Synthesis method of siponimod intermediate
A synthetic method and intermediate technology, applied in the field of pharmaceutical intermediate synthesis, can solve the problems of easy agglomeration of finished products, high production costs, and long synthetic routes, and achieve the effects of easy scale-up production, low production costs, and short synthetic routes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
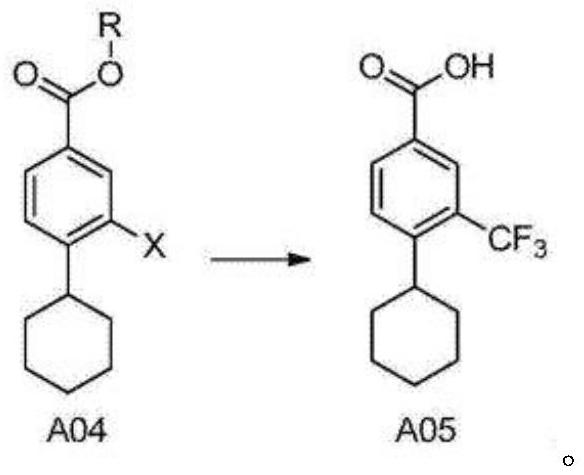
[0040] A kind of synthetic method of Siponimod intermediate, comprises the steps:
[0041] a) Esterification reaction: compound 1 reacts with methanol under concentrated sulfuric acid conditions to obtain compound 2;
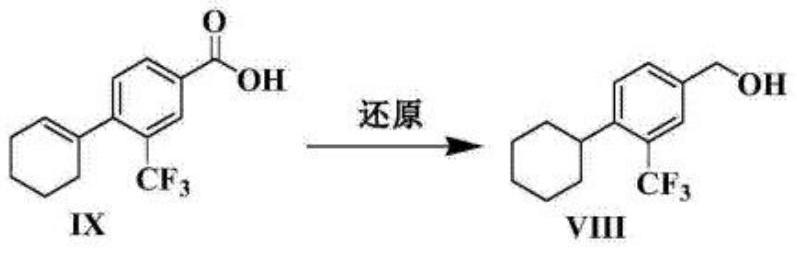
[0042] b) Reduction reaction: compound 2 reacts with absolute alcohol and tetrahydrofuran under reducing agent conditions to obtain compound 3; the absolute alcohol is any one of absolute ethanol, isopropanol, methanol or tert-butanol, the resulting The reducing agent is one of sodium borohydride, potassium borohydride, lithium borohydride or boron trifluoride ether;
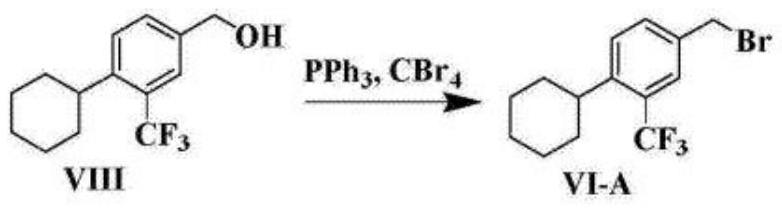
[0043] c) bromination reaction: compound 3 reacts with hydrobromic acid to obtain compound 4;
[0044] d) Coupling reaction: compound 4 reacts with N-hydroxyphthalimide to obtain compound 5;
[0045] e) Deprotection reaction: compound 5 reacts with methanesulfonic acid to obtain compound 6;
[0046] Among them, the structure of compound 1 is 4-cyclohexyl-3-(trifluoromethyl)benzoic acid;
[0...
Embodiment 2
[0056] This implementation carried out relevant parallel tests of the synthesis of Siponimod intermediates:
[0057] The specific operation for the synthesis of compound 2 in this example is as follows: add compound 1 and methanol into the reaction flask, add concentrated sulfuric acid under stirring, and stir until the reaction is complete; add 5 times the volume of water to the reaction solution, add methyl tert-butyl extracted with base ether, dried, and concentrated under reduced pressure to obtain compound 2.
[0058] The synthetic operation of compound 3 is as follows:
[0059] b1. Install a 0-100°C thermometer, mechanical stirring and liquid seal on a clean and dry reaction kettle, and inject nitrogen gas;
[0060] b2. Add 90 mL of anhydrous alcohol and 90 mL of tetrahydrofuran, start stirring, add 31.26 mmol of compound 2, and then add the reducing agent according to the molar ratio of reducing agent to compound 2 (2-3):1;
[0061] b3. After the addition, slowly heat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com