Gonadotropin-releasing hormone antagonist dosing regimens for treating uterine fibroids and reducing menstrual blood loss
A technology for menstrual blood loss and uterine fibroids, which can be used in pharmaceutical formulations, sexual diseases, drug combinations, etc., and can solve problems such as chronic administration incompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
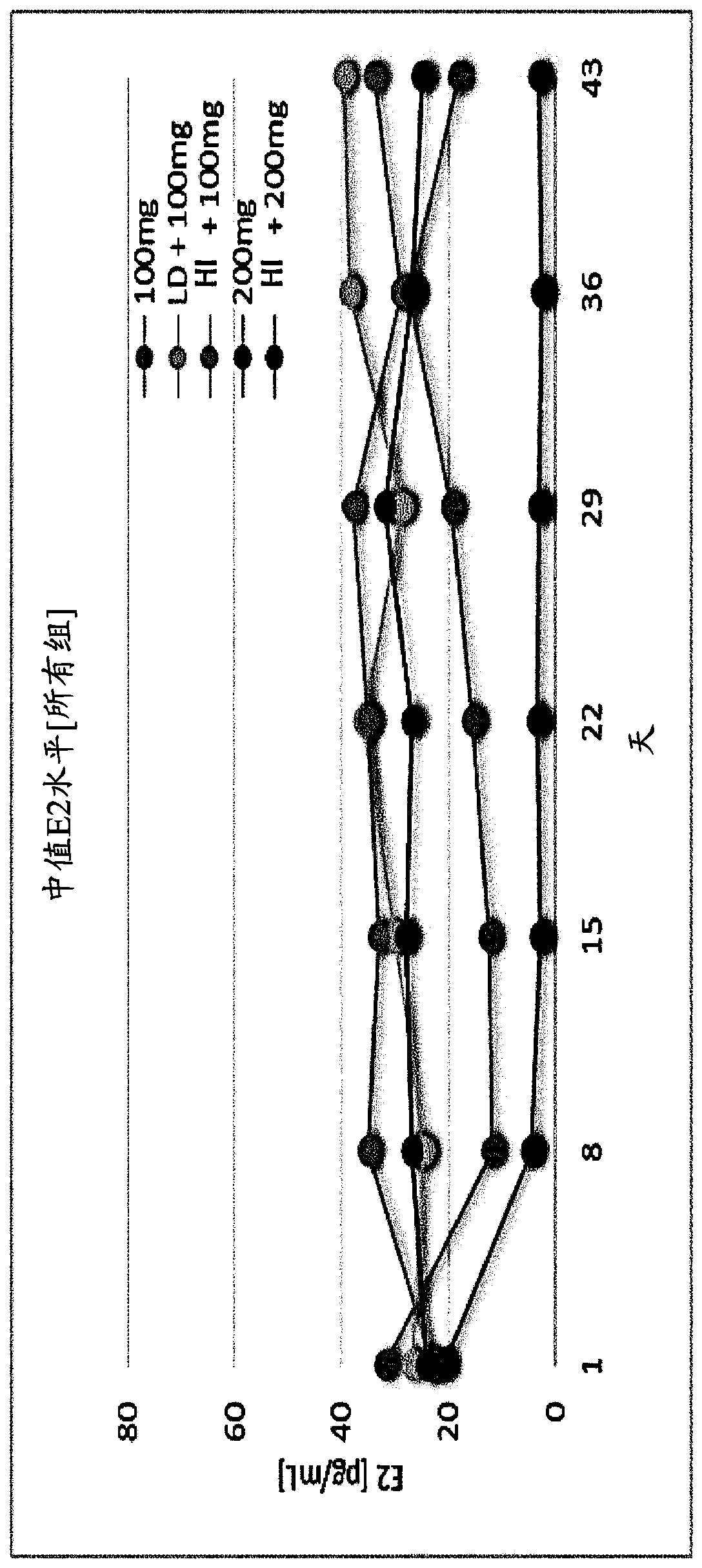
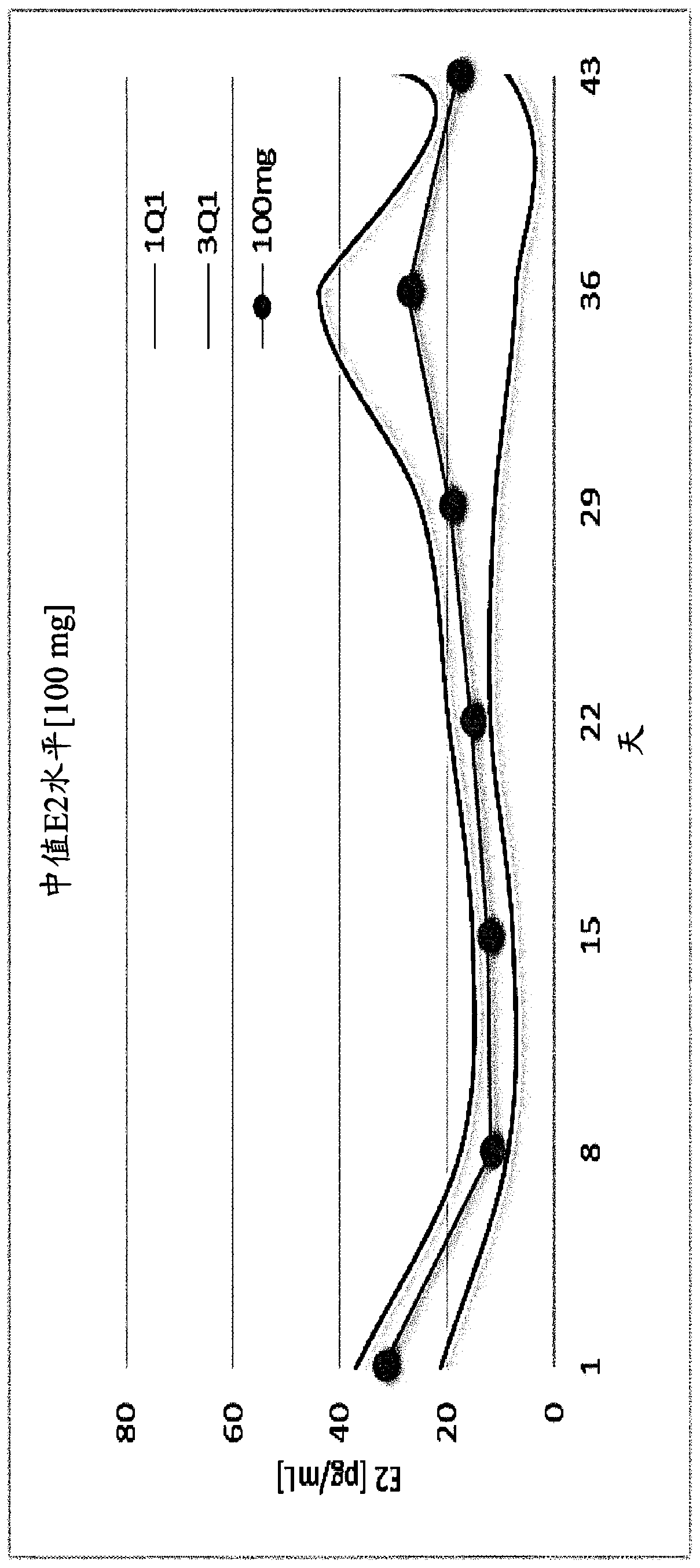
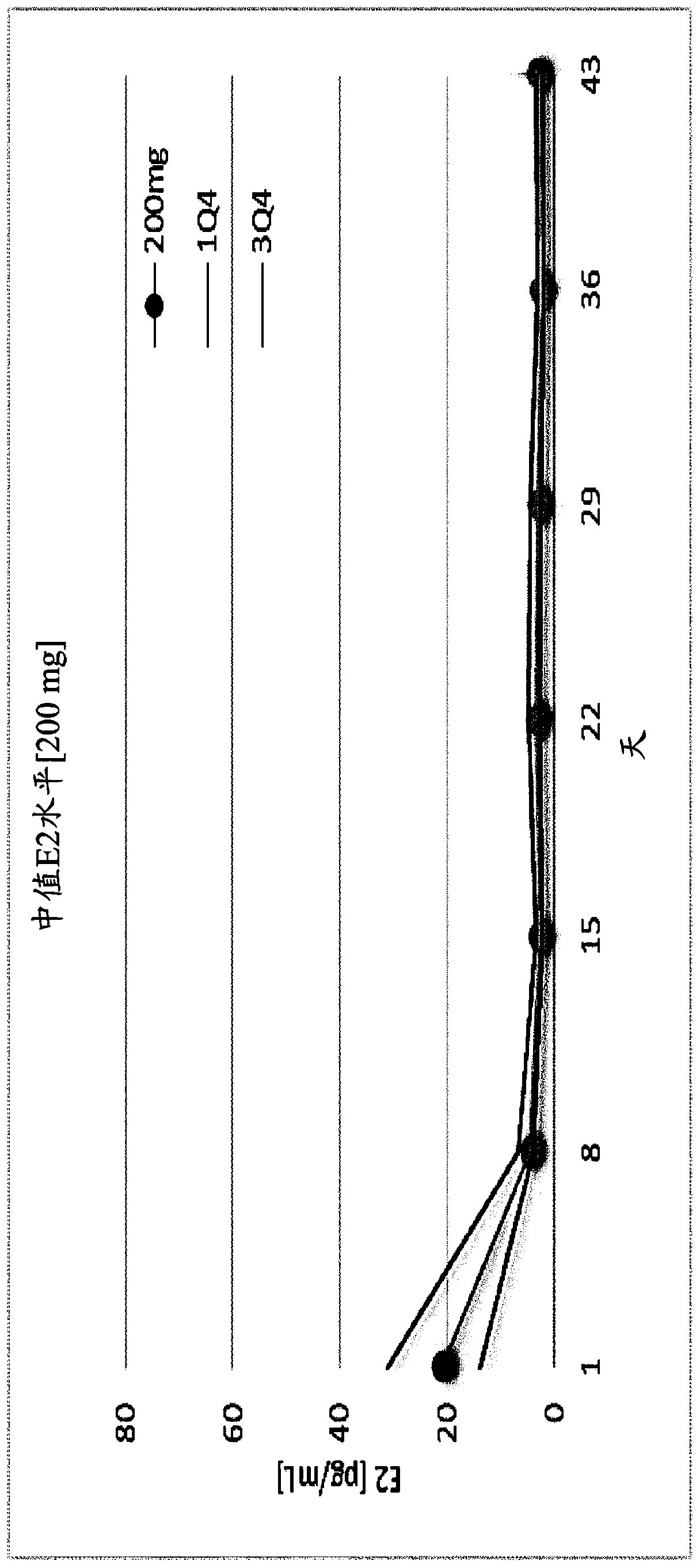
[0345] Example 1. Choline 3-[2-fluoro-5-(2,3-difluoro-6-methoxybenzyloxy)-4-methoxyphenyl]-2,4-dioxo - Evaluation of the effect of 1,2,3,4-tetrahydrothieno[3,4d]pyrimidine-5-carboxylate on serum estradiol and menstrual blood loss in female human subjects
[0346] Subject population and study design
[0347] To assess the effect of Compound (II) on serum β17-estradiol concentrations and menstrual blood loss, a total of 75 healthy female human subjects were divided into five treatment groups, as described in Table 1 below. Subjects were screened for a period of four weeks prior to initiation of treatment with Compound (II). At the end of the four-week screening period, subjects in each treatment group were administered an equal dose of norethindrone acetate, 5 mg x 3 times daily. This 15 mg / day dose was administered for 11 days to allow synchronization of menstruation between subjects. Subjects were then left without treatment for the next 4 days. Following this drug holiday...
Embodiment 2
[0372] Example 2. Use of GnRH Antagonist Dosing Regimen for Treating Patients with Uterine Fibroids and Concomitant Anemia
[0373] Human patients with uterine fibroids with concomitant anemia, such as iron deficiency anemia, can be effectively treated to exhibit reduced menstrual blood loss using the dosing regimens described herein. For example, after a patient is identified as suffering from uterine fibroids and anemia caused by heavy menstrual blood loss, a skilled clinician in the art may prescribe Compound (I) or a pharmaceutically acceptable salt thereof, such as its choline salt, to the patient. daily dose. The compound can be administered to the patient daily, for example, at a dose of 100 mg / day, as a stand-alone treatment or in combination with add-back therapy such as 1.0 mg / day of β17-estradiol and 0.5 mg / day Norethindrone acetate per day or β17-estradiol at 0.5 mg / day and norethindrone acetate at 0.1 mg / day. In an alternative example, a patient may be administe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com