Inhibitors and enhancers of uridine diphosphate-glucuronosyltransferase 2b (UGT2B)
a technology of uridine diphosphate and glucuronosyltransferase, which is applied in the direction of biocide, drug composition, metabolic disorder, etc., can solve the problems of reducing the clearance rate of liver cells, affecting the ability of liver cells to function normally, so as to increase the bioavailability of morphine-like analgesic agents.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
experiment 1
In Vitro Experiment of UGT2B Inhibitor
Material and Method
1. The Preparation of UGT2B Inhibitor
[0076]In the following experiment, 27 different kinds of CHEs and 10 different excipients were used as UGT2B inhibitor in this invention. These CHEs are pure compounds available commercially, and were purchased from Sigma Chemical Co., Nacalai Tesque (Kyoto, Japan) and Indofine Chemical Co., Inc. (Somerville, N.J.). Their category, name, source and chemical formula are listed in Table 1. These UGT2B inhibitors are dissolved in alcohol at the concentration of 1, 10, 100 μM for the following experiment.
[0077]Besides, these excipients are commercially available pure compounds, they are PEG (Polyethylene glycol) 400, PEG 2000, PEG 4000, Tween 20, Tween 60, Tween 80, BRIJ® 58, BRIJ® 76, Pluronic® F68, Pluronic® F127. These excipients are dissolved in water and make up to 0.5%, 5%, 50% (wt %, w / v) for the following experiment. (Note: BRIJ is a registered trademark of ICI Americas, Inc.; Pluronic ...
experiment 2
In Virto Experiment of UGT2B Enhancer
[0103]This experiment uses the same protocol described in Experiment 1, except by testing the 40 CHEs listed in Table 5 as the UGT2B enhancer. Those CHEs are commercially available pure compounds, acquired from Sigma Chemical Co., Nacalai Tesque (Kyoto, Japan) and Indole Chemical Co. Inc (Somerville, N.J. Their categories, names, sources, and chemical compositions are described in Table 5.
TABLE 5Category, name, source, and chemical composition of the UGT2B enhancers.SourceChemical formula andCategoryName(scientific name)molecular weightFlavonoidGenkwaninArtemisiae cpillaris herbaApigeninChamomillaeflosLuteolinDigitals foliumLuteolin-7- GlucosideDigitals foliumHomoorientinSwertiae herbaIsovitexinSwertiae herbaNeohesperidinAurantii fructusimmaturusFormononetinAstragali radixKaempferolSennae foliumIsoquercitrinHydrangeaedulcis folium6-GingerolZingiberisLiquiritinGlycyrrizae radixnaringeninAurantii fructusimmaturusUmbelliferoneAurantii fructusimmatur...
experiment 3
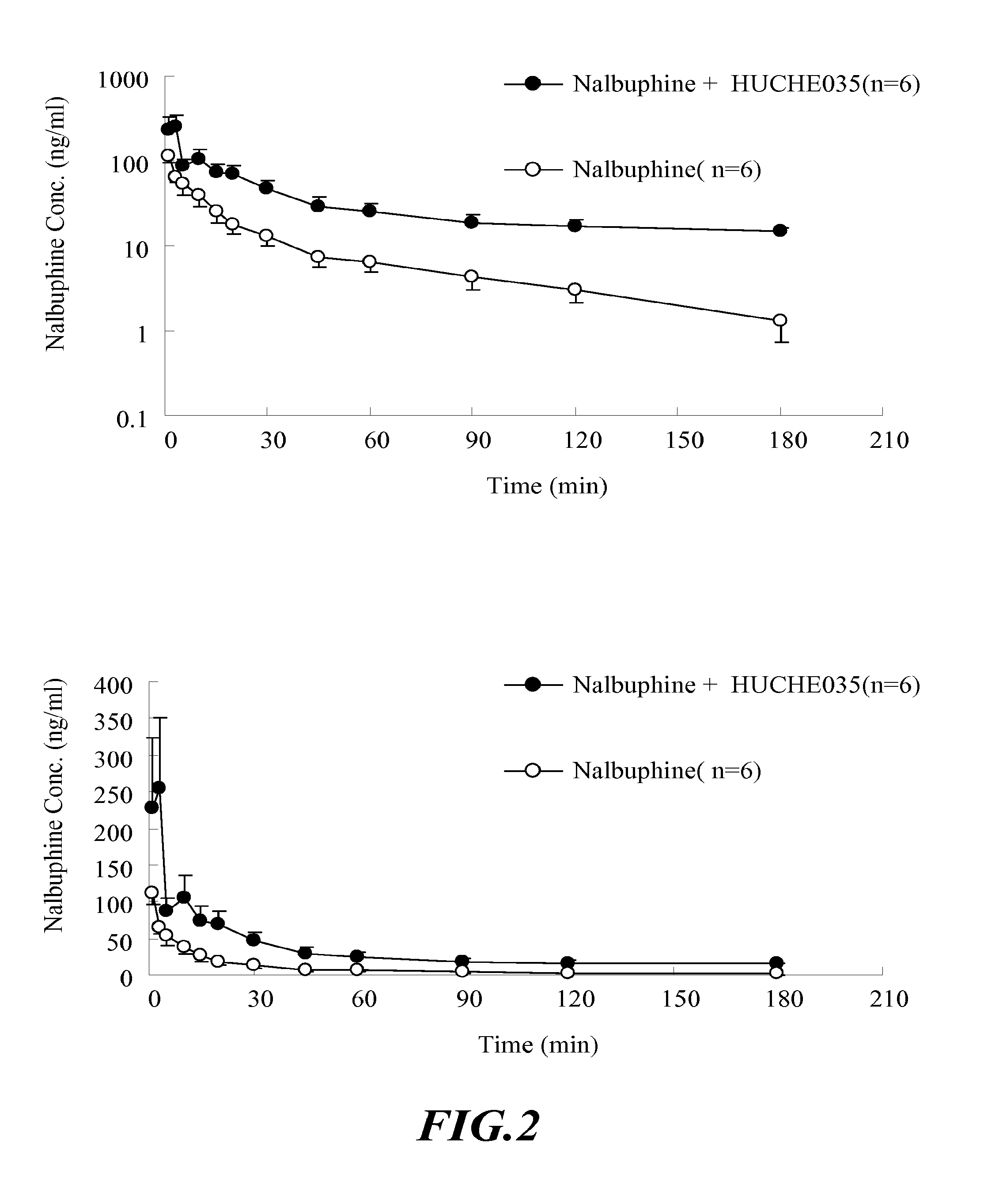
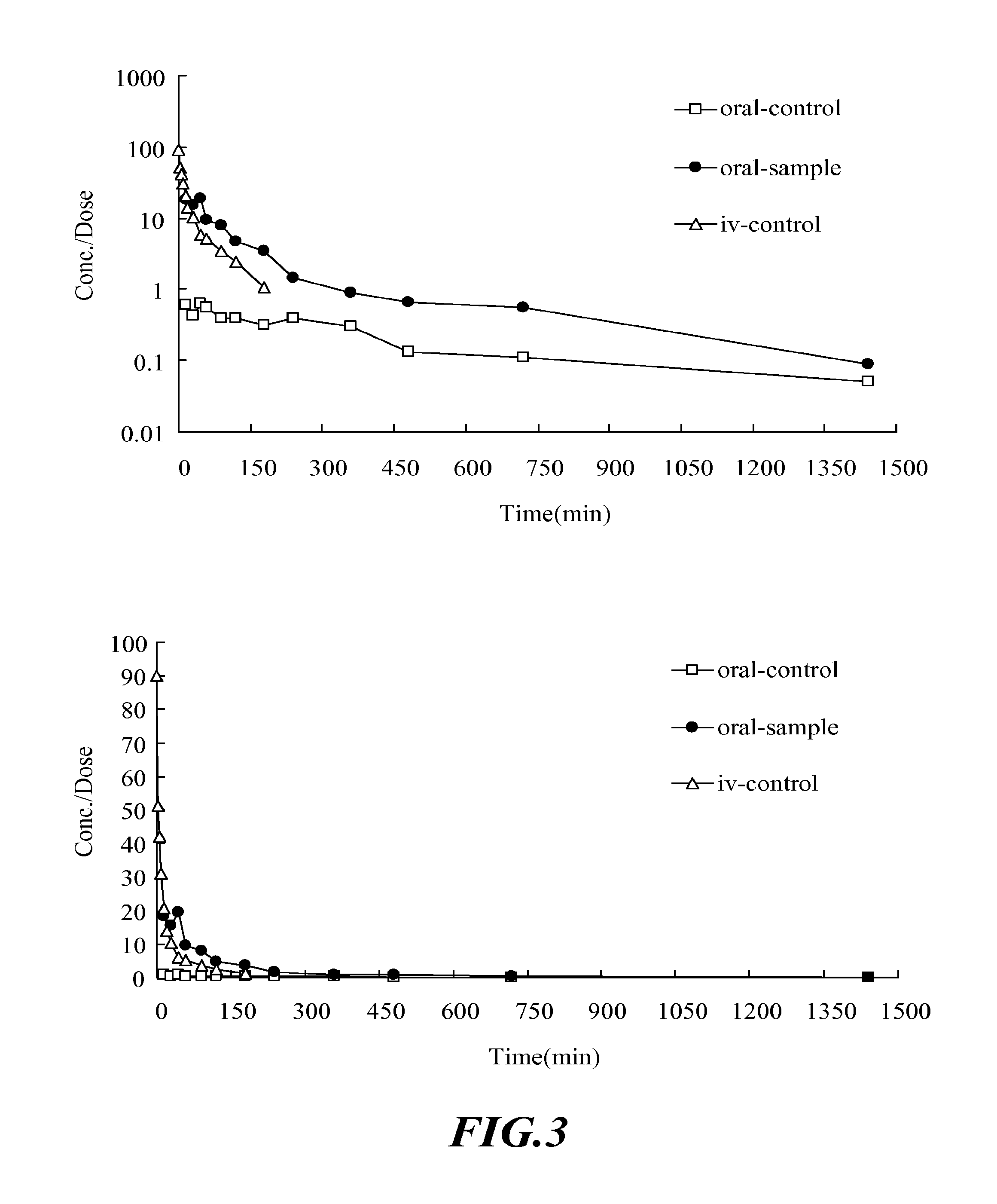
The Effect of UGT2B Inhibitors on the Concentration of Nalbuphine Taken Orally
Material and Methods:
[0105]Healthy male Sprague-Dawley rat, weight 500-600 g, acquired from National Laboratory Animal Breeding and Research Center in Taiwan, are used. After the acquisition, the animals are kept in a room with constant temperature (at 25±1° C.), humidity and day light (12 hours per day) for one week. Before the experiment, the animals are fasted for 12-16 hours. The drugs are administered orally.
2. Preparation of UGT2B Inhibitor and Nalbuphine Solution
[0106]Standard solution of nalbuphine is dissolved in water, and all inhibitors are dissolved in alcohol.
3. Methods:
[0107]i. Anesthetize the rat with 3-5 mg / 100 g body weight of pentobarbital intraperitoneally (I.P.). The rat will be anesthetized in about 20˜30 min.[0108]ii. Insert the PE-50 catheter tube into external jugular vein to sample the blood.[0109]iii. Orally administered 6 rats with UGT2B inhibitor—capillaris...
PUM
| Property | Measurement | Unit |
|---|---|---|
| half life | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
| wt % | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com