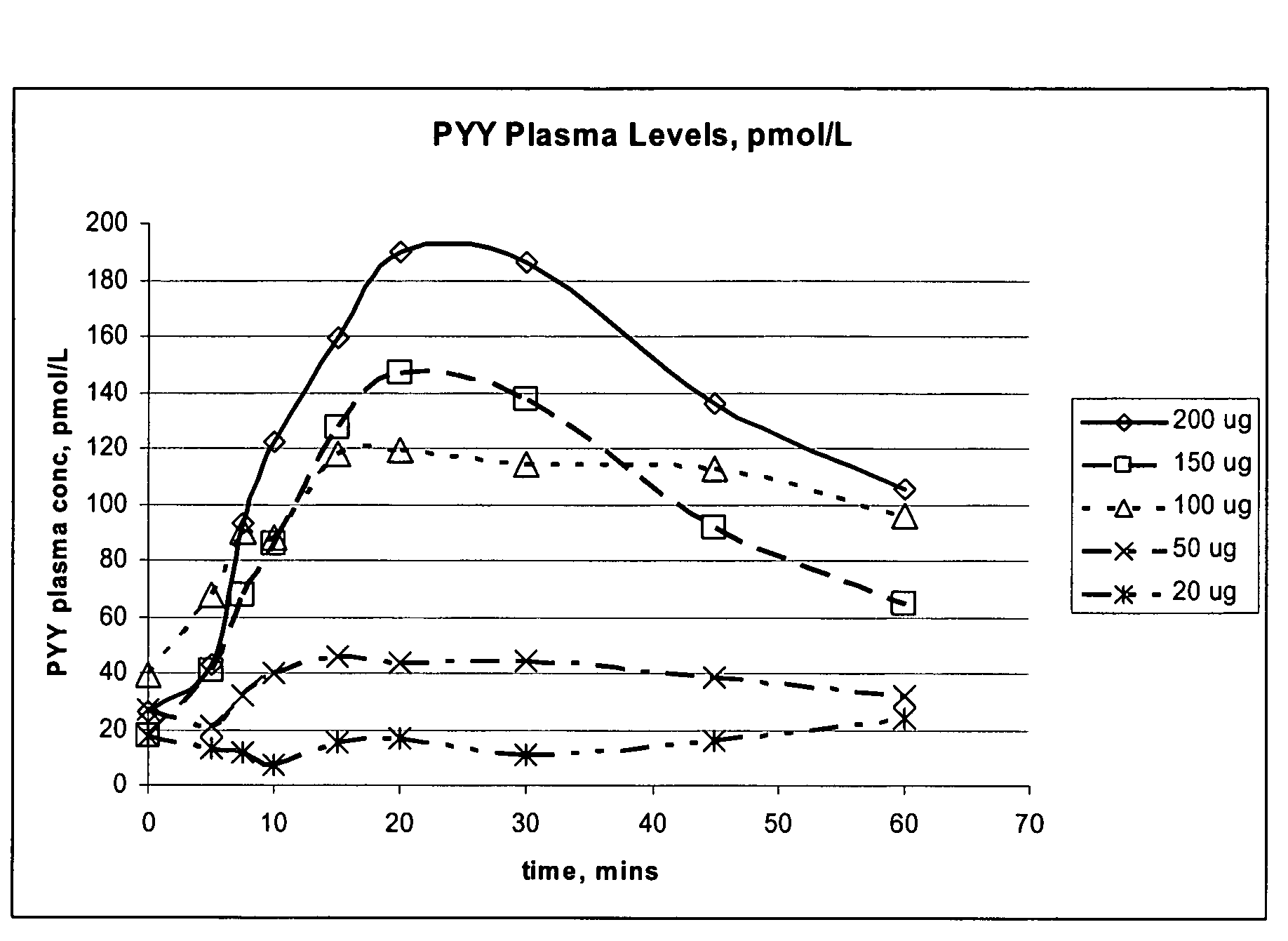
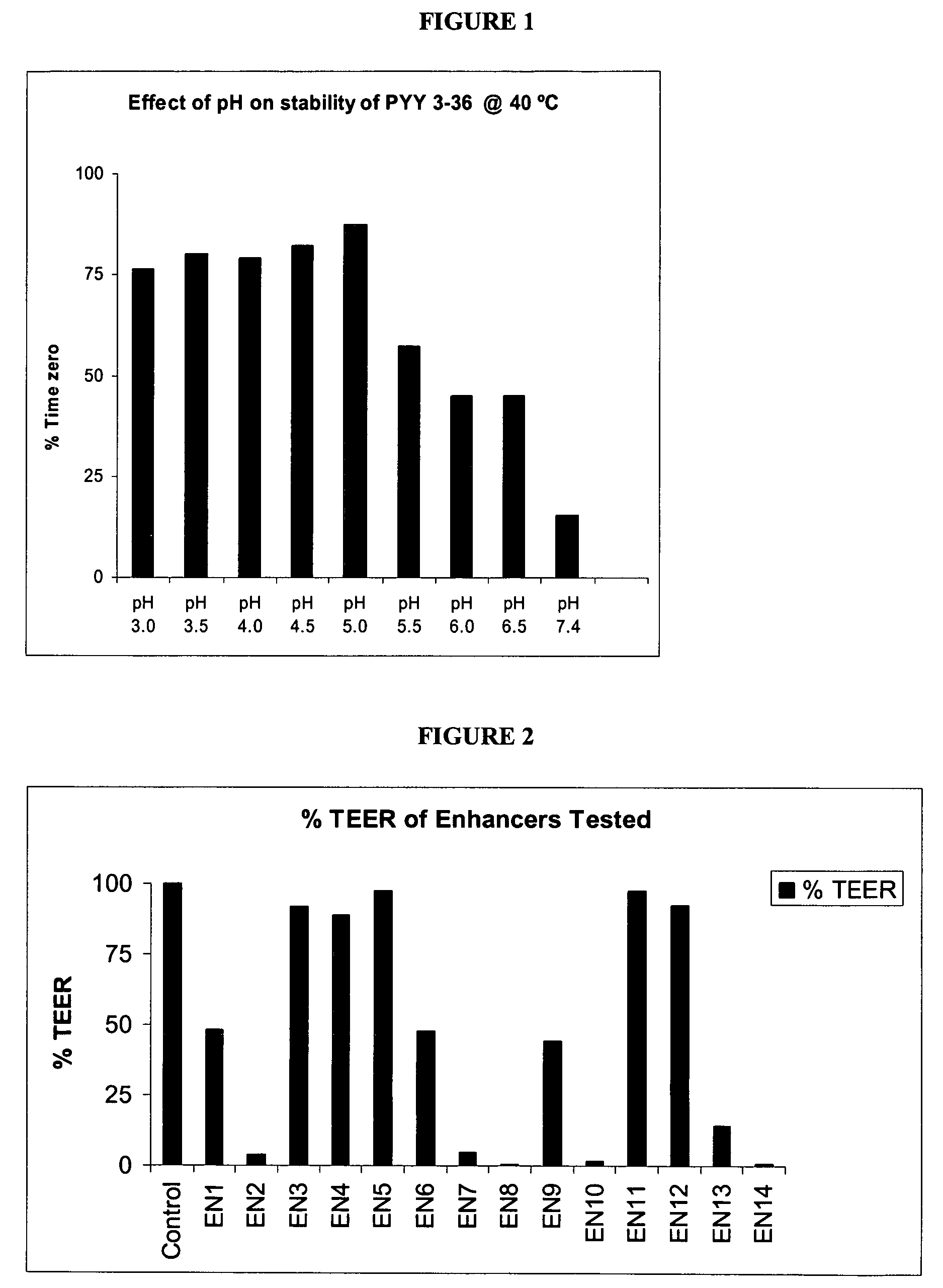
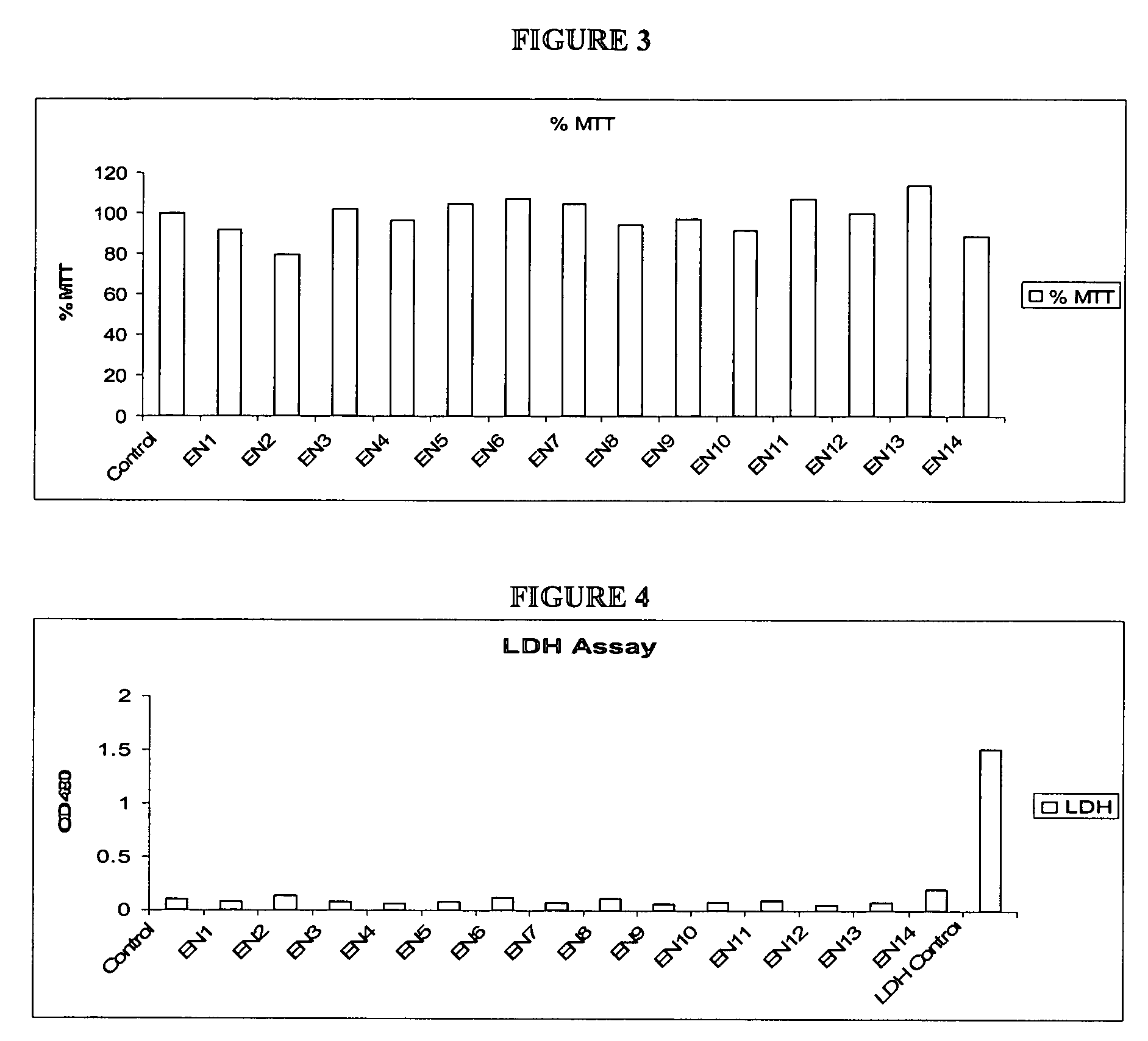
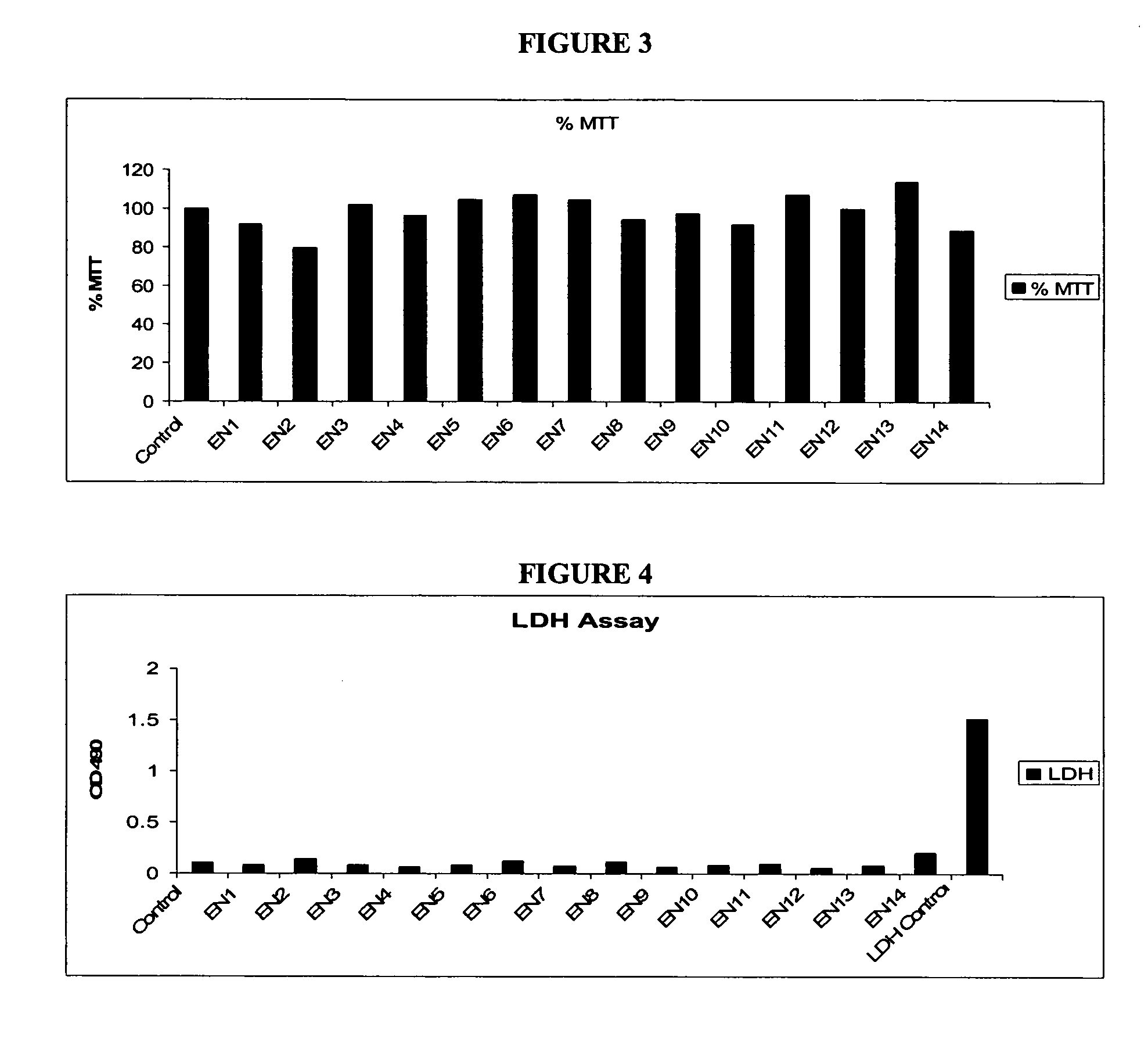
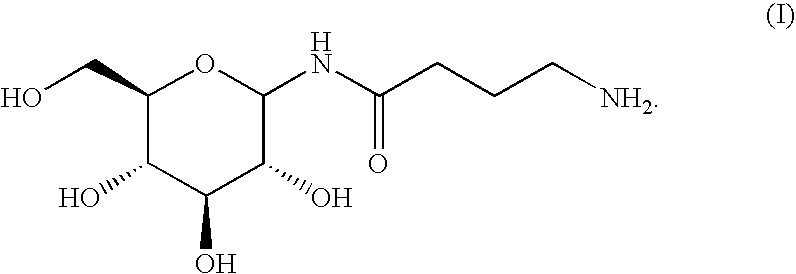
Compositions and methods for enhanced mucosal delivery of Y2 receptor-binding peptides and methods for treating and preventing obesity
InactiveUS7157426B2PromotePrevent and cure diabetesBiocideNervous disorderBinding peptideDense Core Vesicles
Pharmaceutical compositions and methods are described comprising at least one Y2 receptor-binding peptide, such as peptide YY(PYY), Neuropeptide Y (NPY) or Pancreatic Peptide (PP) and one or more mucosal delivery-enhancing agents for enhanced nasal mucosal delivery of the peptide YY, for treating a variety of diseases and conditions in mammalian subjects, including obesity.
Owner:MDRNA
Baclofen conjugate and a pharmaceutical composition for treatment of neuronal disorders
A compound is provided that has the formula NH2CH2CH2CHR1C(O)N—R (I) where R1 is p-chlorophenyl, R is a moiety capable of crossing the blood brain barrier and is as a free compound serotonin, dopamine blood brain barrier (BBB) peptide, membrane translocating protein, TAT peptides, bradykinin, beta-endorphin, bombesin, calcitonin, cholecystokinin, an enkephalin, dynorphin, insulin, gastrin, substance P, neurotensin, glucagon, secretin, somatostatin, motilin, vasopressin, oxytocin, prolactin, thyrotropin, an angiotensin, galanin, neuropeptide Y, thyrotropin-releasing hormone, gonadotropnin-releasing hormone, growth hormone-releasing hormone, luteinizing hormone, vasoactive intestinal peptide transferrin, glucosylamine, amino saccharin, lactylamine, leucine, tryptophan, glutamate and amino cholines.
Owner:MILLER LANDON C G
Compositions and methods for enhanced mucosal delivery of Y2 receptor-binding peptides and methods for treating and preventing obesity
InactiveUS7229966B2Induce satiety in an individualPromote weight-loss in an individualBiocideNervous disorderDiseasePYY Peptide
Pharmaceutical compositions and methods are described comprising at least one Y2 receptor-binding peptide, such as peptide YY(PYY), Neuropeptide Y (NPY) or Pancreatic Peptide (PP) and one or more mucosal delivery-enhancing agents for enhanced nasal mucosal delivery of the peptide YY, for treating a variety of diseases and conditions in mammalian subjects, including obesity.
Owner:MDRNA
Compositions and methods for enhanced mucosal delivery of Y2 receptor-binding peptides and methods for treating and preventing obesity
InactiveUS7186691B2Induce satiety in an individualPromote weight-loss in an individualBiocideOrganic active ingredientsDiseaseBinding peptide
Pharmaceutical compositions and methods are described comprising at least one Y2 receptor-binding peptide, such as peptide YY(PYY), Neuropeptide Y (NPY) or Pancreatic Peptide (PP) and one or more mucosal delivery-enhancing agents for enhanced nasal mucosal delivery of the peptide YY, for treating a variety of diseases and conditions in mammalian subjects, including obesity.
Owner:MDRNA
Aminobutyramide conjugate and a pharmaceutical composition for treatment of neuronal disorders
ActiveUS7074775B2Improve efficiencyEliminate side effectsBiocideDipeptide ingredientsTryptophanSecretin
A compound is provided that has the formulaNH2CH2CH2CH2C(O)N—R (I)where R is a moiety capable of crossing the blood brain barrier and is as a free compound serotonin, dopamine blood brain barrier (BBB) peptide, membrane translocating protein, TAT peptides, bradykinin, beta-endorphin, bombesin, calcitonin, cholecystokinin, an enkephalin, dynorphin, insulin, gastrin, substance P, neurotensin, glucagon, secretin, somatostatin, motilin, vasopressin, oxytocin, prolactin, thyrotropin, an angiotensin, galanin, neuropeptide Y, thyrotropin-releasing hormone, gonadotropnin-releasing hormone, growth hormone-releasing hormone, luteinizing hormone, vasoactive intestinal peptidetransferrin, glucosylamnine, amino saccharin, lactylamine, leucine, tryptophan, glutamate and amino cholines.
Owner:MILLER LANDON C G
Baclofen conjugate and a pharmaceutical composition for treatment of neuronal disorders
A compound is provided that has the formulaNH2CH2CH2CHR1C(O)N—R (I)where R1 is p-chlorophenyl, R is a moiety capable of crossing the blood brain barrier and is as a free compound serotonin, dopamine blood brain barrier (BBB) peptide, membrane translocating protein, TAT peptides, bradykinin, beta-endorphin, bombesin, calcitonin, cholecystokinin, an enkephalin, dynorphin, insulin, gastrin, substance P, neurotensin, glucagon, secretin, somatostatin, motilin, vasopressin, oxytocin, prolactin, thyrotropin, an angiotensin, galanin, neuropeptide Y, thyrotropin-releasing hormone, gonadotropnin-releasing hormone, growth hormone-releasing hormone, luteinizing hormone, vasoactive intestinal peptide transferrin, glucosylamine, amino saccharin, lactylamine, leucine, tryptophan, glutamate and amino cholines.
Owner:MILLER LANDON C G
Compositions for modulating growth of embryonic and adult kidney tissue and uses for treating kidney damage
InactiveUS20080090765A1Protection from damagePeptide/protein ingredientsBone-inducing factorNephronectinMammal
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
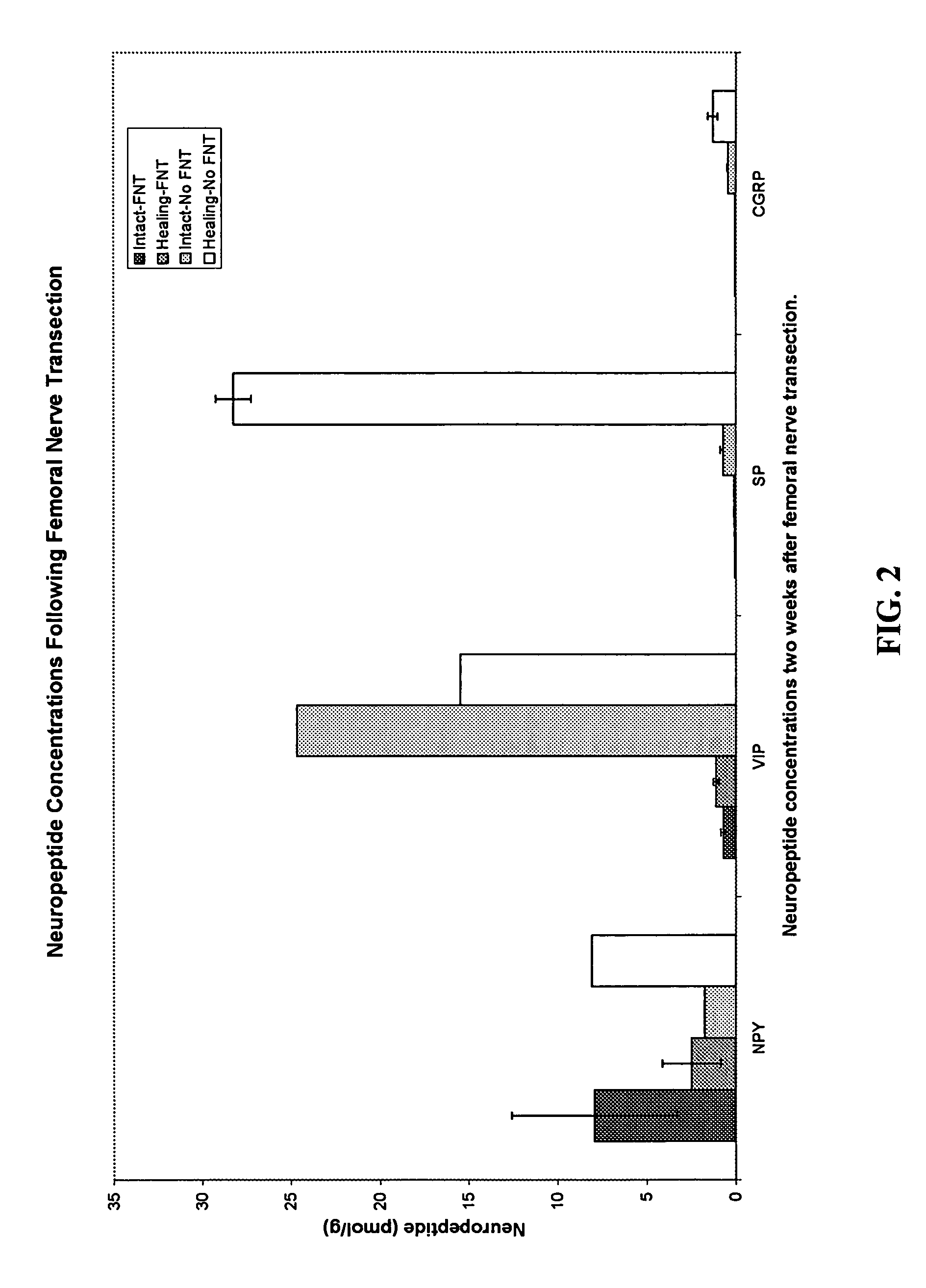
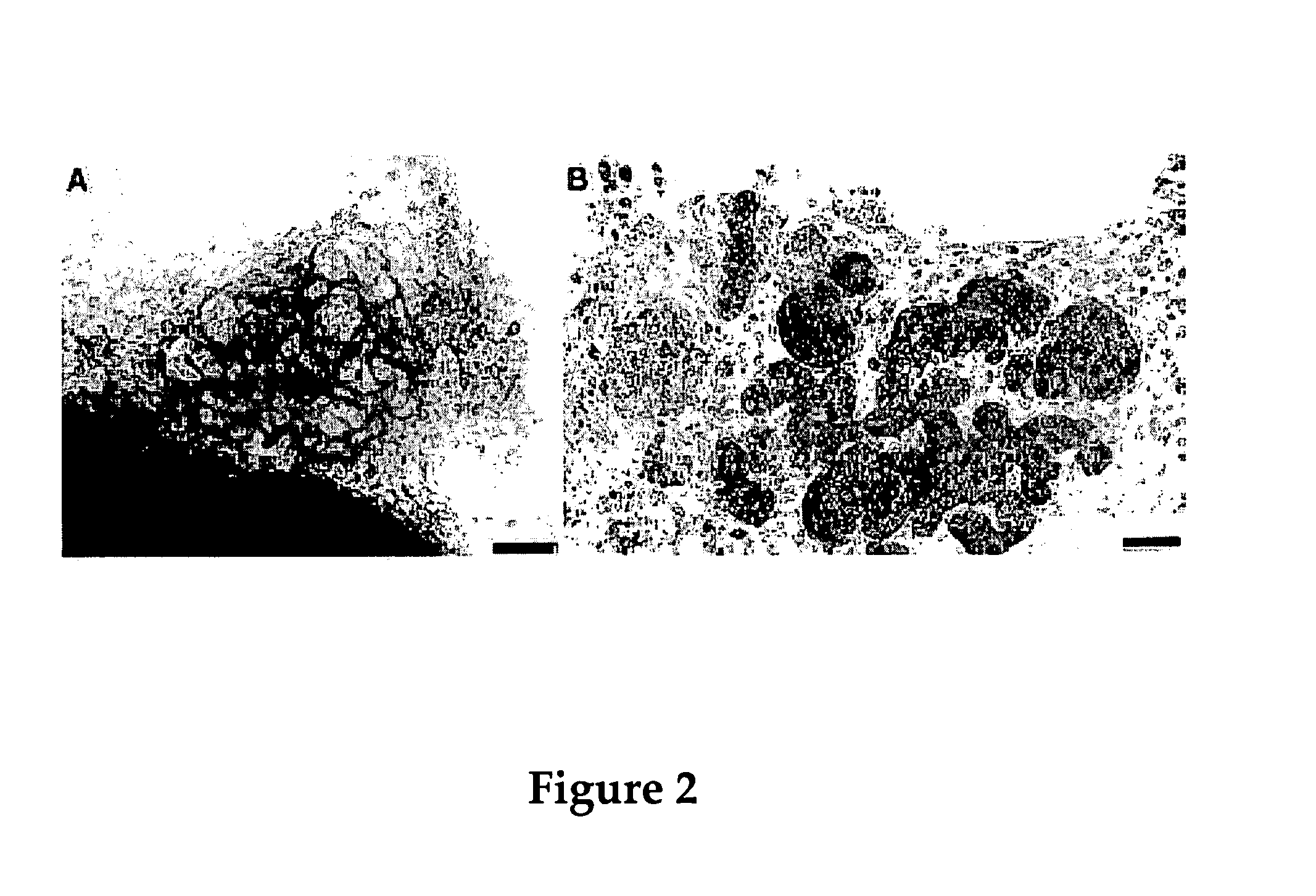
Use of neuropeptides for ligament healing
InactiveUS7776815B2High strengthDistinct utilityTachykinin ingredientsImmunoglobulinsDiseaseThyrotropin-releasing hormone
Disclosed are a method and a corresponding pharmaceutical composition for treating damaged ligaments. Neurogenic compounds in general and neuropeptides in particular have been found to be highly effective in stimulated repair of ligaments damaged due to traumatic injury, ligament disease, and disuse. Preferred active ingredients for use in the method and corresponding pharmaceutical composition include calcitonin gene-related peptide (CGRP), cholecystokinin (CCK), dynorphin, enkephalin, galanin, neuropeptide Y (NPY), neurotensin, somatostatin, substance P (SP), thyrotropin-releasing hormone (TRH), vasoactive intestinal peptide (VIP).
Owner:WISCONSIN ALUMNI RES FOUND
Piracetam and piracetam analog conjugate and a pharmaceutical composition for treatment of neuronal disorders
A compound of the formulawhere R1 is H, C1–C4 alkyl and OH; R2 in is H, C1–C4 alkyl and OH; R3 is H and C1–C4 alkyl; R4 is H and C1–C4 alkyl; n is an integer between 0 and 2 inclusive; R5 is a nullity, NHR7C(O)—, C6H4—, C6H4—O—; R7 is C2–C6 alkyl; and R6 is a moiety capable of crossing the blood brain barrier and is as a free compound serotonin, dopamine, blood brain barrier (BBB) peptide, membrane translocating peptide, TAT peptides, bradykinin, beta-endorphin, bombesin, calcitonin, cholecystokinin, an enkephalin, dynorphin, insulin, gastrin, substance P, neurotensin, glucagon, secretin, somatostatin, motilin, vasopressin, oxytocin, prolactin, thyrotropin, an angiotensin, galanin, neuropeptide Y, thyrotropin-releasing hormone, gonadotropnin-releasing hormone, growth hormone-releasing hormone, luteinizing hormone, vasoactive intestinal peptidegluconate, transferrin, glucosylamine, amino saccharin, saccharin ester, lactylamine, leucine, tryptophan, amino glutamate and amino cholines.
Owner:MILLER LANDON C G
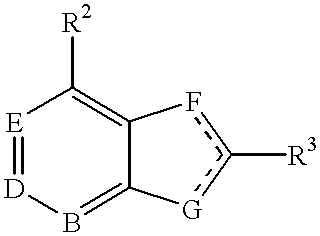
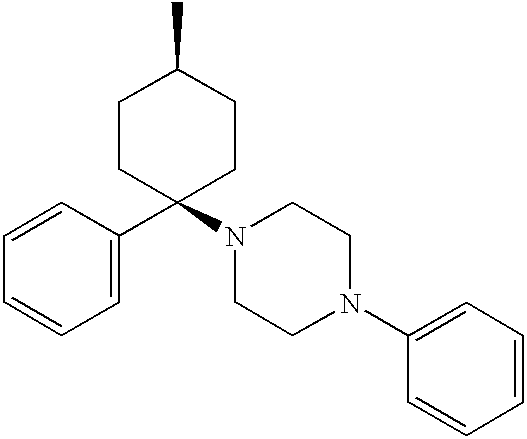
3a,4,5,9b-tetrahydro-1H-benz[e]indol-2-yl amine-derived neuropeptide Y receptors ligands useful in the treatment of obesity and other disorders
Compounds of the formula: are disclosed as ligands for neuropeptide Y receptors and as such are useful in the treatment of obesity and disorders of the central nervous system.
Owner:ORTHO MCNEIL PHARM INC
Novel compositions comprising a phosphodiesterase-5 inhibitor and their use in methods of treatment
InactiveUS20130296324A1Enhance health and appearanceFacilitating accelerating healingBiocideNervous disorderDiseaseSynaptic cleft
The invention relates generally to novel pharmaceutical methods for the treatment of various conditions. Compositions comprising: at least one phosphodiesterase-5-inhibitor in combination with one or more of the following medications: a selective serotonin reuptake inhibitor; a serotonin-norepinephrine reuptake inhibitor; a cholinesterase inhibitor; a dopamine agonist; or a medication suitable to increase the chemical concentrations of the neurotransmitters, selected from amino acids, monoamines, neuropeptides and other agents capable of primary neurotransmission in the synaptic clefts, and their use for treating a neurodegenerative disease in a subject. The invention also relates to: Compositions comprising: at least one phosphodiesterase-5-inhibitor in combination with one or more of the following medications: a selective serotonin reuptake inhibitor; or a cholinesterase inhibitor, and their use for treating damaged skin in a subject.
Owner:HELD JERRY M
Piracetam and piracetam analog conjugate and a pharmaceutical composition for treatment of neuronal disorders
A compound of the formula where R1 is H, C1-C4 alkyl and OH; R2 in is H, C1-C4 alkyl and OH; R3 is H and C1-C4 alkyl; R4 is H and C1-C4 alkyl; n is an integer between 0 and 2 inclusive; R5 is a nullity, NHR7C(O)—, C6H4—, C6H4—O—; R7 is C2-C6 alkyl; and R6 is a moiety capable of crossing the blood brain barrier and is as a free compound serotonin, dopamine, blood brain barrier (BBB) peptide, membrane translocating peptide, TAT peptides, bradykinin, beta-endorphin, bombesin, calcitonin, cholecystokinin, an enkephalin, dynorphin, insulin, gastrin, substance P, neurotensin, glucagon, secretin, somatostatin, motilin, vasopressin, oxytocin, prolactin, thyrotropin, an angiotensin, galanin, neuropeptide Y, thyrotropin-releasing hormone, gonadotropnin-releasing hormone, growth hormone-releasing hormone, luteinizing hormone, vasoactive intestinal peptidegluconate, transferrin, glucosylamine, amino saccharin, saccharin ester, lactylamine, leucine, tryptophan, amino glutamate and amino cholines.
Owner:MILLER LANDON C G
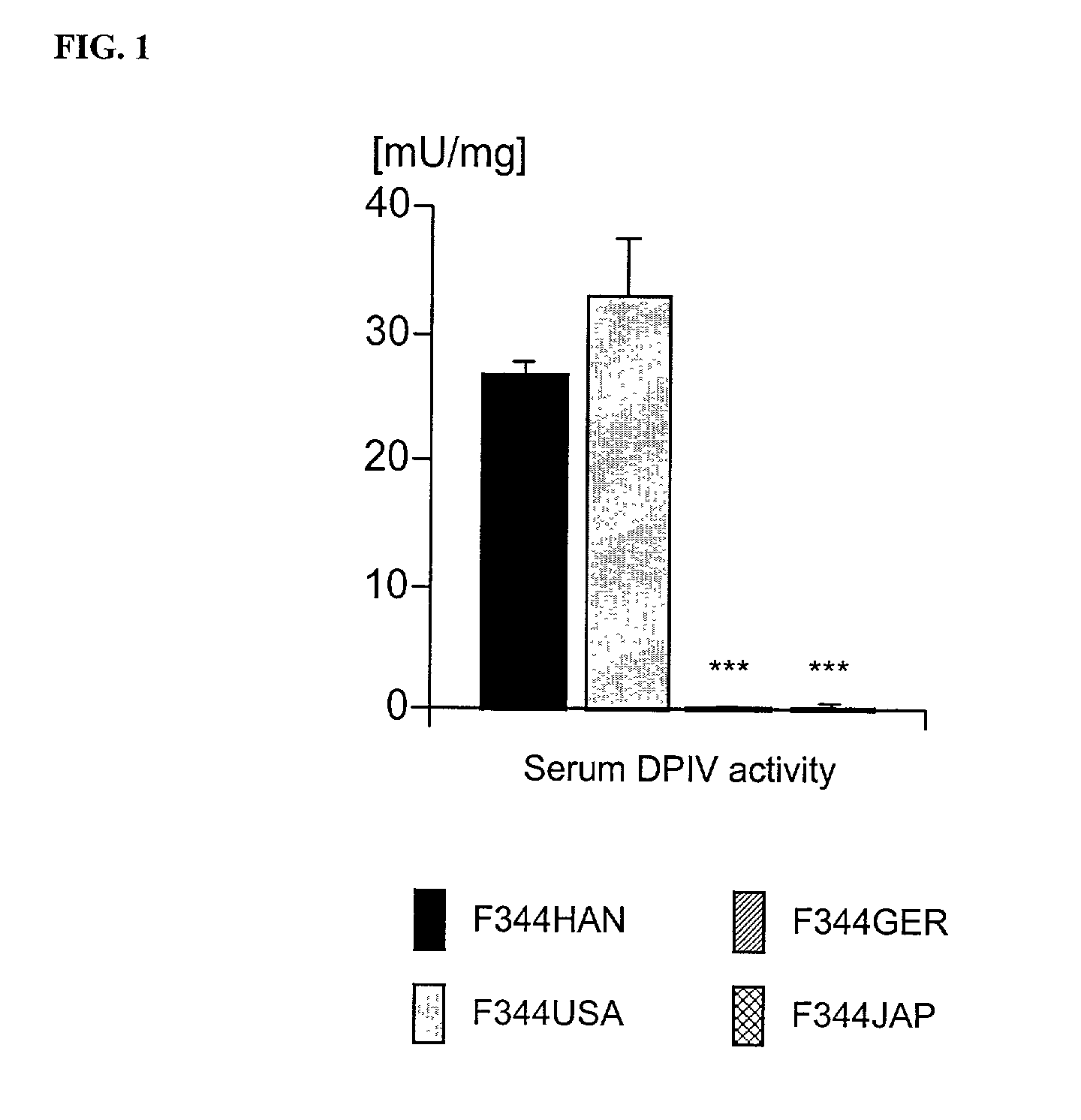
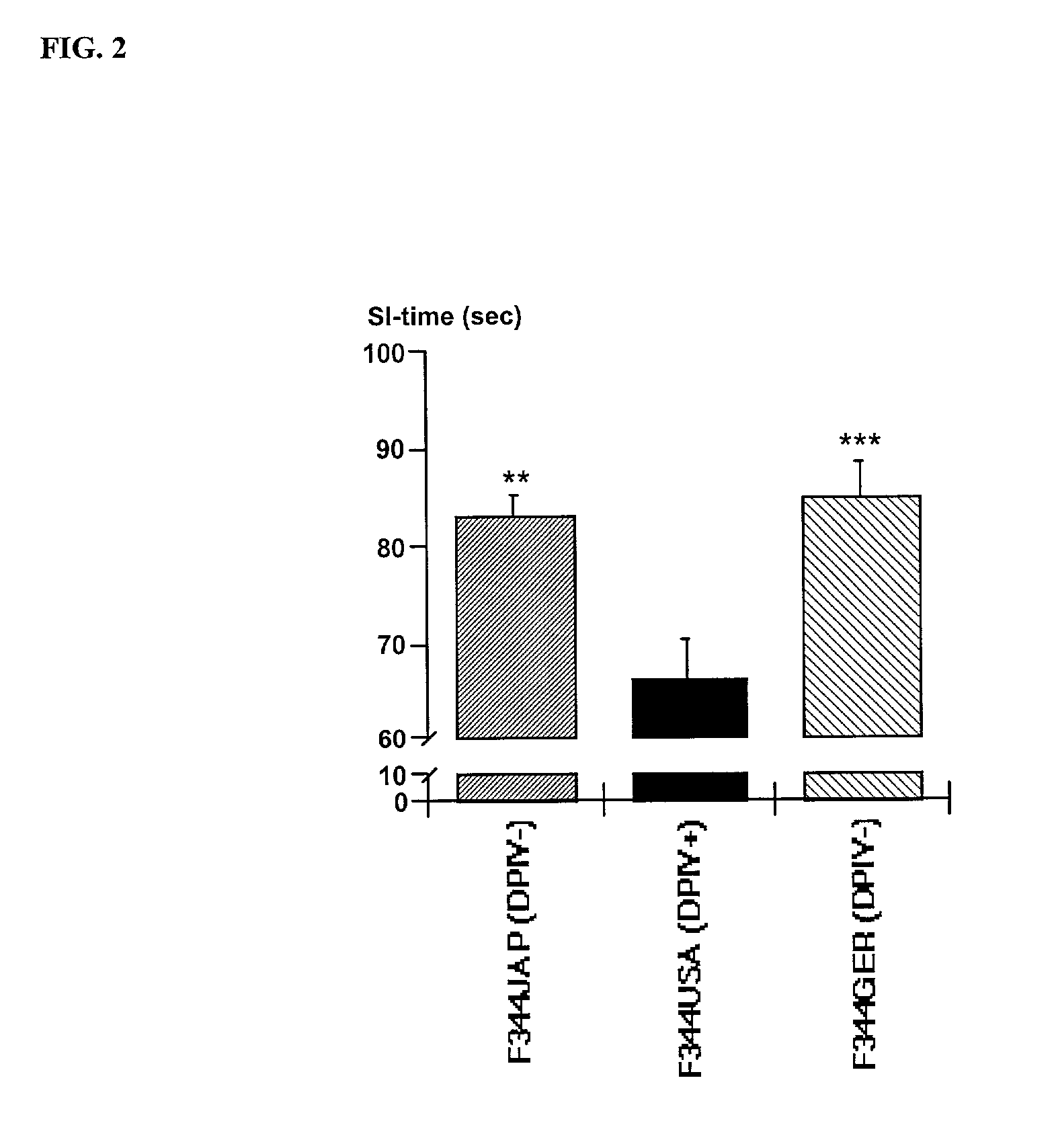
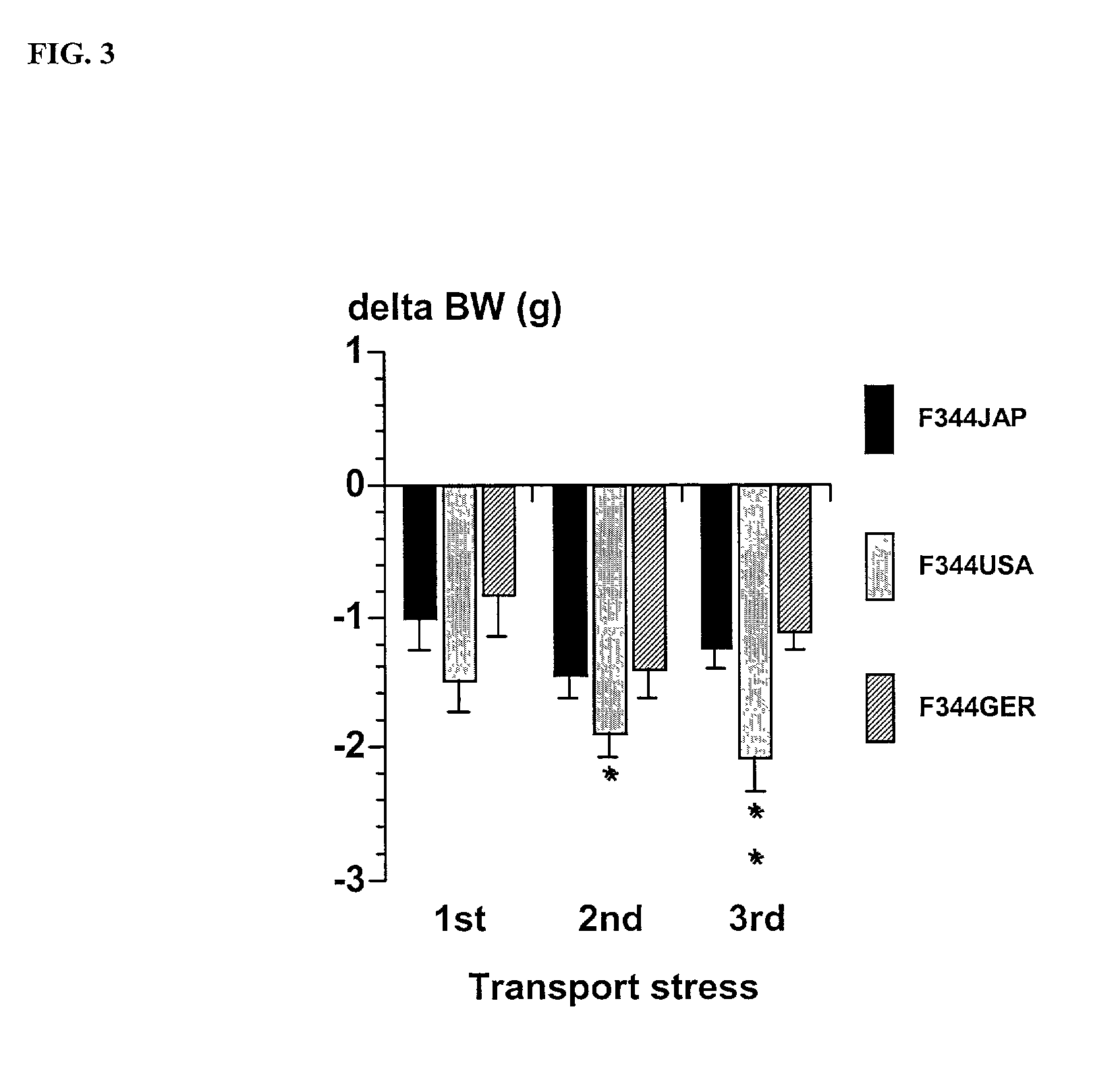
Modulation of central nervous system (CNS) dipeptidyl peptidase IV (DPIV) -like activity for the treatment of neurological and neuropsychological disorders
InactiveUS7132104B1Avoid rapid degradationGood effectBiocideNervous disorderHypertension medicationsNervous system
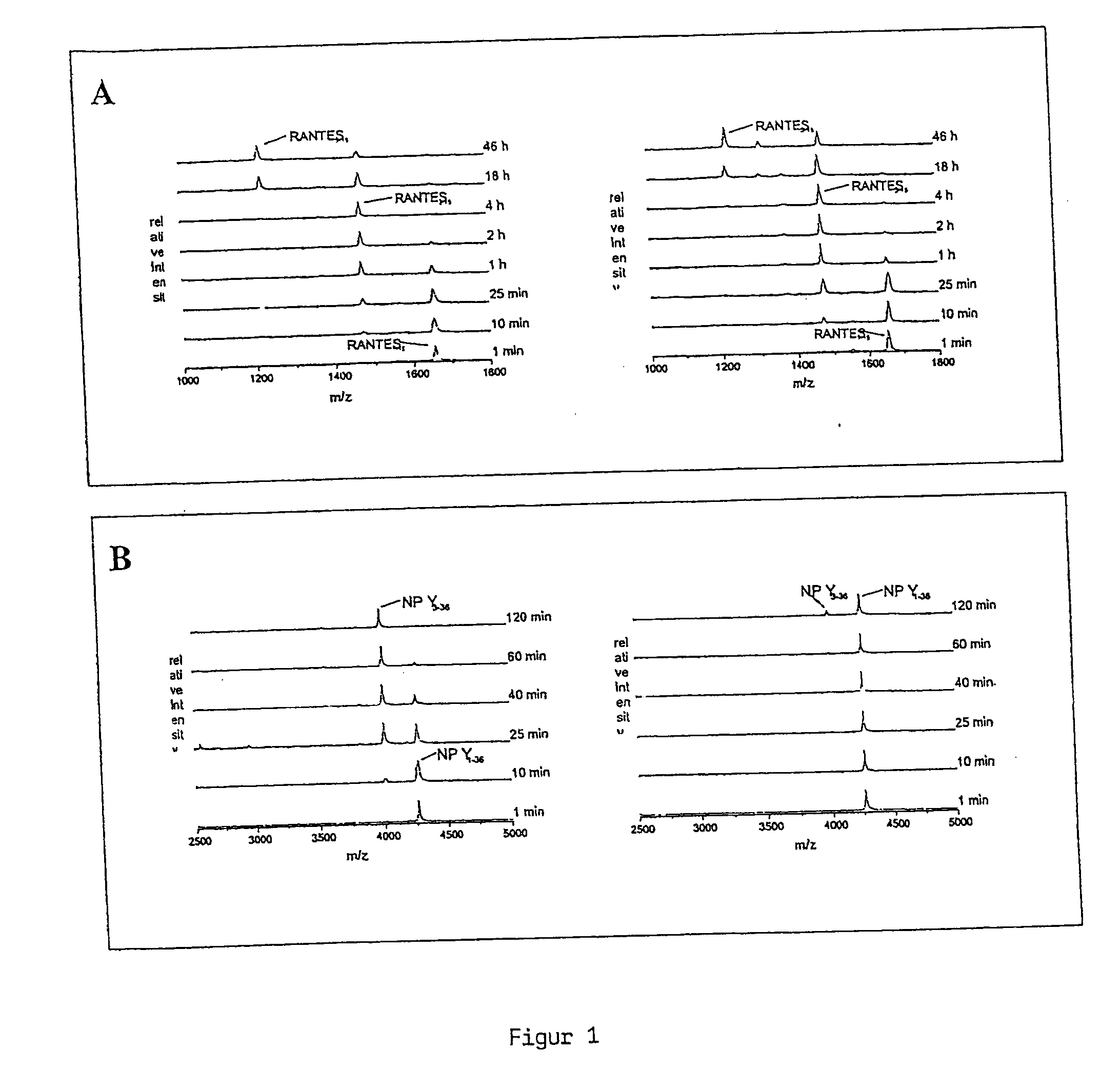
The present invention discloses a method for therapeutically treating an animal, including a human, for psychosomatic, depressive and neuropsychiatric diseases, such as anxiety, depression, insomnia, schizophrenia, epilepsy, spasm and chronic pain. Administration of a suitable DP IV inhibitor causes the reduction of activity in the enzyme dipeptidyl peptidase (DP IV or CD 26) or of DP IV-like enzyme activity in the brain of mammals and leads as a causal consequence to a reduced degradation of the neuropeptide Y (NPY) and similar substrates by DP IV and DP IV-like enzymes. Such treatment will result in a reduction or delay in the decrease of the concentration of functionally active neuronal NPY (1–36). As a consequence of the resulting enhanced stability of the endogenous NPY (1–36) caused by the inhibition of DP IV activity, NPY activity is prolonged thereby resulting among other things in functionally active NPY Y1 receptor activity thereby facilitating anti-depressive, anxiolytic, analgesic, anti-hypertension and other neurological effects.
Owner:FRAUNHOFER GESELLSCHAFT ZUR FOERDERUNG DER ANGEWANDTEN FORSCHUNG EV
Compositions and methods for enhanced mucosal delivery and non-infused administration of Y2 receptor-binding peptides and methods for treating and preventing obesity
InactiveUS7186692B2Induce satietyPromoteHormone peptidesPeptide/protein ingredientsDiseaseBinding peptide
Pharmaceutical compositions and methods are described comprising at least one Y2 receptor-binding peptide, such as peptide YY(PYY), Neuropeptide Y (NPY) or Pancreatic Peptide (PP) and one or more mucosal delivery-enhancing agents for enhanced nasal mucosal delivery of the peptide YY, for treating a variety of diseases and conditions in mammalian subjects, including obesity.
Owner:MDRNA
Cytotoxic Conjugates Having Neuropeptide Y Receptor Binding Compound
InactiveUS20120010154A1Good curative effectReduce complicationsOrganic active ingredientsMetabolism disorderNPY-Y1 receptorDisease
There is provided a series of novel neuropeptide Y-cytotoxic conjugates, compositions comprising the same, and methods relating to their therapeutic use for the treatment of disease or condition states associated with aberrant or undesirable proliferation of cells that express NPY-Y1 receptors.
Owner:IPSEN PHARMA SAS
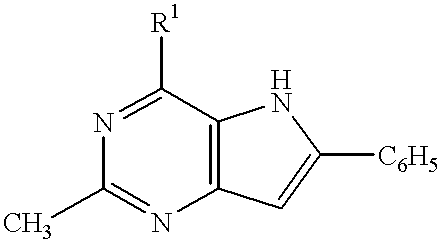
4-aminopyrrole (3, 2-D) pyrimidines as neuropeptide Y receptor antagonists
This invention provides a means of inhibiting or preventing a disease or condition associated with an excess of neuropeptide Y which comprises administering to a mammal in need of such treatment an effective amount of a compound of the formula:wherein R1 is a nitrogen containing heterocyclic compound.
Owner:PFIZER INC
Method for the treatment of neurological and neuropsychological disorders
The present invention discloses a method for therapeutically treating an animal, including a human, for psychosomatic, depressive and neuropsychiatric diseases, such as anxiety, depression, insomnia, schizophrenia, epilepsy, spasm and chronic pain. Administration of a suitable attractin inhibitor causes the reduction of activity in the enzyme attraction or in isoforms thereof in the brain of mammals and leads as a causal consequence to a reduced degradation of the neuropeptide Y (NPY) and similar substrates. Such treatment will result in a reduction or delay in the decrease of the concentration of functionally active neuronal NPY (1-36). As a consequence of the resulting enhanced stability of the endogenous NPY (1-36), NPY activity is prolonged thereby resulting among other things in functionally active NPY Y1 receptor activity thereby facilitating antidepressive, anxiolytic, analgesic, t antihypertension and other neurological effects.
Owner:VIVORYON THERAPEUTICS NV
Trophic Factor Combinations for Nervous System Treatment
The present invention relates to a composition including an effective amount of at least one of an antimicrobial peptide and a substance having an antimicrobial peptide effect and an effective amount of a neurotrophin. The composition can also include an effective amount of at least one of a growth factor and a neuropeptide. The present invention also relates a method of treating an injury to a nervous system of an animal that includes the steps of identifying the injury to the nervous system and applying to the injury an effective amount of at least one of antimicrobial peptide and a substance having an antimicrobial peptide effect. The method can also include applying an effective amount of one or more trophic factors selected from the group consisting of a growth factor, a neurotrophin, and a neuropeptide to the injury.
Owner:WISCONSIN ALUMNI RES FOUND
Urocortin proteins and uses thereof
InactiveUS20020127221A1Reduced food intakeAvoid intakeBacteriaPeptide/protein ingredientsDense Core VesiclesAmino acid peptide
Owner:RES DEVMENT FOUND
Preparations containing an extract of eperua falcata and/or constituents of the latter
A cosmetic, pharmaceutical or dermatological preparation containing extract of the plant Eperua falcata, active principles of the plant Eperua falcata, astilbin or engeletin. The preparation is useful to inhibit release of pro-imflammatory mediators and neuropeptides, including CGRP and SP, for skin and hair treatment, including, sensitive skin, acne, scalp itch and neurogenous inflammation.
Owner:COGNIS FRANCE SA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
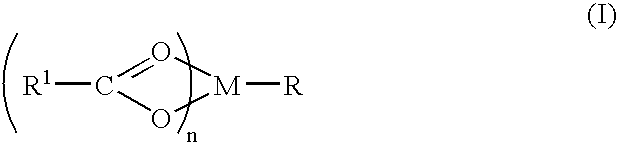
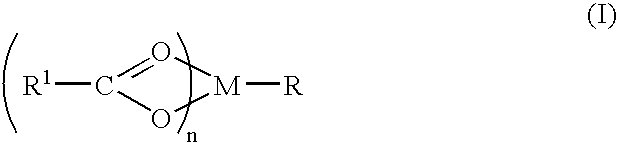


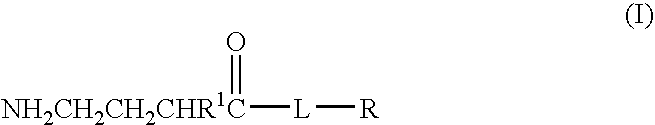
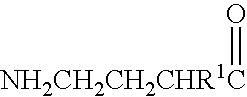
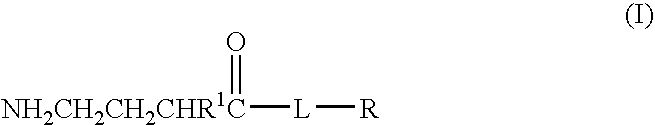
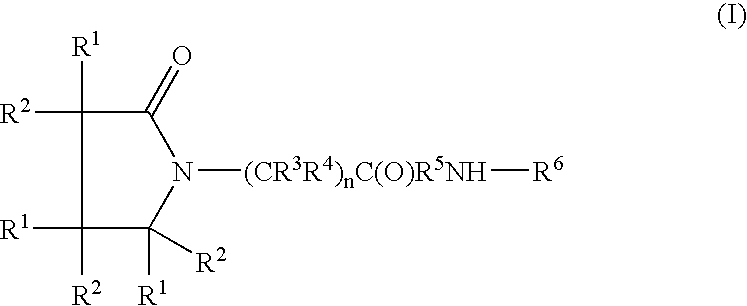
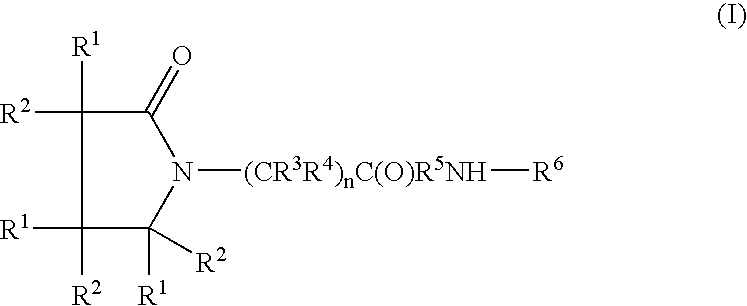
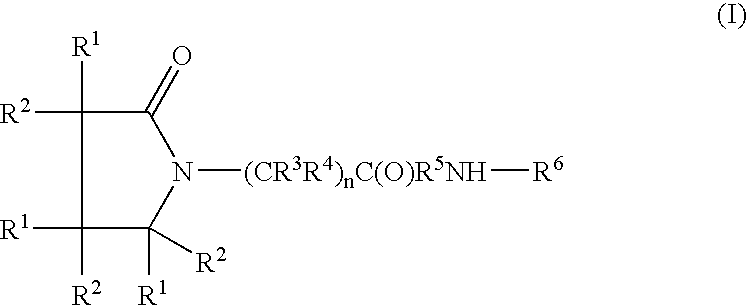
![3a,4,5,9b-tetrahydro-1H-benz[e]indol-2-yl amine-derived neuropeptide Y receptors ligands useful in the treatment of obesity and other disorders 3a,4,5,9b-tetrahydro-1H-benz[e]indol-2-yl amine-derived neuropeptide Y receptors ligands useful in the treatment of obesity and other disorders](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/2040d2fc-ab38-4f93-8f58-2a83fa996f83/US06841552-20050111-C00001.png)
![3a,4,5,9b-tetrahydro-1H-benz[e]indol-2-yl amine-derived neuropeptide Y receptors ligands useful in the treatment of obesity and other disorders 3a,4,5,9b-tetrahydro-1H-benz[e]indol-2-yl amine-derived neuropeptide Y receptors ligands useful in the treatment of obesity and other disorders](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/2040d2fc-ab38-4f93-8f58-2a83fa996f83/US06841552-20050111-C00002.png)
![3a,4,5,9b-tetrahydro-1H-benz[e]indol-2-yl amine-derived neuropeptide Y receptors ligands useful in the treatment of obesity and other disorders 3a,4,5,9b-tetrahydro-1H-benz[e]indol-2-yl amine-derived neuropeptide Y receptors ligands useful in the treatment of obesity and other disorders](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/2040d2fc-ab38-4f93-8f58-2a83fa996f83/US06841552-20050111-C00003.png)