Polymeric coupling agents and pharmaceutically-active polymers made therefrom
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
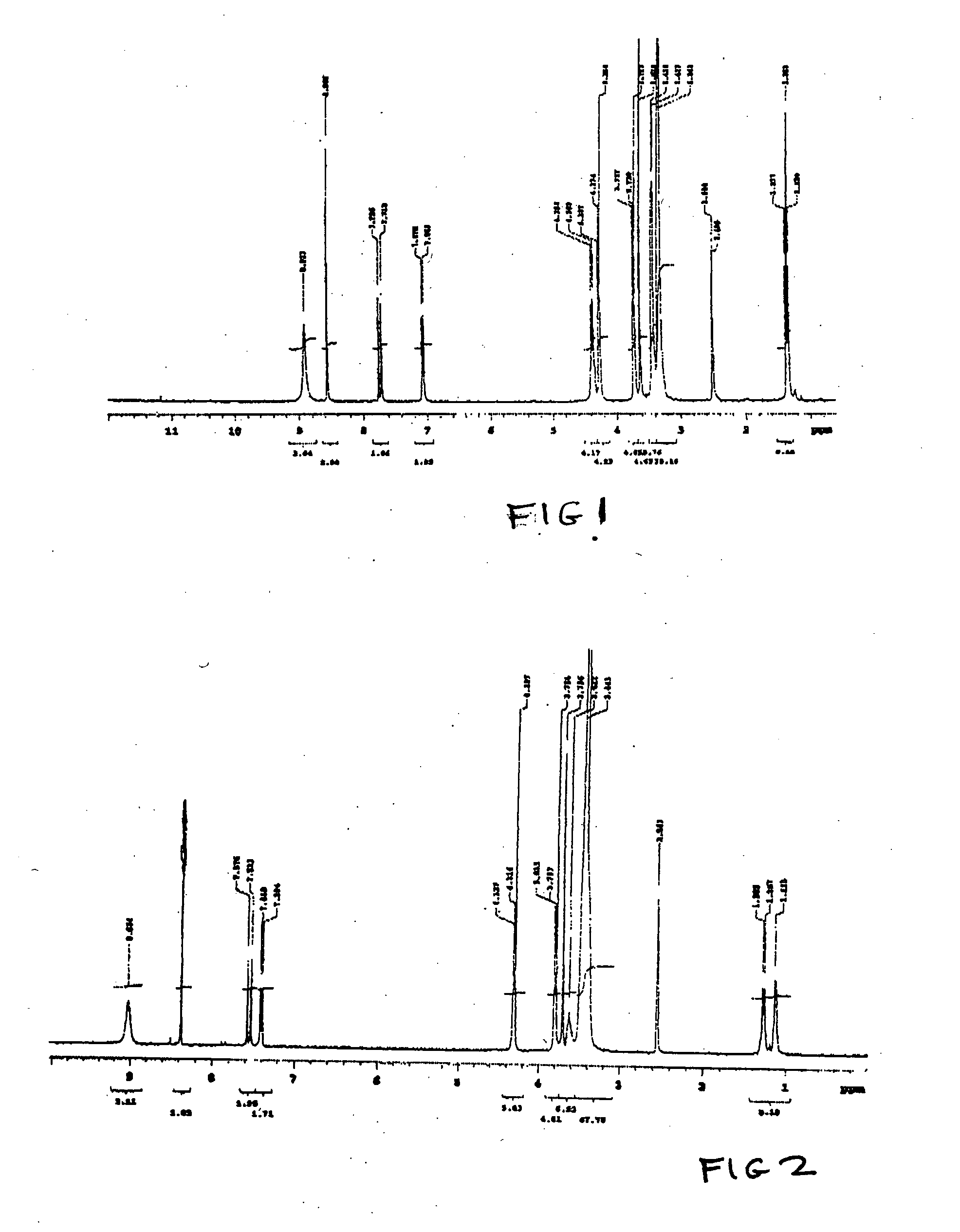
[0143] NORF-TEG-NORF and CIPRO-TEG-NORF are examples of antimicrobial drug containing biomonomers according to the invention. The example shows the use of a single drug or combination of drugs. The conditions of synthesis for this reaction are as follows.
[0144] In step A, of NORF (1.3 g, 4 mmol) / or CIPRO hydrochloride salt (4 mmol) were reacted with trityl chloride (2.7 g, 8.8 mmol) and TEA (0.6 ml, 8 mmol) (Aldrich, 99%) / or 12 mmol of TEA in the case of CIPRO in 40 ml of CHCl3 for four hours at room temperature. A clear solution was obtained.
[0145] In step B, 40 ml of methanol was added into the above clear solution. The mixture was heated to 50° C. and stirred for one hour, a precipitate appeared in the solution. After the reaction mixture was cooled down to room temperature, precipitates were collected by filtration. The precipitate was further purified from CHCl3 / methanol. 3.4 mmol of Product B were obtained. Yield was usually greater than 85%.
[0146] In step C, Product B (20 ...
example 2
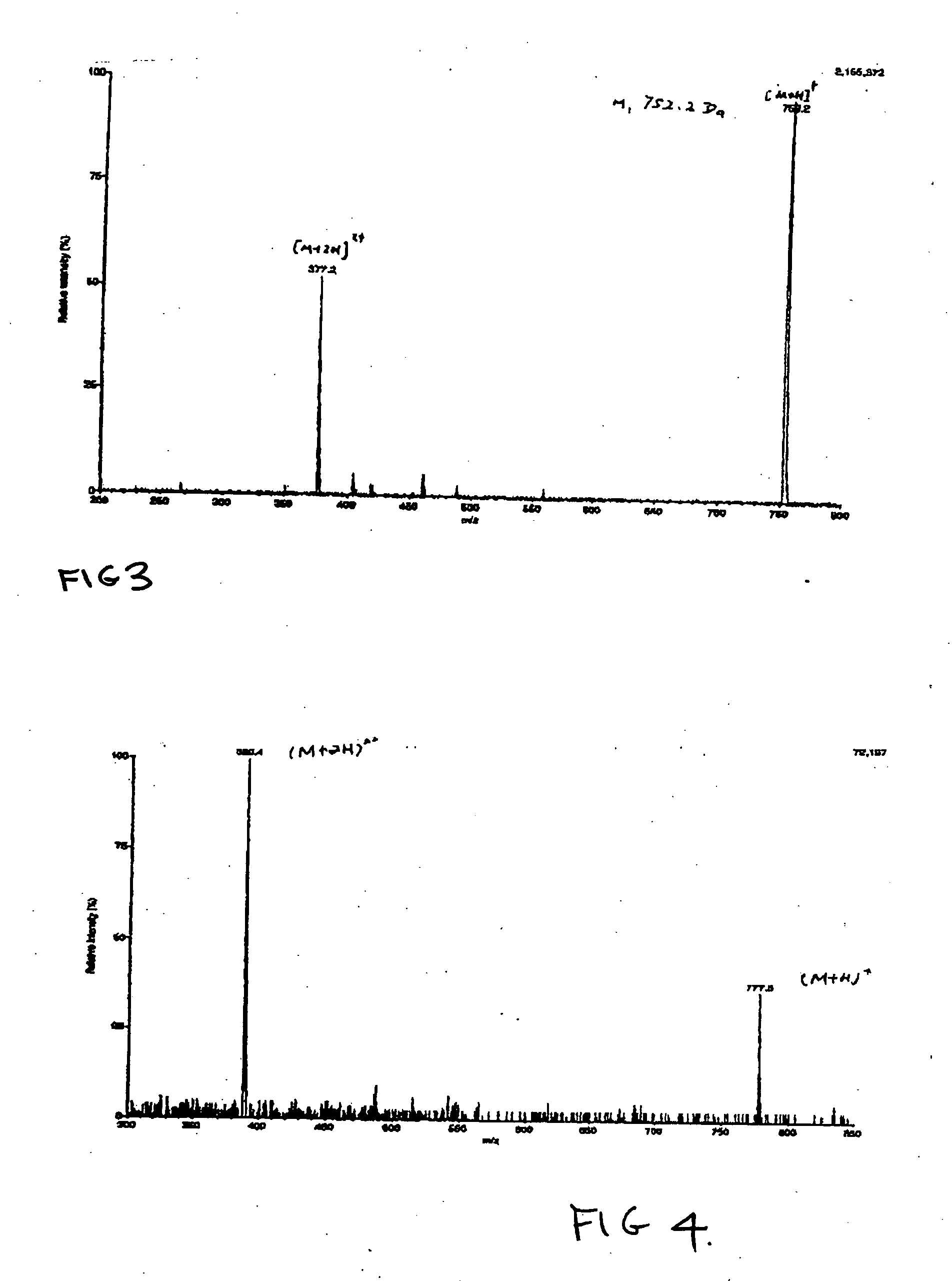
[0152] CIPRO-HDL-CIPRO is an example of biomonomer according to the invention and different from example 1 by the introduction of a hydrophobic link A molecule rather than hydrophilic link A molecule. The conditions of synthesis for this reaction are as follows.
[0153] The reaction conditions for selectively protecting amine groups of CIPRO are the same as the step A and B in Example 1.
[0154] In step C, Product B (20 mmol), HDL (9.5 mmol), DMAP (1.24 g, 10 mmol) are dissolved in 100 ml DCM. EDAC (31 g, 160 mmol) is then added into reaction system. The reaction mixture is stirred at room temperature under a nitrogen atmosphere for one week. After the reaction is finished, DCM is removed by rotary evaporatior. The residues are washed with de-ionized water several times to remove soluble reagents such as the by-product of urea. The solids are then dissolved in chloroform and washed with de-ionized water again. The crude product of the reaction is recovered from the solution by extract...
example 3
[0156] NORF-HDA-NORF is example of biomonomer according to the invention and different from example 1 in that a diamine is used to generate an amide rather than ester linkage in the biomonomer. The conditions of synthesis for this reaction are as follows.
[0157] The reaction conditions for selectively protecting the amine groups of NORF are the same as the step A and B in Example 1.
[0158] In step C, Product B (20 mmol), HDA (9.5 mmol), DMAP (1.24 g, 10 mmol) are dissolved in 100 ml DCM. EDAC (31 g, 160 mmol) is then added into reaction system. The reaction mixture is stirred at room temperature under a nitrogen atmosphere for one week. After the reaction is finished, DCM is removed by rotary evaporatior. The residues are washed with de-ionized water several times to remove soluble reagents such as the by-product of urea. The solids are then dissolved in chloroform and washed with de-ionized water again. The crude product of the reaction is recovered from the solution by extraction....
PUM
| Property | Measurement | Unit |
|---|---|---|
| Composition | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
| Biological properties | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com