Aqueous electrophoretic deposition
a technology of electrophoretic deposition and water, applied in the direction of fluid pressure measurement, liquid/fluent solid measurement, peptide measurement, etc., can solve the problems of low economic value of direct current epd from water at low field, inability to produce coatings, and inability to attract economic value, so as to reduce or prevent water electrolysis, the effect of reducing or preventing water electrolysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Controllability of the Process
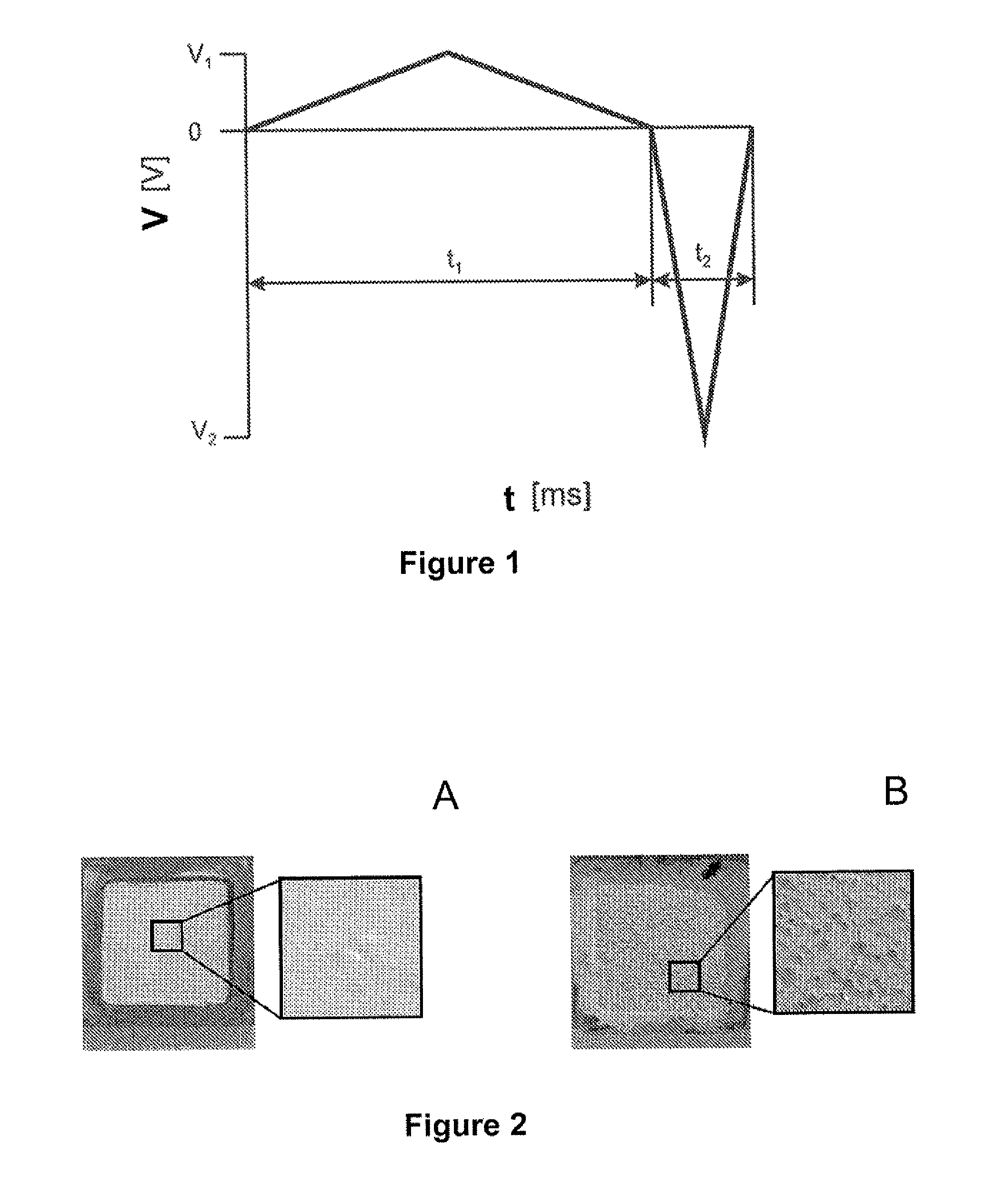
[0121]α-Al2O3 was deposited using the technology of present invention. The deposition rate of α-Al2O3 versus the symmetry of the applied signal V1, V2, t1, t2 (as defined in FIG. 1). The suspension consisted of a 200 g / L SM8 in water containing 4.10−4 M HNO3.
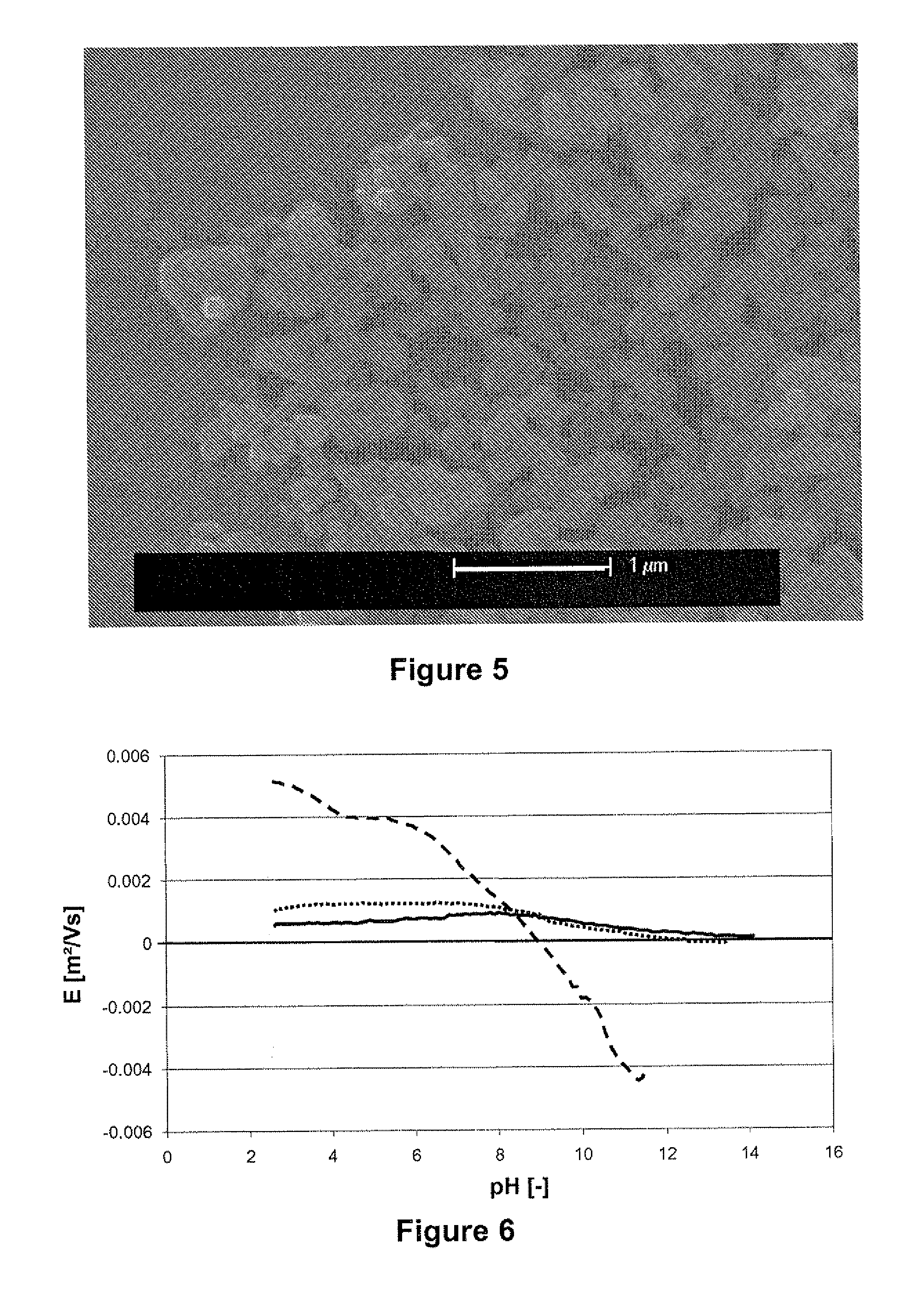
[0122]The more symmetric signals the smaller deposition rates observed. For instance symmetry of the signal was varied, by varying the height (amplitude) and width (pulse duration) of the triangular waves while maintaining a total period of 20 ms, a peak to peak height of 500 V and a net integral lower in absolute value than 4V, preferably lower than 1.24V and most preferably equal to zero, over one period. The asymmetry factor is determined as the ratio between the pulse heights, which is equal to the ratio of the pulse widths.
[0123]FIG. 3 shows that the deposition rate is zero when the applied field is symmetric (asymmetry factor equal to one) because no net electrophoresis occurs under the influe...
example 2
Material and Methods
[0127]Fresh deionised water with an initial conductivity of 0.04 μS / cm and prepared with a commercial ion exchange setup (Sation Aqualab 50) was used for all suspensions. Commercially available α-Al2O3 (Baikowski grade SM8), TiB2 (H.C.Starck grade F) and rutile TiO2 (Kemira grade Rutilex) powders were used for experimentation. The TiO2 was boiled and washed in a dialysis cell prior to use. No charging agent was added to the TiB2 suspension while the TiO2 was charged using KOH (Chem Lab 0.1 mol / l standard). HNO3 (Fluky 65%) was used as a charging agent for the experiments with alumina. For each experiment a new suspension was prepared using 50 ml freshly prepared water and the indicated amount of powder and charging agent (Table 1). The suspensions were magnetically stirred for 15 minutes; followed by 15 minutes of treatment in an ultrasonic bath (Branson 2510), subsequently followed by 15 minutes of stirring on a magnetic plate. A programmable function generator ...
example 3
Characterization
The Archimedes Method for Green Density Determination:
[0132]The green density is a value that relates to the particle packing in the formed object, and therefore is often used as a measure of the quality of the formed object. This value is traditionally stated as a percentage of the theoretical density of the material. For the measurement, the mass of the object is recorded. In order to include the interparticle porosity the object is coated with a lacquer that seals off this porosity, and after drying the mass of the coated object is registered as well. The volume of the coated object is measured by submerging it in a solvent of known density and measuring the buoyancy. The Archimedes principle is used to calculate the total volume of the coated object. From the weight difference before and after coating, the volume of the lacquer can be calculated and subtracted from the total volume. The density of the sealing lacquer is determined previously by coating a substrat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
| Electric potential / voltage | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com