Methods for treating pervasive development disorders
a technology for developing disorders and pdd, applied in the direction of instruments, peptide/protein ingredients, diagnostic recording/measuring, etc., can solve the problems that none of the known methods have tested fecal samples in determining the benefits of administering secretin, other neuropeptides, peptides and/or digestive enzymes to individuals suffering from pdd, etc., to promote normal growth and development, improve protein digestion, and improve the effect of pdd
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
experiment 1
[0038] I. Experiment 1
[0039] In this experiment, 16 children diagnosed as having autism were administered the following Fecal Chymotrypsin Test in accordance with an embodiment of the invention. First, approximately 2 grams of stool were collected from each child and placed in a sterile container (although it is to be understood that any quantity of stool may be collected, as 2 grams of stool is not a required amount). Each stool sample was then analyzed using, e.g., an enzymatic photospectrometry analysis as is known by those skilled in the art, to determine the level of fecal chymotrypsin in the stool. Although the enzymatic photospectrophotometry process is preferred, any suitable conventional method may be used for measuring the fecal chymotrypsin levels. This measured chymotrypsin levels of the 16 autistic children are illustrated in FIG. 13.
[0040] After determining the chymotrypsin levels of the stools, each of these levels were compared with threshold chymotrypsin levels to ...
experiment 2
[0048] II. Experiment 2
[0049] In this experiment, 37 autistic children with abnormal fecal chymotrypsin levels were administered secretin over the course of 6 months using the secretin infusion process described above. Their fecal chymotrypsin (FC) levels were measured weekly using the fecal chymotrypsin test described above.
[0050] Results of Experiment 2
[0051] Out of the 37 autistic children tested, the fecal chymotrypsin levels of 34 children had returned to normal after 6 months, the fecal chymotrypsin levels of 2 children moved to equivocal, and the fecal chymotrypsin level of 1 child remained abnormal. These results of this experiment are listed in the following Table 1.
TABLE IPre-Secretin6 Months Post-Administra-SecretinAutistic Children TestedtionAdministration# Autistic Children w / Abnormal FC levels371# Autistic Children w / Equivocal FC levels02# Autistic Children w / normal FC levels034
experiment 3
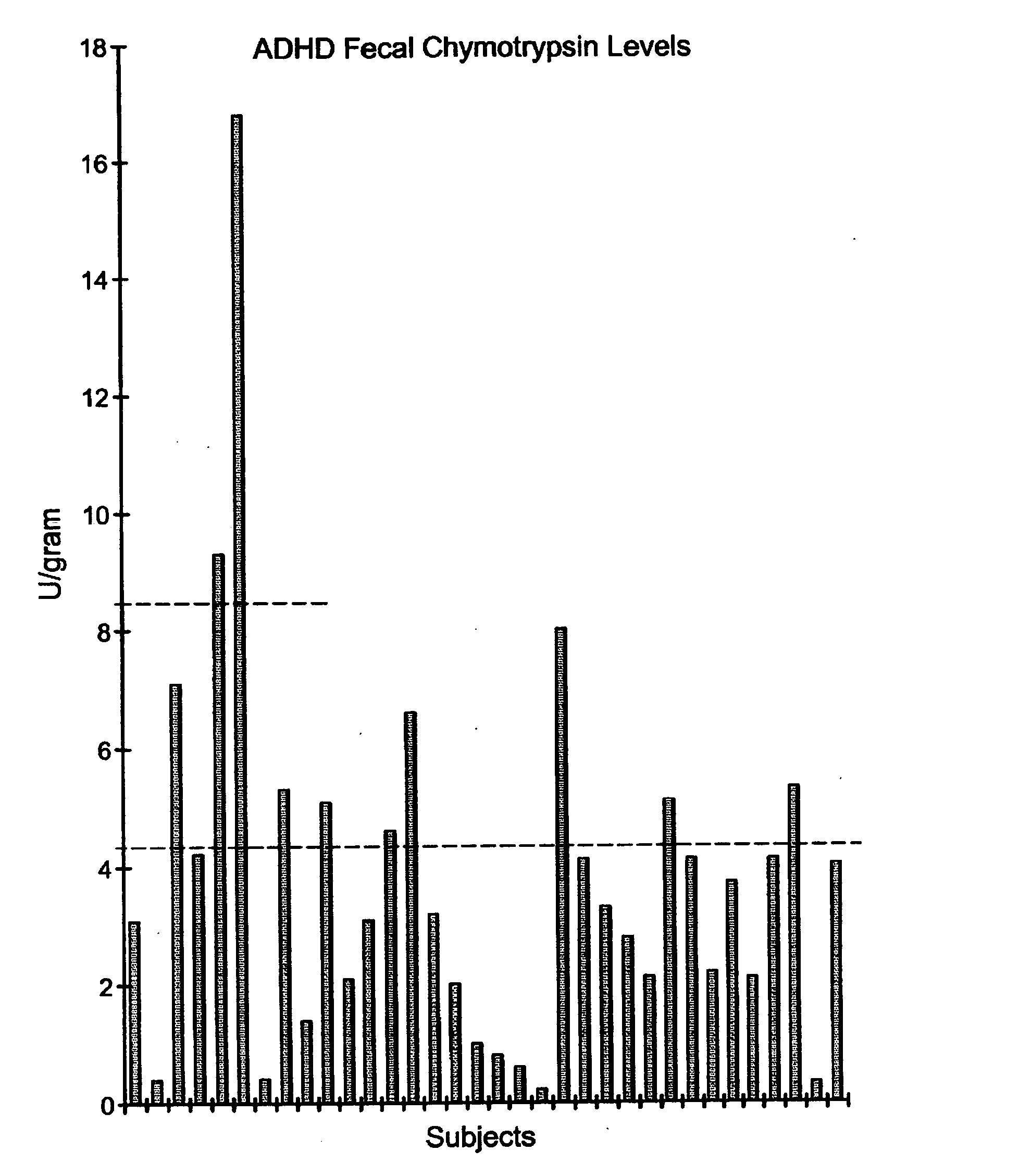
[0052] III. Experiment 3
[0053] In this experiment, the fecal chymotrypsin levels of 28 children diagnosed with ADD were obtained using the fecal chymotrypsin test described above in Experiment 1. FIG. 15 illustrates the measured fecal chymotrypsin levels of these 28 children. It is to be noted that, as shown in FIG. 15, all of the 28 ADD children were found to have sub-normal fecal chymotrypsin levels since all of the values fell below 8.4 U / g. More specifically, 8 out of 28 children were determined to have an equivocal fecal chymotrypsin level and 20 out of the 28 children were determined to have a pathologic level of fecal chymotrypsin. As noted above, a chymotrypsin level of 8.4 U / g is considered a reference value for normal levels of chymotrypsin.
[0054] Of these 28 children who were diagnosed with ADD and abnormal fecal chymotrypsin levels, 10 were administered digestive enzymes comprising amylase, proteases, lipases, sucrase, maltase, and other digestive enzymes. These digesti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Attention Deficit Disorder | aaaaa | aaaaa |
| Attention Deficit Hyperactivity disorder | aaaaa | aaaaa |
| Attention deficit disorder | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com