Recombinant human collagen, coding gene and application of recombinant human collagen in preparation of biodegradable collagen-based corneal substitute
A technology of human collagen and collagen-based cornea, applied in the direction of animal/human protein, application, tissue regeneration, etc., can solve the problem of corneal transplantation effectiveness that cannot take into account biocompatibility, biosafety, clinical application value, and products Difficult to market and other issues, to achieve good mechanical strength, no risk of immunogenicity, and excellent results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
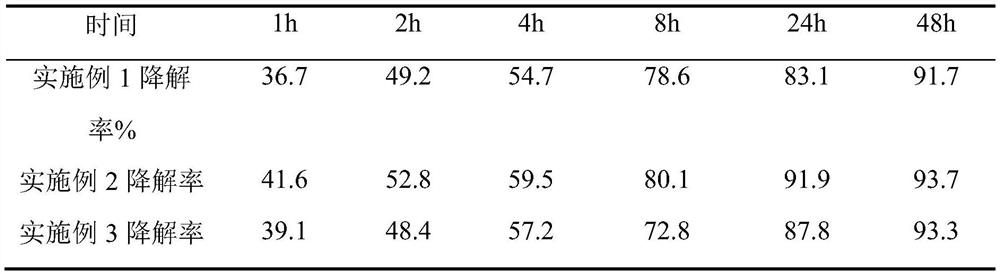
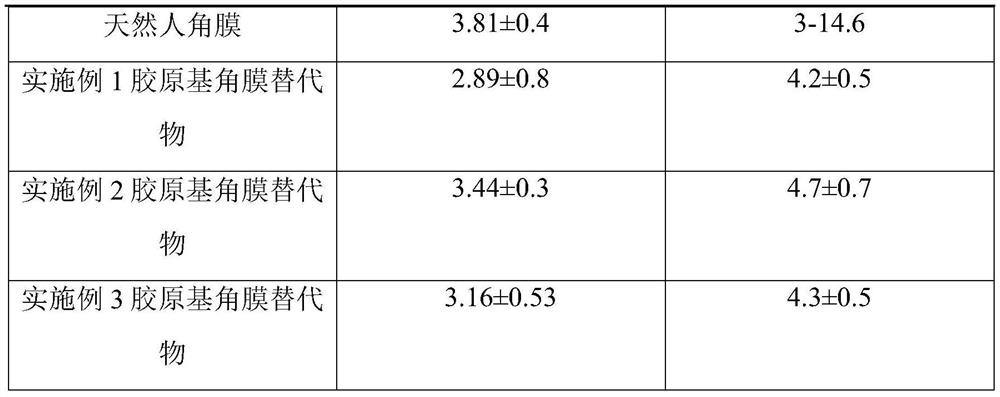
Embodiment 1
[0040] 1. Mix 18 g of recombinant human collagen with a molecular weight of 32 kDa and 2 g of polyvinyl alcohol 124 with a number average molecular weight of 170,000 to obtain a collagen-based blend.
[0041] 2. Dilute the collagen-based blend obtained in step 1 to 100mL with deionized water, stir evenly, and then
[0042] Add 0.02 g of carbodiimide, then place the resulting mixture in a mold, and conduct a crosslinking reaction at 25° C. for 2 hours.
[0043] 3. Put the sample after the cross-linking reaction in step 2 into a 1kDa cut-off dialysis bag for cross-linking agent removal treatment, and replace the ultra-pure water in the external environment every 6 hours, a total of 4 times; the residual cross-linking agent will be removed in a clean environment Samples were placed in phosphate sterile preservation solution and packaged, treated at -10°C for 8 hours, 10kGy Co 60 Sterilized by irradiation to obtain biodegradable collagen-based corneal substitutes.
[0044] 4. Ad...
Embodiment 2
[0047] 1. Mix 8 g of recombinant human collagen with a molecular weight of 32 kDa and 8 g of polyvinyl alcohol 124 with a number average molecular weight of 120,000 to obtain a collagen-based blend.
[0048] 2. Dilute the collagen-based blend obtained in step 1 to 100mL with deionized water, stir well, then add 0.032g of transglutaminase, and then place the resulting mixture in a mold for cross-linking reaction at 4°C 48h; the obtained product was washed with PBS for 30min, then added to 100mL of deionized water, stirred evenly, added 0.016g of glutaraldehyde, and cross-linked at 25°C for 4h.
[0049] 3. Ultrasonic cleaning of the sample after the cross-linking reaction in step 2 to remove the cross-linking agent, and then place the sample in a clean environment in a phosphate sterile preservation solution and package it, and treat it at -20°C for 6 hours. 15kGyCo 60 Sterilized by irradiation to obtain biodegradable collagen-based corneal substitutes.
[0050] 4. Add 1mL of ...
Embodiment 3
[0053] 1. Mix 8 g of recombinant human collagen freeze-dried powder with a molecular weight of 32 kDa, 1 g of polylactic acid with a number average molecular weight of 40,000, and 1 g of polyglycolic acid with a number average molecular weight of 20,000 to obtain a collagen-based blend.
[0054] 2. The above-mentioned collagen-based blend was formed into a scaffold by electrospinning technology to obtain a nanoscale three-dimensional mesh scaffold. The scaffold material was cut to the required size and immersed in 100mL ethanol solution containing 0.3g carbodiimide , cross-linking reaction at 25°C for 10h; the obtained product was rinsed with PBS for 30min, then immersed in 100mL aqueous solution containing 0.2g transglutaminase, and cross-linking reaction at 4°C for 16h.
[0055] 3. The corneal stent after the cross-linking reaction in step 2 was repeatedly washed with PBS to remove the remaining cross-linking agent. After freeze-drying, it was packaged in a medical aluminum f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com